Abstract
In kidney transplantation, microthrombi and fibrin deposition may lead to local perfusion disorders and subsequently poor initial graft function. Microthrombi are often regarded as donor-derived. However, the incidence, time of development, and potential difference between living donor kidneys (LDK) and deceased donor kidneys(DDK), remains unclear. Two open-needle biopsies, taken at preimplantation and after reperfusion, were obtained from 17 LDK and 28 DDK transplanted between 2005 and 2008. Paraffin-embedded sections were immunohistochemically stained with anti-fibrinogen antibody. Fibrin deposition intensity in peritubular capillaries(PTC) and glomeruli was categorized as negative, weak, moderate or strong and the number of microthrombi/mm2 was quantified. Reperfusion biopsies showed more fibrin deposition (20% to 100% moderate/strong, p < 0.001) and more microthrombi/mm2 (0.97 ± 1.12 vs. 0.28 ± 0.53, p < 0.01) than preimplantation biopsies. In addition, more microthrombi/mm2 (0.38 ± 0.61 vs. 0.09 ± 0.22, p = 0.02) and stronger fibrin intensity in glomeruli (28% vs. 0%, p < 0.01) and PTC (14% vs. 0%, p = 0.02) were observed in preimplantation DDK than LDK biopsies. After reperfusion, microthrombi/mm2 were comparable (p = 0.23) for LDK (0.09 ± 0.22 to 0.76 ± 0.49, p = 0.03) and DDK (0.38 ± 0.61 to 0.90 ± 1.11, p = 0.07). Upon reperfusion, there is an aggravation of microthrombus formation and fibrin deposition within the graft. The prominent increase of microthrombi in LDK indicates that they are not merely donor-derived.
Similar content being viewed by others
Introduction
It is well known in kidney transplantation, that microthrombi can be observed in preimplantation biopsies of kidney grafts. However, it is unclear whether the donor-derived microthrombi persist or disappear or if new microthrombi arise when the graft is reperfused. Disappearance is rather unlikely, since ischemia/reperfusion injury (IRI), which is inevitable in transplantation and is associated with a procoagulatory response, potentially enhances formation of microthrombi and deposition of fibrin. IRI induces a series of interactions between the microvasculature, tubular epithelium, and infiltrating inflammatory cells, leading to the activation of the coagulation cascade, the complement system and accumulation of fibrin(ogen) in the peritubular capillaries (PTC) of the kidney1. Fibrinogen, an acute phase protein, is upregulated in case of injury or inflammation2. When converted to fibrin within the kidney, it provides an accumulation site for platelets and red blood cells, facilitating the formation of thrombi, which subsequently can lead to local perfusion disorders and poor initial kidney graft function3,4.
In deceased organ donors, it has been shown that hemostasis is activated and fibrinolysis dysregulated3. Activation of hemostasis with subsequent microthrombus formation in deceased organ donors may be one of the reasons living donor kidneys (LDK) are of better quality than deceased donor kidneys (DDK). Despite the identification of microthrombi in preimplantation biopsies, little is known about the actual incidence, time of development and potential differences between donor types. In addition, it appears that administration of heparin to the recipient prior to reperfusion leads to less fibrin formation, reflected by lower D-dimer levels5. Intervening with unfractionated heparin or other antithrombotic agents might prevent extra formation of fibrin and subsequently microthrombi.
The primary aim of the current study was to investigate whether the amount of fibrin deposition and microthrombi in kidney graft biopsies changes over the course of transplantation. We further investigated whether fibrin deposition and microthrombus formation is associated with donor type or intraoperative use of heparin.
Materials and methods
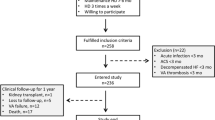
All biopsies and patient data were obtained from the TransplantLines biobank (NCT03272841), a prospective cohort study and biobank that includes all types of solid organ transplant recipients as well as (living) organ donors. The study was approved by both the TransplantLines board and the institutional ethical review board (METc UMC Groningen 2014/077). Patient data were processed and stored according to the Declaration of Helsinki. Clinical and research activities followed the principles of the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Two open-needle biopsies were obtained from 45 kidneys (28 DDK and 17 LDK), transplanted between 2005 and 2008 after static cold storage; one prior to vascular anastomosis (preimplantation) and one 30 min after reperfusion (reperfusion). Biopsies were embedded in paraffin and stored at -80 °C. After procurement, all kidneys were flushed and perfused with cold University of Wisconsin perfusate (ViaSpan, DuPont, Wilmington, NC, USA; Belzer UW, Bridge to life, Columbia SC, USA). According to EuroTransplant protocol DBD donors received 20,000 international units (IU) of heparin prior to systemic flush. DCD donors or living kidney donors were not given any form of antithrombotic prophylaxis. The transplant procedure was performed according to local protocol which was published in detail before6. Per protocol, preemptively transplanted recipients, in contrast to dialysis-dependent recipients, were given 5000 IU of unfractionated heparin prior to reperfusion. Post-reperfusion analyses were performed without these nine recipients, as the effect of heparin on factors IIa and Xa in the coagulation cascade would interfere with the results. Delayed graft function (DGF) was defined as recipients receiving hemodialysis within 7 days of transplantation. Estimated glomerular filtration rate (eGFR) at 3, 6 and 12 months was calculated using the CKD-Epi formula.
Immunohistochemical studies
Microthrombi and fibrin deposition were assessed in 3μ paraffin-embedded preimplantation and post-reperfusion biopsy sections. Sections were deparaffinized with xylene and brought to 70% ethanol. Antigen retrieval was performed with proteinase K 0.1% and an endoblock using 0.3% H2O2. The sections were incubated at room temperature for 60 min with a polyclonal rabbit anti-human fibrinogen antibody (1:750, A0080; Dako, Glostrup, Denmark). Diaminobenzidine (Dako) was used as the chromogen substrate and hematoxylin as counterstain. Slides were then digitally stored using a digital slide scanner (Hamamatsu Nanozoomer HT2.0, Hamamatsu Photonics K.K., Japan) with 40× magnification and analyzed using Aperio Imagescope v12.1 (Leica Biosystems Imaging, Inc. USA). Microthrombus was defined as a cluster of fibrin threads with or without trapped red blood cells occluding parts or the whole of peritubular capillaries or glomerular capillaries (Fig. 1). Scoring was performed by a trained nephropathologist, blinded for donor type or timepoint of the biopsies. Microthrombi were scored when observed in glomeruli and/or PTC in a semi-quantitative matter. To correct for the size of the biopsy, we quantified the number of microthrombi per surface area (microthrombi/mm2), which was traced by hand using Aperio Imagescope. In addition, fibrin staining intensity of endothelium of peritubular capillary walls and glomeruli was assessed (Fig. 2). Generalized fibrin deposition intensity of endothelium of glomerular capillaries and PTC was graded from 0 to 3, with 0 as negative, 1 weak, 2 moderate and 3 as strong staining, according to a previously described method (Fig. 3)7. Additional staining was performed with Martius Scarlet Blue (MSB) stain for demonstration of fibrin, as described previously5.
Fibrin stain intensity differences of the glomerular and PTC endothelium between preimplantation and reperfusion biopsies (IHC stain). (a) Example of the most observed intensity in preimplantation biopsies ( ×40 magnification); (b) example of most observed intensity in reperfusion biopsies (×60 magnification).
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation (SD) or median with interquartile range (IQR) for continuous variables, depending on variable distribution. Outcomes are presented as mean with 95% confidence interval (CI). Tests of significance were two-tailed, and statistical significance is set at p < 0.05. The paired t-test or Wilcoxon matched pairs signed rank test were used to compare matched data. The Mann Whitney U-test was used to compare groups with non-normal distribution. Differences between kidneys of recipients with and without platelet aggregation inhibitor (PAI) use were evaluated by Fisher’s exact test. Associations between DGF and microthrombi were analyzed using Mann Whitney U-test. To determine the correlation between ischemia times, kidney function and microthrombi development, a Spearman correlation analysis was conducted. The results were presented by reporting Spearman’s ρ coefficient and corresponding p-values. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS v23; IBM Corp, Armonk, NY, USA) and GraphPad Prism v5.04 (GraphPad Software Inc., La Jolla, CA, USA). Graphs were created using Graphpad Prism.
Institutional review board statement
All biopsies and patient data were obtained from the TransplantLines biobank (NCT03272841), a prospective cohort study and biobank that includes all types of solid organ transplant recipients as well as (living) organ donors. The study was approved by both the TransplantLines board and the institutional ethical review board (METc 2014/077). Patient data were processed and stored according to the Declaration of Helsinki. Clinical and research activities followed the principles of the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Informed consent statement
Informed consent was obtained from all subjects involved in the TransplantLines biobank.
Results
Baseline characteristics
Baseline characteristics and relevant intraoperative variables of the total cohort are shown in Table 1. Seventeen kidneys were derived from living donors and 28 from deceased donors. Median age of both donors and recipients was 50 ± 14 years. Eleven recipients received single antiplatelet therapy (carbasalate calcium, n = 10; clopidogrel, n = 1) which were continued during surgery. None of the recipients were treated with anticoagulants preoperatively. Eight recipients were transplanted preemptively. These recipients all received an LDK.
Development of fibrin deposition and microthrombi over time
There was an increased glomerular fibrin stain intensity in the reperfusion biopsies compared to the preimplantation biopsies, in which only 20% of the preimplantation biopsies were categorized as moderate/high glomerular fibrin stain which increased to 100% in the reperfusion biopsies (p < 0.001). There also was a difference in PTC fibrin intensity (9% vs. 65% resp., p < 0.001). The increased stain intensity in PTC or glomeruli was accompanied by comparable stain intensity of the interstitium (Figs. 2 and 3). In 37% of preimplantation biopsies and 42% of reperfusion biopsies there was some variance of stain intensity within the biopsy. Regarding staining of glomerular endothelium and PTC endothelium, 23 preimplantation biopsies showed no differences, 9 showed a one-category difference and 2 showed a two-category difference. In the reperfusion biopsies, 7 biopsies showed no differences, 22 reperfusion biopsies showed a one-category difference and 6 a two-category difference (moderate in glomeruli and mild in PTC). Glomerular stains were almost always more pronounced in case of a difference in categories in both preimplantation and reperfusion biopsies.
In addition, an increase in the number of microthrombi was observed upon reperfusion. In total, 16 preimplantation biopsies (38%) contained microthrombi compared to 28 reperfusion biopsies (65%, p < 0.01). This is in line with the significant increase in microthrombi/mm2 observed between preimplantation and reperfusion biopsies (0.28 ± 0.53 microthrombi/mm2 vs. 0.87 ± 1.00 microthrombi/mm2, p = 0.02; Table 2). Microthrombi were mainly observed in PTC in both preimplantation (93% of detected microthrombi) and reperfusion biopsies (80%). Sixteen (36%) patients experienced DGF after transplantation. One patient had primary non-function. This kidney presented 0.25 and 0.26 microthrombi/mm2 in the preimplantation and reperfusion biopsy respectively. During one procedure, there was a tear of the vena renalis, which was reconstructed. Postoperatively, this patient developed deep venous thrombosis reaching into the vena iliaca externa on the second day after KTx, after which transplantectomy was required. In the biopsies of this kidney, there were no microthrombi present at preimplantation, and 1.81/mm2 after reperfusion. There was no significant association between number of microthrombi/mm2 in preimplantation biopsies and DGF (p = 0.08) or in reperfusion biopsies and DGF (p = 0.71). Mean creatinin level after 3 months was 144 ± 70 µmol/L. Mean estimated glomerular filtration rate at 3 months was 53 ± 20 mL/min/1.73m2. There were no correlations between number of microthrombi at preimplantation or after reperfusion and eGFR after 3, 6 or 12 months (Table 3).
Donor type differences
Recipients of an LDK had a significantly shorter mean time on dialysis prior to transplantation (21 ± 11 vs. 54 ± 16 months, p < 0.01), were transplanted preemptively more often (47% vs. 0%, p < 0.001), were less frequently on a platelet aggregation inhibitor (PAI) regimen (n = 10 vs. n = 1, p = 0.03) and cold ischemia time (CIT) of the kidney graft was significantly shorter (157 ± 26 min vs. 1038 ± 287 min, p < 0.001) compared to recipients of a DDK. Preimplantation biopsies of LDK showed significantly lower fibrin intensity in both glomeruli (0% vs. 28%, p < 0.01) and PTCs (0% vs. 14%, p = 0.02) than DDK. In the reperfusion biopsies, this difference disappeared in both the glomeruli (both at 88% high intensity, p > 0.99) and the PTCs (11% vs. 19%, p = 0.89).
LDK had significantly fewer microthrombi/mm2 in preimplantation biopsies than DDK (0.09 ± 0.22 microthrombi/mm2 vs. 0.38 ± 0.61 microthrombi/mm2, p = 0.02, Table 2) but did show a somewhat steeper increase from preimplantation biopsies to reperfusion biopsies (0.09 ± 0.22 to 0.76 ± 0.49 microthrombi/mm2, p = 0.03) than DDK (0.38 ± 0.61 to 0.90 ± 1.11 microthrombi/mm2, p = 0.07). This resulted in a comparable mean number of microthrombi/mm2 between LDK and DDK in the reperfusion biopsies (p = 0.23, Fig. 4).
When stratified for deceased donor type, no differences were observed between DBD and DCD kidneys between preimplantation (0.47 ± 0.79 microthrombi/mm2 vs. 0.31 ± 0.37 microthrombi/mm2, p = 0.90) and reperfusion biopsies (0.92 ± 1.13 microthrombi/mm2 vs. 0.96 ± 1.15 microthrombi/mm2, p = 0.90, Table 2). Deceased donor characteristics are stated in Supplementary Table 1. Furthermore, no differences were observed between DDK that went to recipients who used PAI compared to recipients who did not (0.92 ± 1.16 vs. 1.06 ± 1.11 microthrombi/mm2 at reperfusion, p = 0.56).
MSB stain
Microthrombi and fibrin depositions on endothelial lining of PTC were observed in the MSB stained biopsies (Fig. 5). Preimplantation biopsies showed 0.05 ± 0.24 microthrombi/mm2 compared to 0.15 ± 0.43 microthrombi/mm2 in reperfusion biopsies (p = 0.26). At preimplantation, both DDK and LDK are comparable (0.08 ± 0.29 vs. 0.00 ± 0.00 microthrombi/mm2, p = 0.21). DDK and LDK showed a comparable number of microthrombi/mm2 in the reperfusion biopsies (0.23 ± 0.52 vs. 0.00 ± 0.00 microthrombi/mm2, p = 0.06).
Kidney biopsies stained with Martius Scarlet Blue (MSB) stain. Fibrin stains red, collagen blue, red blood cells yellow. (a) Preimplantation biopsy with microthrombus in peritubular capillary, (b) reperfusion biopsy with microthrombus in peritubular capillary, (c) preimplantation biopsy with fibrin deposition on endothelial lining of peritubular capillary, (d) reperfusion biopsy with multiple microthrombi in glomerulus. (×40 magnification).
Intraoperative use of heparin in the recipient and microthrombi formation
Since preemptively transplanted patients were given 5000 IU of unfractionated heparin prior to reperfusion, and as there were no recipients with a preemptive status in the deceased donor group, only LDK were stratified for heparin administration. Significantly fewer microthrombi/mm2 were observed in the kidneys of recipients who were given intraoperative heparin compared to controls (0.13 ± 0.24 microthrombi/mm2 vs. 0.76 ± 0.49 microthrombi/mm2, p = 0.02. Figure 6). One outlier in the LDK group was removed from the analysis due to a disagreement between the surgical report and the patient file on heparin administration. Baseline characteristics and intraoperative parameters, such as longer ischemia times or surgical complications, were comparable with the kidneys that remained in the analysis, which reduces the risk of selection bias.
Discussion
This study shows that microthrombi not only are present in donor kidneys pre-implantation, but increase upon reperfusion and that there is a generalized increased fibrin deposition. Post-reperfusion, 0.97 microthrombi per mm2 biopsy tissue were observed in glomeruli and peritubular capillaries, as compared to 0.28/mm2 in preimplantation biopsies, and all reperfusion biopsies had a moderate to high intensity of the fibrin stain, compared to 20% in the preimplantation biopsies. In preimplantation biopsies, a higher number of microthrombi were observed in DDK compared to LDK. This difference disappeared after reperfusion, with an equal number of microthrombi/mm2 in both groups. Although the number of microthrombi and fibrin deposition were less pronounced in the MSB stained biopsies, the results of this stain were in line with the IHC stain. Furthermore, kidneys transplanted in recipients who were administrated 5000 IU of unfractionated heparin before clamping of the vessels showed significantly fewer microthrombi upon reperfusion than kidneys transplanted in recipients without heparin. This analysis, however, was restricted to LDK.
The higher number of microthrombi in DDK biopsies compared to LDK biopsies preimplantation, is probably due to the prolonged agonal ischemic injury seen in DCD donors, and the procoagulatory response associated with brain death in DBD donors3,8. The fact that the number of microthrombi/mm2 is equal after reperfusion in both LDK biopsies and DDK biopsies, however, indicates that the majority of microthrombi develop upon reperfusion of the graft and to a lesser extend in the donor. This finding is clinically relevant since previous studies mainly focused on formation of microthrombi in preimplantation biopsies or its association with disseminated intravascular coagulation in the donor, assuming that microthrombi are donor-derived9,10,11,12,13. Our results show that this is not the case, as LDK derived from relatively healthy donors exhibit a steady development from 0.09 microthrombi/mm2 in preimplantation biopsies to 1.19 microthrombi/mm2 in reperfusion biopsies. Extrapolation of these results to the entire cortex of the kidney would result in a considerable amount of microthrombi. These results are unexpected, as LDK are considered the perfect donor in terms of quality and graft survival, which is reflected by the low numbers of microthrombi in the preimplantation biopsies14. Furthermore, recipients of LDK are more often preemptively transplanted as well, contributing to a better outcome15. Nevertheless, we observed a greater increase in microthrombi/mm2 in LDK than in DDK after reperfusion. It seems unlikely that this difference is explained by a difference in thrombogenic environment, as kidneys from DDK usually experience more severe IRI. A possible explanation could be the manipulation of the kidney in hand-assisted live donor nephrectomy, which might result in bruising of the kidney. The use of a pneumoperitoneum could add to further compression. Due to the relative short time between the manipulation and the cold flush and subsequent SCS, which slows down metabolism, it could be that new microthrombi as a result of the manipulation are formed once flow is restored. However, this hypothesis does not justify systemic heparinization of the living donor16,17.
Reperfusion biopsies showed significantly more fibrin deposition than preimplantation biopsies. It appears that upon reperfusion an increase in accumulation of fibrin occurs, which results in a generalized and more diffuse pattern surrounding the endothelial walls of glomeruli, PTC and tubuli. This formation and accumulation of extravascular fibrinogen has been previously associated with IRI, and was found to be initiated by endothelial dysfunction after reperfusion1,18. These fibrin depositions form an accumulation site for microthrombi. This was also demonstrated by a recent study with kidneys undergoing normothermic machine perfusion that had formed microvascular plugs after fibrin deposition in the cortex and medulla, which caused reduced perfusion of the renal cortex. Markers of renal injury were reduced after lysis with plasminogen and tissue plasminogen activator4,19.
The idea of microthrombi development and activation of the coagulation cascade during renal transplantation is not new. As microthrombi are often thought to be donor-derived, several reports tried to inhibit microthrombi development by systemic heparinization of the donor. Improved kidney function or fewer surgical complications were unfortunately not achieved16,20,21. Although unfractionated heparin appears to be a very suitable agent to inhibit microthrombi development, by intervening at the thrombin level and inhibiting fibrinogen formation22, it has a short half-life23. Therefore, it should be administered just prior to the peak of microthrombi formation. In the current study, this peak appears to be upon reperfusion, where a pronounced increase of microthrombi in PTC was observed. This is further illustrated by the data showing that recipients who were given heparin just prior to reperfusion developed significantly fewer microthrombi in PTC. Hence, by administering heparin to the recipient at the most crucial moment of microthrombi formation, microcirculation and potentially even graft outcome could be optimized.
In this study, microthrombi were scored in both PTC and glomeruli. The occurrence of glomerular fibrin thrombi (GFT) has been investigated before, especially since GFT in preimplantation biopsies are sometimes used to accept or decline a deceased donor kidney offer. Incidences of GFT vary from 2.6% to 9.9%11,12,13. In our study GFT were present in 7.1% of the DDK. Contradictory findings regarding the influence of GFT on long-term kidney function have been reported. In two studies, GFT were shown to be a risk factor for delayed or reduced graft function in the first year after transplantation13,24. Two other studies nonetheless report that the majority of GFT resolve within 1 to 3 months after transplantation, and conclude that GFT do not influence kidney function after transplantation or lead to more fibrosis or glomerulosclerosis11,25. A recent study12 came to the same conclusion, although it is debatable whether their cut-off of < 50% fibrin thrombi positive glomeruli—compared to a study using 15%26—does not underestimate the results. In other words, it is still unclear whether a kidney with 30% of glomeruli affected by fibrin thrombi will have a function similar to that of a kidney with 10% or 45%.
It can be debated whether glomeruli are the renal structures of interest when determining the number of microthrombi in renal biopsies. The majority of microthrombi in the current study were observed in the PTC, which suggests a primary focus in these structures and less in glomerular pathogenesis. The microvasculature is especially susceptible to IRI18, and as PTC have limited capacity to regenerate26 (in contrast to tubular cells and glomeruli) they are directly associated with loss of kidney function27. These PTC are often neglected yet are pivotal to maintaining sufficient perfusion. Injury to the microcirculation due to endothelial cell damage may lead to permanent peritubular capillary rarefaction, which causes chronic hypoxia in these regions. This may induce transcription of fibrogenic genes like transforming growth factor-β and connective tissue growth factor together with an accumulation of α-smooth muscle actin which in the end may lead to development of IFTA (interstitial fibrosis and cortical tubular atrophy)28.
At reperfusion, when the renal vein and artery clamps are released, the kidney is reoxygenated after a period of ischemia. During ischemia, and in particular upon reperfusion, reactive oxygen species (ROS) are formed due to dysfunctioning of the mitochondrial respiratory chain29. ROS have a deleterious effect on cells due to activation of various injurious pathways through carbonylation of proteins or lipid peroxidation. At a vascular level, this will lead to swelling of endothelial cells, degradation of the cytoskeleton and loss of the glycocalix. Intercellular contact of endothelial cells is lost and subendothelial cells expressing tissue factor (TF) are exposed28.
Activated subendothelial TF initiates the coagulation cascade resulting in fibrin and microthrombi formation, and, in addition, IRI can also activate intravascular coagulation through activation of the complement system. Therefore, it is probable that microthrombi formation will continue after reperfusion. Although assessment of biopsies taken after the transplant procedure was not possible, due to the absence of these, previous plasma analyses of LDK have shown that coagulation markers between preemptively and non-preemptively transplanted patients are comparable, but upregulated, compared to healthy controls and remain so at least 2 h postoperatively5. Our current study showed that the formation of microthrombi could be reduced from 0.76 to 0.13 microthrombi/mm2 by administering heparin prior to reperfusion. Hypothetically, existing or developing microthrombi are lead points from where bigger thrombi can grow, as fibrin attracts platelets and activated platelets attract other platelets. By removing these lead points, possible further damage could be prevented.
This study has a few limitations that need to be addressed. First, it is a retrospective single-center study with a relatively small sample size. Unfortunately, the sample size could not be increased, since the retrieval of post-reperfusion biopsies was stopped after 2008 due to a change in protocol in our center. Additionally, to formally analyze an effect of intraoperative heparin treatment on kidney function, a larger sample size and preferably a randomized controlled trial are required which, due to the many confounding factors involved in kidney transplantation, would require a large number of patients. However, we do feel that we can draw a firm conclusion from our data due to the evident differences between the donor types, which justifies further research and even policy on heparin use. Second, there were variations in outcome between the immunohistochemical (IHC) and MSB stains. However, MSB staining has a risk of over/understaining or inadeaquate differentiation, depending on the size and maturity of the fibrin deposition30. The outcome of IHC staining seems more robust and has less potential for ambiguous results. Third, follow-up biopsies were not available so we were unable to determine whether a resolution or increase of microthrombi would occur in our patients. The limited sample size also prevents adequate graft survival analysis. This information would contribute to deeper insight into the pathophysiology and a future intervention.
Conclusions
Our results show that kidney transplantation is associated with a generalized deposition of fibrin and an aggravation of microthrombi formation in peritubular capillaries and glomeruli. Although deceased donor kidneys show more preexistent microthrombi, it is mainly the living donor kidneys, which show an increase during the transplantation procedure. This increase in microthrombi and fibrin depositions indicates that microthrombi are not mainly donor-derived, but that the most significant formation occurs upon reperfusion. This might suggest a role for ischemia/reperfusion injury.
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available for privacy reasons.
References
Sörensen-Zender, I. et al. Role of fibrinogen in acute ischemic kidney injury. Am. J. Physiol. Physiol. 305, F777–F785 (2013).
Kattula, S., Byrnes, J. R. & Wolberg, A. S. Fibrinogen and fibrin in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 37(3), e13–e21 (2017).
Lisman, T., Leuvenink, H. G. D., Porte, R. J. & Ploeg, R. J. Activation of hemostasis in brain dead organ donors: An observational study. J. Thromb. Haemost. 9, 1959–1965 (2011).
DiRito, J. R. et al. Lysis of cold-storage-induced microvascular obstructions for ex vivo revitalization of marginal human kidneys. Am. J. Transplant. 21(1), 161–173 (2021).
Nieuwenhuijs-Moeke, G. J. et al. Preemptively and non-preemptively transplanted patients show a comparable hypercoagulable state prior to kidney transplantation compared to living kidney donors. PLoS ONE 13(7), e0200537 (2018).
Zorgdrager, M. et al. Chronic inguinal pain after kidney transplantation, a common and underexposed problem. World J. Surg. 41, 630–638 (2017).
Bösmüller, et al. Detection of the BRAF V600E mutation in serous ovarian tumors: A comparative analysis of immunohistochemistry with a mutation-specific monoclonal antibody and allele-specific PCR. Hum. Pathol. 44(3), 329–335 (2013).
Woodside, K. J. et al. Enhancing kidney function with thrombolytic therapy following donation after cardiac death: A multicenter quasi-blinded prospective randomized trial. Clin. Transplant 29, 1173–1180 (2015).
Takeda, A. et al. Case study of paired cadaver renal allografts from the same donor: influence of local DIC kidney and concomitant acute rejection on early graft outcome. Clin. Transplant. 13(Suppl 1), 13–16 (1999).
Hefty, T. R. et al. Disseminated intravascular coagulation in cadaveric organ donors incidence and effect on renal transplantation. Transplantation 55(2), 442–443 (1993).
Batra, R. K. et al. Rapid resolution of donor-derived glomerular fibrin thrombi after deceased donor kidney transplantation. Am. J. Transplant. 16(3), 1015–1020 (2016).
Gao, G. et al. Donor characteristics, recipient outcomes, and histologic findings of kidney allografts with diffuse donor-derived glomerular fibrin thrombi. Transplantation 103(9), 1921–1927 (2019).
Hansen, D., Rørvig, S., Andersen, C. B. & Sørensen, S. S. Fibrin thrombi in deceased donor kidneys: Prevalence and influence on graft function and graft survival in transplanted patients. APMIS 126(1), 3–8 (2018).
Terasaki, P. I., Cecka, J. M., Gjertson, D. W. & Takemoto, S. High survival rates of kidney transplants from spousal and living unrelated donors. N. Engl. J. Med. 333(6), 333–336 (1995).
Innocenti, G. R. et al. Preemptive living donor kidney transplantation: Do the benefits extend to all recipients?. Transplantation 83(2), 144–149 (2021).
Ramani, A. P. et al. Impact of intraoperative heparin on laparoscopic donor nephrectomy. J. Urol. 174(1), 226–228 (2005).
Crotty, C., Tabbakh, Y., Hosgood, S. A. & Nicholson, M. L. Systemic heparinisation in laparoscopic live donor nephrectomy. J. Transplant 2013, 138926 (2013).
Slegtenhorst, B. R., Dor, F. J. M. F., Rodriguez, H., Voskuil, F. J. & Tullius, S. G. Ischemia/reperfusion injury and its consequences on immunity and inflammation. Curr. Transplant Rep. 1(3), 147–154 (2014).
Tietjen, G. T. et al. Nanoparticle targeting to the endothelium during normothermic machine perfusion of human kidneys. Sci. Transl. Med. 9(418), 6764 (2017).
Friedersdorff, F. et al. No need for systemic heparinization during laparoscopic donor nephrectomy with short warm ischemia time. World J. Urol. 29(4), 561–566 (2011).
Cheng, E. Y., Leeser, D. B., Kapur, S. & Del Pizzo, J. Outcomes of laparoscopic donor nephrectomy without intraoperative systemic heparinization. J Urol. 183(6), 2282–2286 (2010).
Madhusudhan, T., Kerlin, B. A. & Isermann, B. The emerging role of coagulation proteases in kidney disease. Nat. Rev. Nephrol. 12(2), 94–109 (2016).
Garcia, D. A., Baglin, T. P., Weitz, J. I. & Samama, M. M. Parenteral anticoagulants—Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141(2 Suppl), e24S-e43S (2012).
McCall, S. J., Tuttle-Newhall, J. E., Howell, D. N. & Fields, T. A. Prognostic significance of microvascular thrombosis in donor kidney allograft biopsies. Transplantation 75, 1847–1852 (2003).
Sood, P., Randhawa, P. S., Mehta, R., Hariharan, S. & Tevar, A. D. Donor kidney microthrombi and outcomes of kidney transplant: A single-center experience. Clin Transplant. 29(5), 434–438 (2015).
Muczynski, K. A., Ekle, D. M., Coder, D. M. & Anderson, S. K. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: Characterization, isolation, and regulation of MHC class II expression. J. Am. Soc. Nephrol. 14(5), 1336–1348 (2003).
Basile, D. P. Rarefaction of peritubular capillaries following ischemic acute renal failure: A potential factor predisposing to progressive nephropathy. Curr. Opin. Nephrol. Hypertens. 13(1), 1–7 (2004).
Nieuwenhuijs-Moeke, G. J. et al. Ischemia and reperfusion injury in kidney transplantation: Relevant mechanisms in injury and repair. J. Clin. Med. 9(1), 253 (2020).
Martin, J. L., Gruszczyk, A. V., Beach, T. E., Murphy, M. P. & Saeb-Parsy, K. Mitochondrial mechanisms and therapeutics in ischaemia reperfusion injury. Pediatr. Nephrol. 34(7), 1167–1174 (2019).
Davison, A. M. et al. Identification of intrarenal fibrin deposition. J. Clin. Pathol. 26, 102–112 (1973).
Funding
This research was funded by a grand from the Tekke Huizinga Foundation, grant number (STHF-197).
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization, T.B., M.H., T.L., H.G. and R.P.; funding acquisition, T.B., H.G. T.L. and R.P.; methodology, T.B., M.H., T.L., J.A. and J.W.B.; formal analysis, T.B. and M.H.; investigation, T.B., M.H., J.W.B. and J.A.; resources, T.L., J.W.B., J.A., S.B. and H.G.; data curation, T.B., R.P, G.M.; writing—original draft preparation, T.B., M.H., H.G., T.L., S.B. and R.P.; writing—review and editing, all authors; visualization, T.B.; supervision, T.L., H.G., S.B. and R.P.; project administration, T.B., R.P. and T.L.; All authors have reviewed the manuscript and agreed to the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van den Berg, T.A.J., van den Heuvel, M.C., Wiersema-Buist, J. et al. Aggravation of fibrin deposition and microthrombus formation within the graft during kidney transplantation. Sci Rep 11, 18937 (2021). https://doi.org/10.1038/s41598-021-97629-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97629-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.