Abstract
This study evaluated the accuracy of total keratometry (TK) and standard keratometry (K) for intraocular lens (IOL) power calculation in eyes treated with femtosecond laser-assisted cataract surgery. The retrospective study included a retrospective analysis of data from 62 patients (91 eyes) who underwent uneventful femtosecond laser-assisted cataract surgery with Artis PL E (Cristalens Industrie, Lannion, France) IOL implantation by a single surgeon between May 2020 and December 2020 in Severance Hospital, Seoul, South Korea. The new IOLMaster 700 biometry device (Carl Zeiss Meditec, Jena, Germany) was used to calculate TK and K. The mean absolute error (MAE), median absolute error (MedAE), and the percentages of eyes within prediction errors of ± 0.25 D, ± 0.50 D, and ± 1.00 D were calculated for all IOL formulas (SRK/T, Hoffer-Q, Haigis, Holladay 1, Holladay 2, and Barrett Universal II). There was strong agreement between K and TK (intraclass correlation coefficient = 0.99), with a mean difference of 0.04 D. For all formulas, MAE tended to be lower for TK than for K, and relatively lower MAE and MedAE values were observed for SRK/T and Holladay 1. Furthermore, for all formulas, a greater proportion of eyes fell within ± 0.25 D of the predicted postoperative spherical equivalent range in the TK group than in the K group. However, differences in MAEs, MedAEs, and percentages of eyes within the above prediction errors were not statistically significant. In conclusion, TK and K exhibit comparable performance for refractive prediction in eyes undergoing femtosecond laser-assisted cataract surgery.
Similar content being viewed by others
Introduction
Patient satisfaction after cataract surgery is largely dependent upon precise predictions of refractive outcomes, highlighting the importance of rapid technological advancements in methods for measuring ocular parameters and intraocular lens (IOL) power. Among the keys to accurate prediction of IOL power is precise measurement of the keratometric value. Today, standard keratometry (K) relies purely on measurements of the anterior corneal surface, and even when the posterior corneal surface is taken into consideration, the refractive index of the posterior corneal surface has been mostly inferred by calculation based on model eyes or nomograms1. Therefore, recent studies have debated the addition of accurate posterior corneal measurements to formulas for calculating IOL in patients undergoing cataract surgery2,3.
Recently, a new biometry device known as the IOLMaster 700 (Carl Zeiss Meditec, Jena, Germany), based on the principle of swept-source optical coherence tomography (SS-OCT), has been developed. IOLMaster 700 enables the assessment of the posterior corneal surface by combining data from the anterior corneal surface obtained via telecentric keratometry with pachymetry data obtained via SS-OCT4,5,6,7. Subsequently, total keratometry (TK) values are calculated using data from both the anterior and posterior cornea and measurements of corneal thickness, which are combined using the thick lens formula4.
Some studies have reported better refractive outcomes for conventional monofocal IOL implantation when accurate posterior corneal data are used to calculate TK values, whereas few other studies did not show any benefits of TK over K2,4,8,9,10. A recent study also compared refractive outcomes of cataract surgery with diffractive multifocal IOLs using K and TK, and K groups showed better IOL power prediction accuracy than TK groups across most of the formulas (Haigis, Holladay2, Barrett Universal II), except for the SRK/T11. Lawless et al. reported that the Barrett True-K using TK showed the lowest prediction error in eyes with previous laser refractive surgery12. Favorable results have also been documented for calculations associated with toric IOL implantation, which is more often expected to reduce postoperative astigmatism3,13,14. Similar to toric IOLs, femtosecond laser-assisted astigmatic keratotomy during femtosecond laser-assisted cataract surgery is among the preferred solutions for the correction of preoperative astigmatism. Therefore, accurate measurements of keratometry and subsequent optimal IOL power selection are crucial to ensure favorable postoperative refractive results. However, there is a paucity of data evaluating refractive outcomes using TK values in patients undergoing femtosecond laser-assisted cataract surgery. Femtosecond laser-assisted cataract surgery is a new technology which was introduced approximately half a decade ago. With potential benefits of more accurate capsulotomy size, shape, and positioning, and less IOL tilt with fewer higher-order aberrations, many surgeons integrate laser technology into their practice over conventional cataract surgery procedure15,16,17. Therefore, with the growing popularity, there is a need to evaluate the benefit of using TK for IOL power calculation in patients undergoing femtosecond laser-assisted cataract surgery.
Therefore, the present study aimed to evaluate the agreement of K and TK values measured using IOLMaster 700 in eyes undergoing femtosecond laser-assisted cataract surgery. Subsequently, the study compared the accuracy of K and TK in predicting residual refraction after femtosecond laser-assisted cataract surgery using existing standard formulas, including SRK/T, Hoffer-Q, Haigis, Holladay 1, Holladay 2, and Barrett Universal II.
Results
Patient characteristics and preoperative measurements
Patient characteristics and ocular biometry measurements, including axial length (AL), anterior chamber depth (ACD), lens thickness (LT), white-to-white (WTW), corneal center thickness (CCT), obtained using IOLMaster 700 are shown in Table 1. A total of 91 eyes (46 right, 45 left) of 62 patients (32 men, 30 women) with a mean age of 68.01 ± 10.07 years were included for the analysis. The mean AL was 23.89 ± 1.76 mm, and the mean ACD was 3.14 ± 0.40 mm. The mean magnitudes of preoperative K and TK values measured using IOLMaster 700 were 44.20 ± 1.46 D and 44.24 ± 1.48 D, respectively, while the corresponding median K and TK values were 44.22 D and 44.25 D, respectively. The mean preoperative and postoperative best-corrected visual acuity (BCVA) (LogMAR) were 0.39 ± 0.26 and 0.06 ± 0.22, respectively. The mean preoperative and postoperative SE was − 1.55 ± 4.78 and -0.48 ± 0.25, respectively. The mean IOL power selected by the surgeon was 21.18 ± 4.14 D.
Effect of femtosecond laser-assisted keratotomy on keratometric values
The mean magnitudes of preoperative and postoperative K values measured using Scheimpflug-based corneal topography were 44.14 ± 1.57 D and 44.23 ± 1.58 D, respectively. There was no significant difference between preoperative and postoperative keratometric values (P = 0.296). The mean magnitudes of preoperative and postoperative corneal astigmatism values measured using Scheimpflug-based corneal topography were 0.95 ± 0.60 D and 0.80 ± 0.46 D, respectively. There was a significant difference between preoperative and postoperative corneal astigmatism (P = 0.005).
Agreement between K and TK
The mean difference between K and TK (K–TK) was 0.04 D, and the ICC between the two groups was 0.99, indicative of very good agreement. As shown in Fig. 1a, the Bland–Altman plot of K and TK also demonstrates a fine agreement between the two groups. In addition, the Bland–Altman plot and pairwise comparisons of absolute prediction errors (APE) values for K and TK obtained using multiple IOL formulas are shown in Figs. 1b–g and 2.
Agreement between conventional keratometry (K) and total keratometry (TK) for the normal range of keratometry values. A Bland–Altman plot between standard keratometry (K) and total keratometry (TK). (b–g) Agreement between absolute prediction error (APE) of spherical equivalent (SE) from standard keratometry (K) and total keratometry (TK) values. A Bland–Altman plot showing (b) APE of SE calculated using the SRK/T formula. (c) APE of SE calculated using the Haigis formula. (d) APE of SE calculated using the Hoffer-Q formula. (e) APE of SE calculated using the Holladay 1 formula. (f) APE of SE calculated using the Holladay 2 formula. (g) APE of SE calculated using the Barrett Universal II formula.
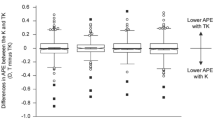
Pairwise comparison of absolute prediction error (APE) of spherical equivalent (SE) from standard keratometry (K) and total keratometry (TK) values. (a) APE of SE calculated using the SRK/T formula. (b) APE of SE calculated using the Haigis formula. (c) APE of SE calculated using the Hoffer-Q formula. (d) APE of SE calculated using the Holladay 1 formula. (e) APE of SE calculated using the Holladay 2 formula. (f) APE of SE calculated using the Barrett Universal II formula.
Refractive outcomes
Refractive errors, mean absolute errors (MAEs) and median absolute errors (MedAEs), calculated using six standard formulas (SRK/T, Hoffer-Q, Haigis, Holladay 1, Holladay 2, and Barrett Universal II) based on optimized IOL constants are shown in Table 2. The MAEs ranged from 0.370 to 0.397 D in the K group and from 0.358 to 0.404 D in the TK group. Although the MAEs tended to be lower in the TK group than in the K group for all formulas (except Hoffer-Q), the magnitudes of differences were small and not statistically significant (Wilcoxon signed-rank tests, all P values > 0.05). Additionally, the MAE and MedAE values were relatively lower for the SRK/T and Holladay 1 formulas than for other formulas; however, there were no significant differences between formulas (Friedman test with Bonferroni adjustment, P = 0.202).
The proportions of eyes within ± 0.25 D, ± 0.5 D, and ± 1.00 D of predicted postoperative spherical equivalent (SE) across all formulas are shown in Fig. 3 and Table 3. The proportion of eyes within ± 0.25 D of the predicted range for postoperative SE was higher in the TK group than in the K group for all formulas except for the Barrett Universal II, and a similar trend was observed for eyes falling within the ± 0.5 D range for the majority of formulas (Haigis, Hoffer-Q, Holladay 1, Holladay 2, Barrett Universal II). However, there were no significant differences between the groups (McNemar’s chi-square test, all P values > 0.05).
Discussion
Today, standard K relies purely on measurements of the anterior corneal surface, and the effect of posterior curvature is only predicted based on mathematical extrapolation using nomograms, such as the Baylor nomogram or Barrett Toric calculator. Due to its nature, the possibility of incorrect estimation of total corneal astigmatism remains a concern.
Therefore, several new technologies enabling direct measurement of posterior corneal curvature have been developed, leading to the introduction of the concept of “total corneal power.” However, depending on the device used to measure posterior corneal astigmatism, the use of total corneal power has been associated with varying outcomes in patients undergoing uncomplicated cataract surgery18. In one study, total corneal power, which was measured using topography from a Scheimpflug camera system, was less accurate in predicting IOL power than conventional K9. However, recent studies have demonstrated improvements in refractive outcomes using “total keratometry” values derived from IOLMaster 700, an SS-OCT-type optical biometry device for IOL calculation. Srivannaboon et al.4 reported strong agreement between K and TK, with a trend toward better refractive outcomes using TK in conventional cataract surgery4. However, other studies which investigated the patients undergoing multi-focal IOL implantation and the patients who underwent cataract surgery after myopic laser in situ keratomileusis did not find critical refractive benefit of TK over K in IOL power calculation10,11. Until now, few studies have aimed to validate the benefits of using TK values derived via SS-OCT-type optical biometry, and no studies assessing the compatibility of TK with IOL calculations in femtosecond laser-assisted cataract surgery have been published. Therefore, we aimed to evaluate the benefit of using TK for IOL power calculation in patients undergoing femtosecond laser-assisted cataract surgery. The analysis of this study indicated that TK and K exhibited comparable performance for refractive prediction in eyes undergoing the procedure.
This study follows the method for IOL power studies recommended by Hoffer et al.19 except that the assessment of accuracy was based on the MAE, as suggested by Kane et al.20 Using a single IOL model, a single surgeon performed all surgeries with an optimized IOL constant to minimize the potential bias from variations in operating styles or techniques21.
Previous studies have reported higher prediction accuracy for IOL calculation in patients undergoing conventional cataract surgery using TK than using K, with strong agreement between K and TK values2,4. Fabian et al. reported that the MAEs in SE within ± 0.5 D calculated using the Barrett Universal II formula were 84% (K group) versus 86% (TK group) in a post hoc analysis including 145 eyes2. Srivannaboon et al. also reported a trend toward lower MAEs and MedAEs for TK when compared with K when using the SRK/T, Hoffer-Q, Haigis, Holladay 1 and 2, and Barrett and Barrett TK Universal formulas. Moreover, they observed strong agreement between K and TK values, with a mean difference of 0.03 D between K and TK4. Despite the difference in the surgical procedure, our results are very similar to those of previous studies, supporting the compatibility of TK with IOL calculation.
We observed a strong agreement between TK and K, and there was a trend toward better refractive outcomes in the TK group than in the K group, considering lower MAE values and bigger percentages of eyes within the above prediction errors. This was not surprising because the conventional K values are based on mathematical extrapolation of the posterior corneal curvature and thus cannot take outliers and irregularities into account2. However, the magnitudes of the differences were small between the two groups and not statistically different. Therefore, although we have demonstrated comparable performance of TK in refractive prediction in eyes undergoing femtosecond laser-assisted cataract surgery, further studies need to be done whether there is superiority of TK over K values. It is unexpected that the Barrett Universal II formula yielded the lowest percentage of eyes (61.5% and 69.2%, respectively) with a prediction of ± 0.5 D in both the TK and K groups when compared with other formulas, given that recent studies have demonstrated the Barrett Universal II formula to have the lowest absolute error when compared with other modern formulas20,22,23. However, Srivannaboon et al., who investigated refractive outcomes using TK and K for IOL power calculation in conventional cataract cases, also reported that the Barrett formula yielded the lowest percentage of eyes within the specified range (K: 59.6%, TK: 65.4%). Srivannaboon et al. pointed out that this may be due to the use of optimized IOL constants instead of personalized IOL constants4. In addition, the effect of astigmatic changes after femtosecond laser-assisted astigmatic keratotomy may have played a role in our patients. And it may be associated with the shape of IOL of this study. The IOL used in the current study is slightly small sized with 10.50 mm ~ 11.00 mm overall diameter, and 5.80 mm ~ 6.15 mm optic diameter, and has four closed loop haptic. Further studies with a larger number of patients in each group should be conducted to verify our results.
This study has some limitations. First, the study was confined to patients with biometric parameters falling within the normal range. Therefore, these results cannot be applied to patients with extreme parameters, such as long/short ALs, post-refractive surgery eyes, cases of keratoconus, and others. Second, we did not consider the effect of femtosecond laser-assisted astigmatic keratotomy. Personalized laser keratotomy settings may have introduced bias in our results. However, previous studies have reported no significant difference in the mean postoperative SE between the femtosecond laser-assisted cataract and manual phacoemulsification groups24. Due to a lack of post-operative IOLMaster 700 measurements, the mean corneal front K values measured via Scheimpflug-based corneal topography were compared before and after cataract surgery, and no statistically significant difference was noted (paired sample t-test, P value = 0.296). Therefore, we assumed that the additional step of femtosecond laser-assisted astigmatic keratotomy would not have changed our results to a great extent.
In conclusion, our preliminary results suggest that TK and K values exhibit comparable performance in IOL calculations for femtosecond laser-assisted cataract surgery when the same optimized IOL constant is used, as has been observed in conventional cataract surgery. As this is the first study to investigate the applicability of TK in femtosecond laser-assisted cataract surgery using multiple current standard IOL formulas, further studies involving more eyes and different IOL models are necessary to elucidate the benefit of TK in IOL calculation.
Methods
Participants
This retrospective, observational case series was approved by the Institutional Review Board (IRB) of the Yonsei University College of Medicine (4-2021-0131) and was conducted in accordance with the tenets outlined in the Declaration of Helsinki. Informed consent was waived by the IRB of Yonsei University College of Medicine due to the retrospective nature of the study. The study included 91 eyes of 62 patients who had undergone uneventful femtosecond laser-assisted cataract surgery by a single surgeon at Severance Hospital in Seoul, Korea, between May 2020 and December 2020.
Inclusion criteria were as follows: availability of preoperative TK data from IOLMaster 700, auto-refraction performed at 3 months after cataract surgery, absence of complications during or after cataract surgery, and Artis PL E (Cristalens industrie, Lannion, France) IOL implantation during surgery. Patients who had undergone previous ocular surgery; those who had experienced ocular trauma, ocular diseases, or opacities that may impair visual acuity; those with active ocular infection or inflammation; and those who failed to keep postoperative follow-up appointments were excluded from the study.
Preoperative and postoperative assessments
All patients underwent a comprehensive preoperative ophthalmologic examination performed within 3 months before cataract surgery. This included assessments of BCVA, intraocular pressure (IOP) (CANON, TX-20, Japan), manifest refraction, keratometry, auto-refraction, slit-lamp examination, fundoscopy, retinal examination using Heidelberg spectralis optical coherence tomography (software V.5.4.7.0; Heidelberg Engineering, Heidelberg, Germany), specular microscopy (Tomey, EM4000, GmbH, Germany), and Scheimpflug-based corneal topography (Pentacam HR, Oculus, Wetzlar, Germany). All optical biometric parameters, including K, TK, AL, CCT, LT, WTW, and ACD, were measured using IOLMaster 700. A single experienced surgeon selected the IOL power for each patient according to surgical preferences, and predicted postoperative spherical equivalent refractions for all formulas were documented. The patients were examined 1 day, 1 week, 1 month, and 3 months postoperatively. Slit-lamp examination, IOP measurements, K, auto-refraction assessments, manifest refraction assessments, and corneal topography were performed at each follow-up visit.
Surgical technique
All patients underwent scheduled cataract surgery, which was performed by a single, experienced surgeon (I.J.). Femtosecond laser-assisted cataract surgery was performed using the LenSx platform (Alcon Laboratories, Inc., Fort Worth, TX, USA) for capsulorhexis, nucleus fragmentation, and penetrating arcuate keratotomy. The keratotomies were centered on the steep corneal axis, and the length of the arcuate keratotomy was determined using a Verion system (Alcon Laboratories, Inc.). After all pattern selections and parameter choices were made, patients were instructed to lie on a built-in bed beneath the laser device. The disposable vacuum interface was positioned to the operation eye with a suction ring, and laser emission was initiated, following which the patient was transported to the operation room. At the start of cataract surgery, laser corneal incision sites were carefully dissected using a Sinskey hook. The incised anterior capsule button was removed using forceps. Patients underwent conventional phacoemulsification using a Centurion Vision System (Alcon Laboratories, Inc.). Following phacoemulsification, a single type of IOL, Artis PL E, was implanted in the capsular bag using an injector, and the remaining ophthalmic visco-surgical device was removed. All incision sites were hydrated to prevent leakage.
Statistical analyses
Data were analyzed using SPSS Statistics software (version 25; IBM Corporation, Armonk, NY) and Microsoft Excel 2016 (Microsoft Corp, USA). P values < 0.05 were considered statistically significant. Descriptive statistics including standard deviations, means, medians, and frequencies were determined.
The APEs, MAEs, MedAEs, and distributions of eyes within ± 0.25 D, ± 0.5 D, and ± 1.00 D of predicted postoperative SE refraction were calculated. The APE of SE was defined as the absolute difference between the actual postoperative SE and predicted postoperative SE. Similarly, the MAE of SE was defined as the mean absolute difference between the actual postoperative SE and predicted postoperative SE, while the MedAE of SE was defined as the median absolute difference between the actual postoperative SE and predicted postoperative SE25.
The percentages of eyes within ± 0.25 D, ± 0.5 D, and ± 1.00 D of predicted postoperative SE in the K and TK groups were compared using paired McNemar’s chi-square tests. The differences between the APEs obtained from the K and TK data using each formula was compared using Wilcoxon signed-rank tests. The APEs from the K and TK data of all formulas were compared using the Friedman test for multiple comparisons. Agreement between K and TK was assessed using the Bland–Altman plot method, and intraclass correlation coefficients (ICCs) with 95% confidence intervals were calculated to compare K and TK values26.
References
Koch, D. D., Jenkins, R. B., Weikert, M. P., Yeu, E. & Wang, L. Correcting astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J. Cataract. Refract. Surg. 39, 1803–1809 (2013).
Fabian, E. & Wehner, W. Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J. Refract. Surg. 35, 362–368 (2019).
Sano, M. et al. Influence of posterior corneal astigmatism on postoperative refractive astigmatism in pseudophakic eyes after cataract surgery. BMC Ophthalmol. 16, 212 (2016).
Srivannaboon, S. & Chirapapaisan, C. Comparison of refractive outcomes using conventional keratometry or total keratometry for IOL power calculation in cataract surgery. Graefes Arch. Clin. Exp. Ophthalmol. 257, 2677–2682 (2019).
Akman, A., Asena, L. & Gungor, S. G. Evaluation and comparison of the new swept source OCT-based IOLMaster 700 with the IOLMaster 500. Br. J. Ophthalmol. 100, 1201–1205 (2016).
Koch, D. D. et al. Contribution of posterior corneal astigmatism to total corneal astigmatism. J. Cataract. Refract. Surg. 38, 2080–2087 (2012).
LaHood, B. R. & Goggin, M. Measurement of posterior corneal astigmatism by the IOLMaster 700. J. Refract. Surg. 34, 331–336 (2018).
Saad, E., Shammas, M. C. & Shammas, H. J. Scheimpflug corneal power measurements for intraocular lens power calculation in cataract surgery. Am. J. Ophthalmol. 156, 460–467 (2013).
Shammas, H. J., Hoffer, K. J. & Shammas, M. C. Scheimpflug photography keratometry readings for routine intraocular lens power calculation. J. Cataract. Refract. Surg. 35, 330–334 (2009).
Sandoval, H. P., Serels, C., Potvin, R. & Solomon, K. D. Cataract surgery after myopic laser in situ keratomileusis: objective analysis to determine best formula and keratometry to use. J. Cataract. Refract. Surg. 47, 465–470 (2021).
Lee, H., Chung, J. L., Kim, Y. J., Kim, J. Y. & Tchah, H. Prediction accuracy of standard and total keratometry by swept-source optical biometer for multifocal intraocular lens power calculation. Sci. Rep. 11, 4794 (2021).
Lawless, M. et al. Total keratometry in intraocular lens power calculations in eyes with previous laser refractive surgery. Clin. Exp. Ophthalmol. 48, 749–756 (2020).
Reitblat, O., Levy, A., Kleinmann, G., Abulafia, A. & Assia, E. I. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses: Comparison of methodologies. J. Cataract. Refract. Surg. 42, 217–225 (2016).
Savini, G. & Naeser, K. An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest. Ophthalmol. Vis. Sci. 56, 827–835 (2015).
Friedman, N. J. et al. Femtosecond laser capsulotomy. J. Cataract. Refract. Surg. 37, 1189–1198 (2011).
Kranitz, K. et al. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J. Refract. Surg. 27, 558–563 (2011).
Nagy, Z. Z. et al. Comparison of intraocular lens decentration parameters after femtosecond and manual capsulotomies. J. Refract. Surg. 27, 564–569 (2011).
Tonn, B., Klaproth, O. K. & Kohnen, T. Anterior surface-based keratometry compared with Scheimpflug tomography-based total corneal astigmatism. Invest. Ophthalmol. Vis. Sci. 56, 291–298 (2014).
Abulafia, A., Barrett, G. D., Koch, D. D., Wang, L. & Assia, E. I. Protocols for studies of intraocular lens formula accuracy. Am. J. Ophthalmol. 164, 149–150 (2016).
Kane, J. X., Van Heerden, A., Atik, A. & Petsoglou, C. Intraocular lens power formula accuracy: Comparison of 7 formulas. J. Cataract. Refract. Surg. 42, 1490–1500 (2016).
Rocha-de-Lossada, C. et al. Intraocular lens power calculation formula accuracy: Comparison of 12 formulas for a trifocal hydrophilic intraocular lens. Eur J Ophthalmol, 1120672120980690 (2020).
Kane, J. X., Van Heerden, A., Atik, A. & Petsoglou, C. Accuracy of 3 new methods for intraocular lens power selection. J. Cataract. Refract. Surg. 43, 333–339 (2017).
Melles, R. B., Holladay, J. T. & Chang, W. J. Accuracy of intraocular lens calculation formulas. Ophthalmology 125, 169–178 (2018).
Lawless, M. et al. Outcomes of femtosecond laser cataract surgery with a diffractive multifocal intraocular lens. J. Refract. Surg. 28, 859–864 (2012).
Aristodemou, P., Cartwright, N. K., Sparrow, J. M. & Johnston, R. Intraocular lens calculations. Ophthalmology 118, 1221 (2011).
Giavarina, D. Understanding Bland Altman analysis. Biochem Med (Zagreb) 25, 141–151 (2015).
Acknowledgements
This study was supported by a faculty research grant of Yonsei University College of Medicine [6-2020-0222] and a grant of the Basic Science Research Program National Research Foundation of Korea [NRF-2019R1F1A1063311] funded by the Ministry of Science, ICT and Future Planning. The funding organization had no role in the design or conduct of this study.
Author information
Authors and Affiliations
Contributions
S.Y. and I.J. designed the project. S.Y., T.K. and I.J. analysed the results. S.Y. and I.J. wrote the main manuscript. T.K., K.Y.S. and E.K.K. performed the critical revision of the manuscript for important intellectual content. All authors contributed to the review of the literature and drafting of the manuscript, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ryu, S., Jun, I., Kim, Ti. et al. Prediction accuracy of conventional and total keratometry for intraocular lens power calculation in femtosecond laser-assisted cataract surgery. Sci Rep 11, 12869 (2021). https://doi.org/10.1038/s41598-021-92354-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92354-1
This article is cited by
-
Calculation of the total corneal astigmatism using the virtual cross cylinder method on the secondary principal plane of the cornea
Scientific Reports (2024)
-
Lower refractive prediction accuracy of total keratometry using intraocular lens formulas loaded onto a swept-source optical biometer
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Application of total keratometry in ten intraocular lens power calculation formulas in highly myopic eyes
Eye and Vision (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.