Abstract
Pleuroparenchymal fibroelastosis (PPFE) progresses slowly but sometimes relatively quickly, leading to decreased activities of daily living (ADL) and muscle weakness. Skeletal muscle atrophy and muscle weakness in chronic obstructive pulmonary disease (COPD) patients may be caused by cachexia and are associated with reduced ADLs and increased risk of death. However, the association between skeletal muscle mass and the prognosis of PPFE patients remains unknown. We retrospectively analysed the clinical significance of the cross-sectional area of the erector spinae muscle (ESMCSA), a skeletal muscle index, and predictors of mortality within 3 years in PPFE 51 patients, idiopathic pulmonary fibrosis (IPF) 52 patients and COPD 62 patients. PPFE patients had significantly lower ESMCSA than IPF or COPD patients, and lower ESMCSA (< 22.57 cm2) was associated with prognosis within 3 years (log-rank test; p = 0.006), whereas lower body mass index (BMI) showed no association. Multivariate analysis showed that ESMCSA was an independent predictor of mortality within 3 years in PPFE patients (hazard ratio, 0.854; 95% confidence interval: 0.737–0.990, p = 0.036). These results suggest the importance of monitoring ESMCSA in PPFE patients and that assessing ESMCSA in PPFE patients could be a more useful prognostic indicator than BMI.
Similar content being viewed by others
Introduction
Among types of idiopathic interstitial pneumonia, idiopathic pulmonary fibrosis (IPF) is the most frequent, causing chronic progressive fibrosis predominantly at the lung base1,2. Pleuroparenchymal fibroelastosis (PPFE) is another chronic pulmonary fibrosis proposed by Frankel et al. in 20043. PPFE differs from IPF in that there is more fibrosis in the upper lobe, and the histopathology shows fibrosis in the alveolar space, aggregation of elastic fibres in the alveolar walls, and fibrous thickening of the visceral pleura. The European Respiratory Society (ERS), American Thoracic Society (ATS), Japanese Respiratory Society (JRS), and Latin American Thoracic Society (ALAT) 2013 guidelines2 rank idiopathic PPFE (IPPFE) as a rare type of interstitial lung disease (ILD). PPFE is generally considered to progress slowly; however, the rapid onset of dyspnoea sometimes leads to a decline in activities of daily living (ADL), making hospital visits difficult. IPPFE typically presents with changes in body composition, and patients often complain of an emaciated physique with weight loss4; this weight loss often includes muscle weakness. Skeletal muscle atrophy and loss of muscle mass in COPD have been associated with decreased ADL and risk of death5. However, only a few studies have compared skeletal muscle mass with the prognosis of PPFE, although a lower body mass index (BMI) has been implicated as a poor prognostic factor4. Antigravity muscles have been reported to reflect physical activity more readily than other muscle groups6. The cross-sectional area of the erector spinae muscle (ESMCSA), part of the antigravity muscles, can recently be quantitatively assessed using computed tomography (CT) and is considered a strong predictor of survival in COPD and IPF7,8,9. Therefore, we investigated the association of the quantification of ESMCSA obtained by CT with PPFE.
Results
Characteristics
The baseline characteristics of the included patients (PPFE [n = 51], IPF [n = 52], COPD [n = 62]) are shown in Table 1. In this study, eight definite PPFE cases were diagnosed by surgical lung biopsy, and 33 PPFE cases had fibrosis in the lower lobes. PPFE included 14 patients with secondary PPFE (secondary causes comprised hypersensitivity pneumonitis [n = 4], non-tuberculous mycobacteria [n = 4], haematopoietic stem cell transplantation [n = 3], asbestos [n = 2], and ulcerative colitis [n = 1]); secondary factors were determined by pathology or clinical diagnosis. The Japanese severity classification of patients with IPF10, consisting of arterial partial pressure of oxygen and oxygen saturation during exercise, was Stage I/II/III/IV in 11/1/12/28 patients. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage of patients with COPD was I/II/III/IV in 12/22/19/9 patients. Most patients with PPFE were underweight and tended to be thinner than those with IPF or COPD. Laboratory findings showed a mild increase in Krebs von den Lunge-6 (KL-6) and an increase in surfactant protein D. The pulmonary function test results, %forced vital capacity (FVC), and % diffusing capacity of the lung carbon monoxide (DLCO) were not reduced, but residual volume/total lung capacity (%RV/TLC) was increased.
Image analysis
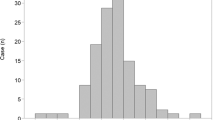
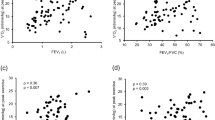
The distributions of the ESMCSA and BMI are shown in Fig. 1 and Supplementary Figures 1 and 2. The ESMCSA of patients with PPFE was significantly smaller than that of patients with IPF or COPD (PPFE; 24.38 [19.62–28.75] cm2, IPF; 30.77 [24.78–34.90] cm2 (Stage I/II; 33.86 [28.07–37.51] cm2, Stage III/IV; 30.77 [24.78–34.90] cm2), COPD; 30.58 [25.82–35.99] cm2 (GOLD I/II; 32.36 [25.96–36.66] cm2, GOLD III/IV; 29.31 [25.80–34.61] cm2); IPF vs PPFE: p < 0.001, IPF Stage I/II vs PPFE: p < 0.001, IPF Stage III/IV vs PPFE: p = 0.022, COPD vs PPFE: p < 0.001, COPD GOLD I/II vs PPFE: p < 0.001 and COPD GOLD III/IV vs PPFE: p = 0.003). BMI was significantly lower in the patients with PPFE than in those with IPF or COPD (PPFE; 18.6 [16.5–19.7] kg/m2, IPF; 21.7 [20.1–24.9] kg/m2; COPD; 23.1 [21.1–25.1] kg/m2; IPF vs PPFE: p < 0.001, IPF Stage I/II vs PPFE: p < 0.001, IPF Stage III/IV vs PPFE: p < 0.001; COPD vs PPFE: p < 0.001, COPD GOLD I/II vs PPFE: p < 0.001 and COPD GOLD III/IV vs PPFE: p = 0.005). The correlations among ESMCSA, BMI, and clinical parameters are shown in Fig. 2 and Table 2. In patients with PPFE, there was a significant correlation between ESMCSA and BMI (r = 0.543, p < 0.001) or RV/TLC (r = −0.509, p < 0.001), but no correlation with KL-6 or %FVC.
Distributions of the cross-sectional area of the erector spinae muscle (ESMCSA) (A) and body mass index (BMI) (B) in patients with pleuroparenchymal fibroelastosis (PPFE), idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD). Patients with PPFE had lower ESMCSA and BMI than patients with IPF or COPD.
Correlations between the cross-sectional area of the erector spinae muscle (ESMCSA) and body mass index (BMI) (A), albumin (B), Krebs von den Lunge-6 (KL-6) (C) and % forced vital capacity (FVC) (D) in pleuroparenchymal fibroelastosis (PPFE). In patients with PPFE, there was a correlation between ESMCSA and BMI but not between ESMCSA and albumin, KL-6 or %FVC.
Prognostic value of ESMCSA and BMI in PPFE
Over 3 years, 12 patients with PPFE died (the causes of death in patients with PPFE were respiratory failure [n = 5], acute exacerbation [n = 2], and unknown [n = 5]). The prognosis of patients with PPFE was evaluated using the Kaplan–Meier method and log-rank test, based on the ESMCSA cutoff value determined by the receiver operating characteristic curve to detect the risk of death (Fig. 3) and on the BMI value (WHO definition of underweight status). The cutoff value for ESMCSA was 22.57 cm2, with a sensitivity of 0.83, specificity of 0.31, and area under the curve of 0.801 (95% confidence interval: 0.663–0.940). In patients with PPFE, a significant difference was observed in the 3-year prognosis for ESMCSA < 22.57 cm2 (p = 0.006) at diagnosis but not for BMI < 18.5 kg/m2 (p = 0.129) (Fig. 4).
Determination of the cross-sectional area of the erector spinae muscle (ESMCSA) cutoff value for the risk of death within 3 years using receiver operating characteristic curves. The area under the curve was 0.801, and the ESMCSA cutoff value was 22.57 cm2, with a sensitivity of 0.83 and a specificity of 0.31.
Prognostic impact of the cross-sectional area of the erector spinae muscle (ESMCSA) (A) and body mass index (BMI) (B) in patients with pleuroparenchymal fibroelastosis (PPFE). Low ESMCSA was associated with death within 3 years in patients with PPFE. However, BMI showed no association with death within 3 years.
Univariate and multivariate analyses of ESMCSA in PPFE
To determine the prognostic impact of measurements related to skeletal muscle changes, a preliminary Cox proportional hazards regression analysis was performed. In the univariate analysis of patients with PPFE, ESMCSA, age, albumin, KL-6, %FVC, and RV/TLC were associated with mortality within 3 years. In the multivariate analyses, ESMCSA was independently associated with mortality within 3 years in patients with PPFE (Table 3).
Discussion
This study is one of the few to compare changes in skeletal muscle mass with prognosis in patients with PPFE. This study highlights the importance of these two evaluation aspects. First, a decrease in ESMCSA was associated with prognosis in patients with PPFE. Second, the assessment of skeletal muscle mass in PPFE may reflect a better prognosis than BMI.
It has been suggested that a lower ESMCSA may be associated with the prognosis of PPFE, including secondary PPFE (SPPFE). SPPFE is caused by haematopoietic stem cell transplantation, hypersensitivity pneumonitis, non-tuberculous mycobacterial infection, and chemotherapy11. However, it is possible that such comorbidities may simply be comorbid and not affect the patient and that IPPFE is included in the SPPFE. There were no significant differences in the laboratory data, respiratory complications, or survival rates between the idiopathic and secondary PPFE groups12. Known prognostic factors for IPPFE include older age, male, lower BMI, elevated KL-6, and lower FVC4,13. This study also found that older age, elevated KL-6 levels, and lower FVC were associated with prognosis; however, no previous reports have shown an association between lower ESMCSA and prognosis. Suzuki et al. also reported an association between PPFE and ESMCSA, but no significant difference was found in prognosis. There are two possible reasons for the different results. First, the diagnostic criteria are different. Both studies had small numbers of patients and may have observed different phenotypes. Second, the disease behaviour could be different. Many of the patients in the present study were diagnosed by imaging, which means that the time axis has not been assessed. Approximately half of the patients with PPFE enrolled in this study were underweight, whereas most patients with PPFE reported by Suzuki et al. were underweight, with a BMI of < 18.5 kg/m2. Therefore, it is possible that the patients with PPFE in the report by Suzuki et al. had a longer disease-modifying period than those in this study and already had a more reduced skeletal muscle mass. The ESMCSA in this study was independent of laboratory findings and pulmonary function tests in the multivariate analysis. Therefore, it is important to monitor ESMCSA in patients with PPFE.
In this study, ESMCSA was significantly correlated with age, BMI, and RV/TLC but not with %FVC or KL-6 values. The correlation of ESMCSA with age and BMI could be related to a decrease in skeletal muscle mass with increasing age or decreasing body weight14. However, it is noteworthy that despite a significant difference in survival within 3 years for ESMCSA, there was no significant difference in BMI. This finding may be related to body weight components. Body weight comprises muscle mass, body fat mass, and the amount of inorganic matter in the skeleton. The lack of a significant difference in prognosis with respect to BMI may be because BMI is calculated based on body weight, including body fat and muscle mass. Weight loss can occur for various reasons, including starvation caused by reduced food intake preserving skeletal muscle mass and cachexia resulting in reduced skeletal muscle mass15.
Cachexia is caused by increased energy expenditure due to inflammatory-induced cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6, decreased appetite and increased protein catabolism associated with leptin and ghrelin, and skeletal muscle atrophy due to angiotensin II16,17. Cachexia occurs in cancer patients and is a cause of reduced skeletal muscle mass in COPD patients18. Compared to healthy controls, patients with COPD, IPF, and PPFE have lower ESMCSA6,8, and those with PPFE have even lower amounts of ESMCSA than those with IPF8. In this study, the patients with PPFE had an even lower ESMCSA than those with COPD or IPF. This suggests that PPFE may be more strongly affected by cachexia than COPD or IPF. Oral nutritional supplements are often used to treat cachexia, and the ghrelin receptor agonist, anamorelin, has recently proved effective in cancer patients19,20. Ghrelin is an appetite-enhancing peptide secreted from the stomach, which binds to the growth hormone secretagogue receptor-1a (GHSR-1a) and regulates appetite and energy metabolism21,22. Ghrelin and its analogues exhibit appetite-stimulating effects by promoting the expression of agouti-related neuropeptides and neuropeptide Y23,24,25 and preventing weight loss26. In addition to its appetite-enhancing effects, ghrelin also suppresses energy expenditure by suppressing leptin-induced inflammatory cytokines such as IL-1β, IL-6, and TNF-α, which are central to the pathogenesis of cachexia27, and reduces skeletal muscle catabolism induced by angiotensin II28. Ghrelin may also be effective for PPFE patients with possible skeletal muscle loss due to cachexia. However, non-cancer cachexia is still difficult to study compared to major diseases because of issues such as research funding and lack of public awareness. Therefore, the relationship between PPFE and cachexia, including inflammatory cytokines, remains uncertain and there are few reports on nutritional therapy and anamorelin in interstitial pneumonia, including PPFE. The symptoms of cachexia, such as severe weight loss, anorexia, early satiety, and oedema, are not clear in the early stages of the disease, and the time required for symptom onset greatly depends on the rate of progression of the underlying disease and host responses, such as activation of the systemic inflammatory response and metabolic, immune, and neuroendocrine changes29. It is difficult to correct undernutrition in advanced cachexia, and prevention at an early stage is considered important30. Although body weight and BMI are often used to assess cachexia31,32, the quantitative analysis of body composition using ESMCSA is a better prognostic parameter than body weight or BMI8. The results of this study suggest that in PPFE, as in COPD, ESMCSA may be a stronger prognostic factor than BMI.
This study has several limitations. First, it was a retrospective study; thus, it was impossible to assess patients' clinical symptoms, such as dyspnoea, or ADLs at the time of diagnosis of PPFE. Second, although this was not a single-centre study, the number of patients included was relatively small. Third, the study included patients with PPFE who were not pathologically diagnosed. Although pathological evaluation is necessary for a definitive diagnosis of IPPFE, surgical lung biopsy is often not performed due to the risk of postoperative lung leaks, pneumothorax, and acute exacerbation33,34. Therefore, Watanabe et al.35 proposed a method for diagnosis without surgical biopsy. Finally, the cross-sectional area of the erector spinae muscle was measured as an antigravity muscle that may influence ADL; however, other antigravity muscles, such as the iliopsoas and quadriceps muscles36, were not assessed. Future prospective studies are required to evaluate various skeletal muscles to overcome these limitations.
In conclusion, we investigated the relationship between ESMCSA and PPFE. This study highlights the importance of monitoring ESMCSA in predicting the prognosis of patients with PPFE. This suggests that ESMCSA may be a better prognostic factor for PPFE than BMI.
Methods
Patients
This retrospective study was conducted in a cohort of 51 consecutive patients with PPFE at Chiba University Hospital and JR Tokyo General Hospital between July 2004 and June 2023. To compare skeletal muscle mass, the study enrolled 52 patients with IPF and 62 patients with COPD who visited our institute as a control group, and they were evaluated using physical measurements, pulmonary function tests, and chest CT. The control group had no malignancy, resected lungs, active infection, or neuromuscular disease.
The study protocol was approved by the Ethics Committee of Chiba University Graduate School of Medicine (M10117) and JR Tokyo General Hospital (R03-23). This retrospective study was conducted in accordance with the amended Declaration of Helsinki, and informed consent was obtained from all participants.
Image diagnosis
The clinical diagnosis of PPFE was based on the following criteria35. Major criteria included (1) the presence of subpleural airspace consolidation with traction bronchiectasis of the upper lobe and (2) subpleural zonal or wedge-shaped dense fibrosis consisting of collapsed alveoli and collagen-filled alveoli with septal elastosis, with or without collagenous thickening of the visceral pleura in surgical lung biopsy specimens. Minor criteria included (1) bilateral upward migration of pulmonary hilar structures and/or volume loss in the upper lobes; (2) dry cough or exertional dyspnoea with insidious onset; and (3) RV/TLC% ≥ 115% and/or BMI ≤ 20 kg/m2 plus RV/TLC% ≥ 80%. The presence of major criteria 1 and 2 indicated a diagnosis of definite PPFE, and the presence of major criteria 1 and minor criteria 1, 2 and/or 3 indicated a diagnosis of clinical PPFE.
Image analysis
Electronically stored CT images were used to assess skeletal muscle mass. All the CT images were obtained for diagnostic purposes during routine clinical practice. Chest CT was performed in the supine position with full inspiration breath-hold at 120 kV and approximately 200 mA. Scan data were analysed using the HOPE LifeMark-PACS (Fujitsu, Tokyo, Japan). Axial CT images (without contrast, 5 mm thick, 5 mm apart) taken at the inferior margin of the 12th thoracic vertebra were selected for ESMCSA measurements based on previous studies. The areas of the left and right ESMs were measured by manual tracing. The sum of the left and right muscle areas was defined as ESMCSA. All CT analyses were independently performed by trained individuals (SK, CK, and NS) blinded to the patients' survival statuses; the results were then averaged.
Data collection
Clinical data were obtained from the patients’ medical records. The laboratory findings and pulmonary function test results obtained at the time of diagnosis were recorded.
Statistical analysis
Survival time was measured from the date of PPFE diagnosis and differentiated between death within 3 years and 3-year survival as early prognosis deterioration. All data are expressed as the median [interquartile range]. The Mann–Whitney U test was used to evaluate differences in means between the two groups, and the Kruskal–Wallis test and post-hoc analyses were used for multiple comparisons. The relationships between continuous variables were evaluated using Spearman's rank correlation coefficients. Univariate and multivariate analyses were performed using Cox proportional hazards regression models to determine the effect of body composition changes on prognosis. A multivariate Cox proportional hazards regression model was performed using a stepwise approach with the Akaike information criterion on explanatory variables. The ESMCSA cutoff values for detecting the risk of death were determined using receiver operating characteristic curves, and BMI cutoff values were defined as underweight according to the World Health Organization. All statistical analyses were performed using R (R version 4.2.2, The R Foundation for Statistical Computing, Vienna, Austria), with p < 0.05 considered statistically significant.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Raghu, G. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183, 788–824 (2011).
Travis, W. D. et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 188, 733–748 (2013).
Frankel, S. K., Cool, C. D., Lynch, D. A. & Brown, K. K. Idiopathic pleuroparenchymal fibroelastosis: description of a novel clinicopathologic entity. Chest. 126, 2007–2013 (2004).
Hayashi, H. et al. Body Mass Index and arterial blood oxygenation as prognostic factors in patients with idiopathic pleuroparenchymal fibroelastosis. Sarcoidosis Vasc. Diffuse Lung. Dis. 34, 35–40 (2017).
Kennedy, C. C. et al. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 16, 217–224 (2019).
Ikezoe, T., Mori, N., Nakamura, M. & Ichihashi, N. Effects of age and inactivity due to prolonged bed rest on atrophy of trunk muscles. Eur. J. Appl. Physiol. 112, 43–48 (2012).
Tanimura, K. et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. Novel chest computed tomography-derived index for prognosis. Ann. Am. Thorac. Soc. 13, 334–341 (2016).
Nakano, A. et al. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci. Rep. 10, 2312 (2020).
Suzuki, Y. et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci. Rep. 8, 14074 (2018).
Kondoh, Y. et al. Disease severity staging system for idiopathic pulmonary fibrosis in Japan. Respirology. 22, 1609–1614 (2017).
Chua, F. et al. Pleuroparenchymal fibroelastosis. A review of clinical, radiological, and pathological characteristics. Ann. Am. Thorac. Soc. 16, 1351–1359 (2019).
Oda, T. et al. Comparison of clinical characteristics and outcomes between idiopathic and secondary pleuroparenchymal fibroelastosis. J. Clin. Med. 10, 846 (2021).
Kinoshita, Y. et al. A proposed prognostic prediction score for pleuroparenchymal fibroelastosis. Respir. Res. 22, 215 (2021).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 39, 412–423 (2010).
Chasen, M. R. & Bhargava, R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer. 17, 1345–1351 (2009).
Yoshida, T. et al. Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia. Int. J. Biochem. Cell Biol. 45, 2322–2332 (2013).
Nishikawa, H. et al. Cancer cachexia: its mechanism and clinical significance. Int. J. Mol. Sci. 22, 8491 (2021).
Schols, A. M., Broekhuizen, R., Weling-Scheepers, C. A. & Wouters, E. F. Body composition and mortality in chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 82, 53–59 (2005).
Temel, J. S. et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 17, 519–531 (2016).
Katakami, N. et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer. 124, 606–616 (2018).
Lv Y, Liang T, Wang G, Li Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci. Rep. 38, BSR20181061 (2018).
Liu, H. et al. Growth hormone secretagogue receptor-1a mediates ghrelin’s effects on attenuating tumour-induced loss of muscle strength but not muscle mass. J. Cachexia Sarcopenia Muscle. 12, 1280–1295 (2021).
Jarkovska, Z., Krsek, M., Rosicka, M. & Marek, J. Endocrine and metabolic activities of a recently isolated peptide hormone ghrelin, an endogenous ligand of the growth hormone secretagogue receptor. Endocr. Regul. 38, 80–86 (2004).
Dwarkasing, J. T. et al. Hypothalamic food intake regulation in a cancer-cachectic mouse model. J. Cachexia Sarcopenia Muscle. 5, 159–169 (2014).
Baldini, G. & Phelan, K. D. The melanocortin pathway and control of appetite-progress and therapeutic implications. J. Endocrinol. 241, R1–R33 (2019).
DeBoer, M. D. et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 148, 3004–3012 (2007).
Dixit, V. D. et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Invest. 114, 57–66 (2004).
Kadoguchi, T. et al. Angiotensin II can directly induce mitochondrial dysfunction, decrease oxidative fibre number and induce atrophy in mouse hindlimb skeletal muscle. Exp. Physiol. 100, 312–322 (2015).
Donohoe, C. L., Ryan, A. M. & Reynolds, J. V. Cancer cachexia: mechanisms and clinical implications. Gastroenterol. Res. Pract. 2011, 601434 (2011).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Martin, L. et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 31, 1539–1547 (2013).
McDonald, M. N. et al. Chest computed tomography-derived low fat-free mass index and mortality in COPD. Eur. Respir. J. 50, 1701134 (2017).
Kono, M. et al. Pneumothorax in patients with idiopathic pleuroparenchymal fibroelastosis: Incidence, clinical features, and risk factors. Respiration 100, 19–26 (2021).
Bonifazi, M., Montero, M. A. & Renzoni, E. A. Idiopathic pleuroparenchymal fibroelastosis. Curr. Pulmonol. Rep. 6, 9–15 (2017).
Watanabe, K. et al. Criteria for the diagnosis of idiopathic pleuroparenchymal fibroelastosis: a proposal. Respir. Investig. 57, 312–320 (2019).
Rahimi, M., Torkaman, G., Ghabaee, M. & Ghasem-Zadeh, A. Advanced weight-bearing mat exercises combined with functional electrical stimulation to improve the ability of wheelchair-dependent people with spinal cord injury to transfer and attain independence in activities of daily living: a randomized controlled trial. Spinal Cord. 58, 78–85 (2020).
Acknowledgements
We would like to thank Editage (www.editage.jp) for the English language editing.
Author information
Authors and Affiliations
Contributions
S.K. designed the research; S.K., M.A., C.K., N.S., D.I., T.K., and J.I. conducted the research; S.K., M.A., C.K. and N.S. analysed the data; S.K. and M.A. wrote the manuscript; and T.S. had the primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kitahara, S., Abe, M., Kono, C. et al. Prognostic impact of the cross-sectional area of the erector spinae muscle in patients with pleuroparenchymal fibroelastosis. Sci Rep 13, 17289 (2023). https://doi.org/10.1038/s41598-023-44138-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44138-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.