Abstract
Previous studies have largely failed to clarify the relationship between p16INK4A status and cervical adenocarcinoma prognosis. The current study aimed to examine the clinical and pathological significance of p16INK4A expression in several cervical adenocarcinoma subtypes. Eighty-two samples collected from patients with cervical adenocarcinoma were formalin fixed and paraffin embedded. Next, p16INK4A levels were analyzed with immunohistochemistry. Additionally, the relationship between p16INK4A expression and clinicopathological factors as well as prognosis was evaluated. The expression of p16INK4A was mostly detected in all usual cervical adenocarcinoma subtypes. In the gastric type, only a few cases were positive for p16INK4A expression. Results of the Kaplan–Meier analysis indicated that the positive p16INK4A expression in tumor cells was significantly associated with favorable progression-free survival and overall survival in patients with cervical adenocarcinoma (p = 0.018 and p = 0.047, respectively, log-rank test). Our findings suggest that the status of p16INK4A expression may influence prognosis. Thus, p16INK4A expression could be used as a biomarker for improving the prognosis of patients with cervical adenocarcinoma.
Similar content being viewed by others
Introduction
According to a report of the National Cancer Institute, cervical cancer is the second most common cancer and the second leading cause of cancer-related deaths in women in their twenties and thirties1. Adenocarcinoma accounts for approximately 10‒25% of uterine cervical carcinoma cases2,3,4. Adenocarcinoma of the cervix is less common than squamous cell carcinoma (SCC), but the relative incidence of adenocarcinoma is increasing, particularly in young women5,6,7. Despite preventive measures, such as HPV vaccines and screening for cervical cancer via cytological examinations, adenocarcinoma is currently estimated to account for up to 25% of all cervical cancers8,9.
Currently, both these preventive measures are not effective for patients in Japan. Although a program encouraging vaccination was launched in June 2013, the HPV vaccine has since been withdrawn owing to many reports of adverse effects, such as complex regional pain syndrome (CPRS). Many junior high school students and their parents are wary of this syndrome, which has resulted in a decrease in new vaccination rates (0.97%) compared with those before these reports10,11. The World Health Organization (WHO) criticized the policy of the Japanese Government and declared that the government must resume vaccination for HPV; however, it has not been resumed. In Japan, the government recommends cervical cytology for cancer screening every 2 years for women from the age of 20 years. Nevertheless, monitoring of cervical cancer has been disregarded, leading to a cancer-screening rate of only 28.3% in 201612. Thus, the prevention of cervical cancer in Japan has become problematic, and the development of new treatments and biomarkers for cervical cancer in a clinical setting is of utmost importance.
P16INK4A acts as not only a surrogate marker of HPV infection but also a tumor suppressor gene, which regulates the cell cycle by specifically inhibiting cyclin D/CDK4/6 activity. Results of previous studies indicating a relationship between p16INK4A expression and prognosis of cervical adenocarcinoma are considered controversial. Therefore, in the current study, we investigated the relationship between p16INK4A expression and patient prognosis. Furthermore, we evaluated the potential relationship between p16INK4A expression and immune-checkpoint inhibitor-related therapy in cervical adenocarcinoma.
Materials and methods
Tissue samples
Tissue samples were obtained from the Department of Obstetrics and Gynecology, Shimane University School of Medicine (Shimane, Japan) and Seirei Hamamatsu General Hospital (Shizuoka, Japan) between 2003 and 2017. Samples of all patients with cervical adenocarcinoma treated during the study period were included. The clinical information of patients was retrospectively obtained from electronic medical records.
The acquisition of tumor tissues was approved by the Institutional Review Board, Shimane University (IRB Nos. 20070305-1 and 20070305-2). After appropriate explanation, written informed consent was obtained from the patients for the procedure and for participation in the study. For those who could not visit the hospitals again, we had clearly announced that the opportunity to opt out of the study is always available to patients by taking measures such as providing information regarding opting out, on the website.
All experiments were performed in accordance with relevant guidelines and regulations. Furthermore, the study was performed in accordance with tenets of the Helsinki Declaration.
A total of 82 samples were collected from patients with uterine cervical adenocarcinoma who underwent surgical resection or biopsy. Diagnoses were confirmed by a gynecopathologist (IN) trained at our institution. Many patients were primarily treated with surgery, and most of them had received adjuvant therapies such as chemotherapy, radiotherapy, and concurrent chemoradiotherapy (CCRT) with a platinum drug (weekly cisplatin; 40 mg/m2). These treatment strategies were selected based on the Japanese guidelines for cervical cancer.
Immunohistochemistry
The expression of p16INK4A and immune escape mechanism-related factors (CD8, PD-L1, and PD-1) was evaluated with immunohistochemistry (IHC). Formalin-fixed and paraffin-embedded sections (4 μm thick) were dewaxed in xylene and hydrated via an alcohol gradient. Following antigen retrieval in a sodium citrate buffer, the sections were incubated overnight at 4 °C with antibodies against p16INK4A (1:30; cloneE6H4; mtm-Roche Diagnostics, Heildelberg, Germany), CD-8 (1:100; Roche, Basel, Switzerland), PD-L1 (1:400; ab205921; Abcam, Cambridge, United Kingdom), and PD-1 (1:100; Roche). Samples were evaluated under a light microscope by a pathologist, who was blinded to clinicopathological factors.
Definition of p16INK4A and CD8/PD-1/ PD-L1 positivity by IHC
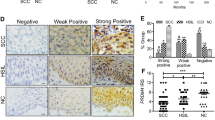
The expression of p16INK4A in both cytoplasm and nucleus was evaluated by staining for three tumor density categories as follows: 0 (undetectable), 1 + (low density), and 2 + (high density). Intensities of + 1 and + 2 were considered as strong staining. In a previous study, over 70% of cervical cancer cells with strong p16 staining of the nuclei and cytoplasm were regarded as p16 positive13. Therefore, in the current study, most of the tumor cells with strong p16 staining were considered as p16 positive. The population density of tumor infiltrating lymphocytes was stratified by CD8 staining into three categories as follows: 0 (undetectable), 1 + (low density, 0–30%), and 2 + (moderate-high density, ≥ 30%). Samples that were categorized as 2 + were considered positive. For PD-L1, tumors with ≥ 5% of stained tumor cells (membranous and cytoplasmic staining) were considered positive. For PD-1, tumors with ≥ 5% of tumor-infiltrating stained lymphocytes were considered positive.
Statistical analysis
The correlation between p16INK4A expression and clinicopathological characteristics and patient prognosis was analyzed by chi-square test. Furthermore, the correlation between p16INK4A expression and other immune escape mechanism-related factors was analyzed using the chi-squared test. Progression-free survival (PFS) and overall survival (OS) were analyzed using the Kaplan–Meier method with the log-rank test. The univariate and multivariate Cox proportional hazard regression analyses were followed by binomial logistic regression for ordered categorical variables. Statistical analyses were performed using IBM SPSS (IBM, Armonk, NY, USA), version 23. Statistical significance was set at p < 0.05.
Ethics declaration
The acquisition of tumor tissues was approved by the Shimane University Institutional Review Board (IRB Nos. 20070305-1 and 20070305-2).
Consent to participate
After appropriate explanation, patients provided written informed consent for the procedure and for participation in the study. For patients who could not visit hospitals again, we had clearly announced that the opportunity to opt out is always available by taking measures such as carrying the information on the opportunity to opt out on the website, as well as making arrangements so that patients can opt out via the website at any time.
Results
Clinicopathological characteristics of the patients
Histological subtypes of the test cases are shown in Table 1 and Fig. 1.
There were several histological subtypes in cervical adenocarcinoma, including the usual type, gastric type, and others. The usual type was the most popular type (59.0%), followed by the gastric type (16%). The clinicopathological characteristics of the test patients are summarized in Table 2 and Supplementary Table S1.
In the present study, we identified clinical stages according to the definition of the International Federation of Gynecology and Obstetrics (FIGO) 2014 as stages IA, IB1, B2, IIA, IIB, IIIB, and IVB. Treatments received by the test patients were as follows: 72 underwent radical hysterectomy and received adjuvant therapy, such as concurrent chemoradiotherapy (CCRT); 9 patients with advanced stage cancer received CCRT; and 1 patient received chemotherapy without surgery due to multiple distant metastases. Radiotherapy (whole pelvic irradiation) or chemotherapy (paclitaxel 175 mg/m2 and carboplatin area under the curve = 5 mg/m2) was administered postoperatively in patients with a high recurrence risk because of locally advanced stage, non-SCC type of histology, bulky tumor > 4 cm, deep infiltration depth of cervical tumor, grade 2 or 3, lymph node metastasis, or lympho-vascular space invasion.
The chemotherapy regimens adopted were paclitaxel plus carboplatin, paclitaxel plus cisplatin, docetaxel plus carboplatin, irrinotecan plus cisplatin, and gemcitabine. In the CCRT regimen, 5‒6 courses of 40 mg/m2 cisplatin were administered weekly.
Relationship between p16INK4A expression and clinicopathological factors in cervical adenocarcinoma
In the present study, 60/82 (73.1%) patients displayed strong p16INK4A expression. Representative cases with positive or negative p16INK4A expression are shown in Fig. 2. Significant relationships were observed between p16INK4A expression and age (p = 0.001), FIGO stage (p = 0.002), histological subtype (p < 0.0001), pelvic lymph node metastasis (p = 0.034), LVSI metastasis (p = 0.003), and disease recurrence (p = 0.021) (Table 3).
HE staining and immunohistochemistry of specimens obtained from patients with cervical adenocarcinoma. The expression of p16INK4A was evaluated in three categories of tumor density via staining: 0 (undetectable); 1 + (low density); 2 + (high density). Cases that were 2 + were considered positive and those with 0 and + 1 were considered negative.
Relationships between p16INK4A expression and CD8, PD-L1, and PD-1 expression in cervical adenocarcinoma
The relationships between p16INK4A expression and CD8, PD-L1, or PD-1 expression were assessed using the chi-squared test. The positive rates of expression of immune checkpoint-related factors, such as CD8, PD-1, and PD-L1, were not significantly different according to p16INK4A expression (Supplementary Table S2).
Clinical features of cervical adenocarcinoma with p16INK4A expression
Kaplan–Meier analysis was performed to determine the potential correlation between p16INK4A expression and prognosis. Cervical adenocarcinoma patients with p16INK4A negativity presented significantly worse PFS and OS than those with p16INK4A positivity (p = 0.018 and p = 0.047, respectively, log-rank test; Fig. 3a,b).
Kaplan–Meier analysis of progression-free (a) and overall (b) survival between the p16INK4A-positive and negative groups. PFS was significantly extended in the p16INK4A-positive group compared with that in the negative group (p = 0.018, log-rank test; a). OS was also extended in the p16INK4A-positive group compared with that in the negative group (p = 0.047, log-rank test; b).
Univariate analysis of prognostic factors in patients with cervical adenocarcinoma
The univariate and multivariate Cox regression analyses of the prognostic factors in patients with cervical adenocarcinoma are shown in Tables 4 and 5. The univariate and multivariate logistic regression models were used for proportional hazards analysis of prognostic factors with a hazard ratio (HR) and 95% confidence interval.
The results of univariate analysis indicated a significant correlation between PFS and p16INK4A expression (HR: 0.376, p5%; CI 0.162‒0.873, p = 0.023) (Table 4). A significant correlation was also observed between PFS and FIGO stage, LVSI, tumor size, and metastasis of pelvic lymph node, paraaortic lymph node, or distance. The multivariate analysis revealed a significant correlation between PFS and LVSI or tumor size. We also performed a stratified multivariate analysis in early-stage cases; however, no significant correlation was observed between PFS and p16 expression (Supplementary Table S3). The univariate analysis revealed a significant correlation between OS and FIGO stage, LVSI, or tumor size, and the multivariate analysis demonstrated a significant correlation between LVSI and OS.
Discussion
This study demonstrated that strong p16INK4A expression is related to a favorable prognosis of cervical adenocarcinoma. In contrast, the results of previous studies on the association between p16INK4A expression and cervical adenocarcinoma have been unclear13,14,15.
The 2018 International Endocervical Adenocarcinoma Criteria and Classification (IECC) distinctively explained the criteria pertaining to the pathological diagnosis of cervical adenocarcinoma, compared with the WHO criteria16. According to the IECC, diagnosis was defined using the HPV infection status, and it reflected patient prognosis. Furthermore, they substantiated their opinion by publishing clinical outcomes of patients with cervical adenocarcinomas associated or unassociated with HPV infection17. In the current study, we replicated their results. At the start of this study, we assumed that tumors with strong p16INK4A expression predicted good prognosis because p16INK4A is a surrogate marker of HPV infection. As viruses express antigens, lymphocytes expressing CD8 attack cancer cells18. According to our predictions, cervical adenocarcinoma patients with strong p16INK4A expression showed the trend of favorable prognosis. However, a positive relationship between p16INK4A expression and the expression of immune-check point-related molecules, such as CD8, PD-1, and PD-L1, was not verified (Supplementary Table S2). Previously, we reported the association between cervical adenocarcinoma and immune characteristics19. We demonstrated that a high PD-1 expression may be associated with a poor prognosis in patients with cervical adenocarcinoma. However, in that study, we did not analyze the relationship between the expression of p16 and immune-check point-related molecules. The lack of a significant positive relationship between p16INK4A expression and immune-check point-related molecules can be attributed to the fact that cervical adenocarcinomas with HPV infection possibly do not continue to express p16INK4A following malignant alteration. A previous study reported that some patients with cervical adenocarcinoma have reduced expression of p16 when the tumor is malignant20. In the current study, the positive rate of p16INK4A expression was considerably higher in early-stage cervical adenocarcinoma than in advanced-stage cervical adenocarcinoma (Table 3).
Several studies have reported that p16INK4A overexpression may be considered as a surrogate marker for high-risk HPV infection in the cervix21,22,23. As HPV infection induces tumor immune-environmental activity, immunotherapy has been recognized as an attractive treatment strategy for cervical carcinoma, as for other malignant tumors24.
Although carcinogenesis is associated with HPV infection, these tumors do not maintain steady p16INK4A (HPV infection) expression during carcinogenesis and tumor growth. The expression of p16INK4A changes under varying conditions, and there are currently no markers associated with the loss of p16 expression in some HPV-positive tumors before confirming malignant tumors. In addition, microenvironmental immunoactivity around HPV-infected tumors has not yet been conclusively proven, owing to which an association between the condition (stage, tumor size, and propensity of invasion) of the tumors themselves and the function of immune-related lymphocytes has not been demonstrated. Thus, further examination may be required to assess the changes in microenvironmental immunoactivity around tumors that have been already infected with HPV and have outgrown their previous conditions.
Another possibility is that p16INK4A also functions as a tumor suppressor gene, and the loss of p16INK4A accentuates the phosphorylation of the retinoblastoma (RB) protein (pRB). This pathway may be more pathophysiologically important. P16INK4A is a tumor suppressor gene, and it regulates the cell cycle by specifically inhibiting the cyclin D/CDK4/6 activity. P16INK4A and pRB form a negative feedback loop and the inactivation or mutation of pRB leads to the overexpression of p16INK4A, resulting in CDK4 and CDK6 dysregulation25,26.
Therefore, cell proliferation is suppressed and tumors that express p16INK4A would have a good prognosis. Llucia et al. further indicated that p16INK4A expression reflected not only the status of HPV infection but also dysregulation of the RB pathway, particularly in head and neck malignant tumors27.
Overall, the findings of previous studies and the current study indicate that p16INK4A expression may be a favorable prognostic factor, associated with the pRB pathway, rather than with HPV infection, in cervical adenocarcinoma. Several studies have indicated that immunocyte invasion by HPV infection may contribute to the effectiveness of its treatment, whereas p16INK4A expression reflects tumor suppressor properties, as well as its role in inhibiting CDK4 and maintaining pRB. Missaoui et al. reported that p16INK4A expression primarily affects the RB protein-related pathway, rather than the HPV-independent pathway28. In the future, we aim to study mechanism(s) underlying the changes in p16INK4A expression at the onset of HPV infection, during tumorigenesis and tumor growth. We will also investigate the changes in the expression of immune-related factors associated with tumor condition and p16INK4A expression.
Another notable point is that the prognosis of gastric type was very poor. Mucinous gastric type was associated with a very poor prognosis; however, we could not unravel the mechanism underlying the correlation between poor prognosis and negative HPV infection in gastric-type tumors. The expression of p16 could be affected by various factors. We want to emphasize that p16-negative tumor subtypes such as gastric-type tumors are not associated with HPV infection and show a poor prognosis because of the inactivation of a tumor suppressor gene. Currently, we are conducting genetic analysis of gastric-type tumor using whole-exome sequencing.
Our study had some limitations. Our series of cervical adenocarcinomas with confirmed p16INK4A-negativity were frequently found in an advanced FIGO stage, showing a higher rate of lymph node metastasis. Therefore, the possibility of better responses of p16INK4A-positive cases in advanced FIGO stage to chemotherapy and radiation therapy, as observed in head and neck cancer cases27,29, cannot be excluded. Availability of a higher number of cases may have allowed comparisons within the same FIGO stage, leading to more information regarding each p16INK4A condition.
A further limitation was that only p16INK4A expression was examined. Addition of PCR analyses or IHC of HPV may have aided in the clarification of the precise relationship between p16INK4A expression and HPV infection status. However, some studies have reported that while p16INK4A expression reflects HPV infection30, it may not necessarily reflect tumors with HPV infection31. Due to difficulties in correctly and easily establishing whether a tumor has HPV infection, only p16INK4A expression was taken into consideration.
In summary, results of the current study indicated that p16INK4A expression might be associated with favorable outcomes in patients with cervical adenocarcinoma. Thus, p16INK4A expression could serve as a biomarker for improving the prognosis of patients with cervical adenocarcinoma. Further research is needed to clarify that the expression of p16INK4A may function as a tumor suppressor rather than an HPV infection suppressor, activating invasive immune system cells.
Data availability
Data of current study was available from corresponding author (K.N.).
References
Katanoda, K. et al. Childhood, adolescent and young adult cancer incidence in Japan in 2009–2011. Jpn. J. Clin. Oncol. 47, 762–771 (2017).
Hori, M. et al. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the monitoring of cancer incidence in Japan (MCIJ) project. Jpn. J. Clin. Oncol. 45, 884–891 (2015).
Vinh-Hung, V. et al. Prognostic value of histopathology and trends in cervical cancer: A SEER population study. BMC Cancer 7, 164 (2007).
Wilbur, D. C. et al. Glandular tumors and precursors. In World Health Organization Classification of Tumors Pathology and Genetics Tumors of Female Reproductive Organs 4th edn (eds Kurman, R. J. et al.) 183–189 (IARC Press, 2014).
Smith, H. O., Tiffany, M. F., Qualls, C. R. & Key, C. R. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based study. Gynecol. Oncol. 78, 97–105 (2000).
Mathew, A. & George, P. S. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix—worldwide. Asian Pac. J. Cancer Prev. 10, 645–650 (2009).
Shiliang, L., Semenciw, R. & Mao, Y. Cervical cancer: The increasing incidence of adenocarcinoma and adenosquamous carcinoma in younger women. CMAJ 164, 1151–1152 (2001).
Adegoke, O., Kulasingam, S. & Virnig, B. Cervical cancer trends in the United States: A 35-year population-based analysis. J. Womens Health 21, 1031–1037 (2012).
Australian Institute of Health and Welfare (AIHW). Cervical screening in Australia 2012–2013. National cervical screening program. Cancer Series No. 93, Cat No CAN 91. Canberra: AIHW 2015.
Hanley, S. J., Yoshioka, E., Ito, Y. & Kishi, R. HPV vaccination crisis in Japan. Lancet 385, 2571 (2015).
Yagi, A. et al. Realistic fear of cervical cancer risk in Japan depending on birth year. Hum. Vaccin. Immunother. 8, 1–5 (2017).
Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. https://ganjoho.jp/reg_stat/statistics/dl_screening/index.html#a16.
Kawachi, A. et al. Tumor-associated CD204+ M2 macrophages are unfavorable prognostic indicators in uterine cervical adenocarcinoma. Cancer Sci. 109, 863–870 (2018).
Eleutério, J. Jr., Lima, T. S., Cunha, M. D., Cavalcante, D. I. & Silva, A. M. Immunohistochemical expression of the tumor suppressor protein p16INK4a in cervical adenocarcinoma. Rev. Bras. Ginecol. Obstet. 39, 21–25 (2017).
Hodgson, A. et al. International endocervical adenocarcinoma criteria and classification: Validation and interobserver reproducibility. Am. J. Surg. Pathol. 43, 75–83 (2019).
Stolnicu, S. et al. International endocervical adenocarcinoma criteria and classification (IECC): A new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am. J. Surg. Pathol. 42, 214–226 (2018).
Stolnicu, S. et al. Clinical outcomes of HPV-associated and unassociated endocervical adenocarcinomas categorized by the international endocervical adenocarcinoma criteria and classification (IECC). Am. J. Surg. Pathol. 43, 466–474 (2019).
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707 (1993).
Ishikawa, M. et al. High PD-1 expression level is associated with an unfavorable prognosis in patients with cervical adenocarcinoma. Arch. Gynecol. Obstet. 302, 209–218 (2020).
Sano, T., Oyama, T., Kashiwabara, K., Fukuda, T. & Nakajima, T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am. J. Pathol. 153, 20 (1998).
Silva, D. C. et al. Immunohistochemical expression of p16, Ki-67 and p53 in cervical lesions −a systematic review. Pathol. Res. Pract. 213, 723–729 (2017).
Kurshumliu, F., Thorns, C. & Gashi-Luci, L. p16INK4A in routine practice as a marker of cervical epithelial neoplasia. Gynecol. Oncol. 115, 127–131 (2009).
Missaoui, N. et al. p16INK4A overexpression in precancerous and cancerous lesions of the uterine cervix in Tunisian women. Pathol. Res. Pract. 206, 550–555 (2010).
Lheureux, S. et al. Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. JAMA Oncol. 4, e173776 (2018).
Rocco, J. W. & Sidransky, D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp. Cell. Res. 264, 42–55 (2001).
LaPak, K. M. & Burd, C. E. The molecular balancing act of p16(INK4a) in cancer and aging. Mol. Cancer Res. 12, 167–183 (2014).
Alos, L. et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: Potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 469, 277–284 (2016).
Missaoui, N. HPV infection and p16 and TP53 expression in rare cancers of the uterine cervix. (Clinical Outcomes of HPV-associated and unassociated endocervical adenocarcinomas categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC) (2018).
Rajendra, S. et al. Survival rates for patients with barrett high-grade dysplasia and esophageal adenocarcinoma with or without human papillomavirus infection. JAMA Netw. Open. 1, e181054 (2018).
Bergeron, C. et al. The clinical impact of using p16(INK4a) immunochemistry in cervical histopathology and cytology: An update of recent developments. Int. J. Cancer 136, 2741–2751 (2015).
Chen, W. et al. HPV(-) The variable clinicopathological categories and role of human papillomavirus in cervical adenocarcinoma: A hospital based nation-wide multi-center retrospective study across. China Chinese HPV typing group. Int. J. Cancer. 139, 2687–2697 (2016).
Acknowledgements
The authors wish to thank the Department of Pathology for supporting this work.
Author information
Authors and Affiliations
Contributions
M.I. and K.N. drafted the manuscript. K.N., H.Y., T.I., T.M., K.S., Y.Y., K.I., N.I., and S.R. carried out the IHC analysis. K.N. participated in designing the study. S.N., Y.O., and N.I. carried out the pathological diagnosis. S.K. conceived of the study, participated in its design and coordination, and helped in drafting the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishikawa, M., Nakayama, K., Nakamura, K. et al. P16INK4A expression might be associated with a favorable prognosis for cervical adenocarcinoma via dysregulation of the RB pathway. Sci Rep 11, 18236 (2021). https://doi.org/10.1038/s41598-021-97703-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97703-8
This article is cited by
-
Molecular markers predicting the progression and prognosis of human papillomavirus-induced cervical lesions to cervical cancer
Journal of Cancer Research and Clinical Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.