Abstract
Men have higher circulating levels of uric acid than women. This sex difference is suspected to be a result of suppressive effects of estradiol on uric acid. If so, estradiol would be inversely associated with circulating uric acid. This study aimed to test this hypothesis. This cross-sectional study included 9472 participants (weighted sample size of 184,342,210) aged 12–80 years from the 2013 to 2016 US National Health and Nutrition Examination Survey. Associations of sex hormones with uric acid were analyzed using weighted least squares regression, adjusting for demographic characteristics, lifestyle risk factors, and comorbidities. Neither free nor bioavailable estradiol was inversely associated with circulating uric acid in adolescent boys or girls, or adult men or women, or perimenopausal women after full adjustment. The sex difference in uric acid was established during adolescence as a result of a dramatic increase in uric acid in adolescent boys. During adolescence, the increase in estradiol in girls over time was accompanied by a relatively unchanged level of uric acid. All three fractions of estradiol (free, bioavailable, and total) were positively associated with uric acid in adolescent boys and girls after full adjustment. In adolescent boys, all three fractions of testosterone were positively associated with serum uric acid, and sex hormone-binding globulin was inversely associated with uric acid after full adjustment. These results suggest that estradiol is not inversely associated with circulating uric acid in adolescents and the establishment of sex difference in circulating uric acid during adolescence is associated with higher testosterone and lower sex hormone-binding globulin in adolescent boys.
Similar content being viewed by others
Introduction
Uric acid is the end product of metabolic breakdown of purine compounds1 which is catalyzed by xanthine oxidase2. Uric acid is excreted mainly via the kidney3. Many factors affect circulating uric acid levels, such as age, sex, ethnicity, body mass index4,5,6,7 as well as lifestyle factors including alcohol consumption, smoking, and physical activity6,8,9. In addition, kidney disease can impair the excretion of uric acid by the kidney and thus can increase uric acid levels in the circulation6,10. Moreover, high circulating uric acid has been reported to be associated with many health problems such as hypertension11, diabetes12, hypercholesterolemia13, coronary heart disease14, stroke15, sleep disorders16, gout17, and cancer18, which may be associated with the pro-inflammatory effect of uric acid19.
It is well known that men have higher circulating levels of uric acid than women20,21,22,23. Experts in the field suspect that this physiological difference in uric acid may be a result of estrogen20,21 because estradiol can inhibit the uric acid-generating enzyme xanthine oxidase isolated from rat liver24; in addition, estradiol replacement therapy can decrease circulating uric acid pharmacologically25. However, whether estradiol regulates uric acid levels under physiological conditions is unknown. If estradiol decreases uric acid levels under physiological conditions, estradiol is expected to be inversely associated with circulating uric acid in a general population. This study aimed to test this hypothesis using a representative US cohort of adolescents and adults who attended the National Health and Nutrition Examination Survey (NHANES) from 2013 to 2016.
Results
The characteristics of the cohort
This study included a total of 9472 noninstitutionalized US residents (weighted to a national sample size of 184,342,210) aged 12–80 years, with a mean (SD) age of 40.7 (17.9) years. Table 1 and Supplementary Table S1 describe the characteristics of the cohort. Compared to adolescents, adult participants had a higher body mass index and a lower eGFR, were less physically active, and had a higher prevalence of hypertension, diabetes, hypercholesterolemia, coronary heart disease, stroke, gout, sleep disorder, and cancer (Table 1).
Patterns of circulating levels of uric acid and sex hormones over the lifetime
Uric acid in males increased during adolescence (Fig. 1 and Table 2) and slightly decreased during adulthood (Fig. 1 and Table 2), whereas in females its level maintained relatively unchanged during adolescence (Fig. 1 and Table 2) and increased during adulthood (Fig. 1 and Table 2). Sex difference in circulating uric acid was established during adolescence as a result of a dramatic rise in uric acid in adolescent boys (Fig. 1).
Total, free, and bioavailable estradiol and testosterone increased during adolescence and then decreased during adulthood in both males and females, except that total estradiol remained relatively unchanged during adulthood in men (Figs. 2 and 3 and Table 2). Sex hormone-binding globulin (SHBG) in males decreased during adolescence and then increased during adulthood; whereas its level in females increased during adolescence and remained relatively unchanged during adulthood (Figs. 2 and 3 and Table 2).
Serum levels of sex hormones and uric acid in adolescents aged 12–19 years. Left panel, 1042 boys which represented a weighted sample size of 13,448,725. Right panel, 993 girls which represented a weighted sample size of 12,291,504. bE bioavailable estradiol; bT bioavailable testosterone; fE free estradiol; fT free testosterone; SHBG sex hormone-binding globulin; tE total estradiol; tT total testosterone. Data represent the weighted mean values over each year of age.
Serum levels of sex hormones and uric acid in adult men and women aged 20–80 years. Left panel, 4037 men which represented a weighted sample size of 88,248,186. Right panel, 3400 women which represented a weighted sample size of 70,353,795. bE bioavailable estradiol; bT bioavailable testosterone; fE free estradiol; fT free testosterone; SHBG, sex hormone-binding globulin; tE total estradiol; tT total testosterone. Data represent the weighted mean values over each year of age.
Establishment of sex difference in circulating uric acid is associated with higher testosterone and lower SHBG in adolescent boys
The establishment of sex difference in uric acid during adolescence coincided with an increase in estradiol in girls without a significant change in uric acid levels in girls during this period (Fig. 2 and Table 2). During the establishment of the sex difference in uric acid, estradiol was not inversely associated with uric acid in either boys or girls (Table 3). Instead, all three fractions of estradiol (free, bioavailable, and total) were positively associated with uric acid in adolescent boys and girls after adjustment for all tested confounders.
This establishment of sex difference in uric acid also coincided with a dramatic increase in testosterone and a decrease in SHBG in boys (Fig. 2). All three fractions of testosterone (log-transformed) were positively associated with serum uric acid in adolescent boys (β = 0.195, P < 0.001, for total testosterone; β = 0.250, P < 0.001, for free testosterone; and β = 0.258, P < 0.001, for bioavailable testosterone; Table 3) after full adjustment. In addition, SHBG (log-transformed) was inversely associated with uric acid in boys (β = − 0.246, P < 0.001, Table 3) after full adjustment.
Association of sex hormones with circulating uric acid in adult men and women
In adult men, all three fractions of estradiol were positively associated with uric acid (Table 3). In this sub-cohort, total testosterone and free testosterone were inversely associated with uric acid, whereas there lacked an association between bioavailable testosterone with uric acid (Table 3).
In adult women and perimenopausal women (aged 47–56 years), free and bioavailable estradiol were not associated with uric acid after full adjustment; however, total estradiol was inversely associated with uric acid in both sub-cohorts after full adjustment (Tables 3 and 4). All three fractions of testosterone were positively associated with uric acid in adult women after full adjustment and so were free and bioavailable testosterone in perimenopausal women (Table 4). Total testosterone was not associated with uric acid in perimenopausal women (Table 4).
SHBG (log-transformed) remained inversely associated with uric acid in adult men (β = − 0.160, P < 0.001) and women (β = − 0.168, P < 0.001, Table 3) and perimenopausal women (β = − 0.194, P < 0.001, Table 4) after full adjustment.
Discussion
Using data from a large representative US cohort, this study found that neither free nor bioavailable estradiol was inversely associated with circulating uric acid in adolescents or adults from a general US population after full adjustment. It also found that the sex difference in uric acid was established during adolescence as a result of a dramatic increase in uric acid in adolescent boys. The establishment of sex difference in circulating uric acid was associated with higher testosterone and lower SHBG in adolescent boys.
It is well known that the level of circulating uric acid in males is higher than that in females20,21,22,23. Experts in the field suspect that this physiological difference may be due to the suppressive effect of estradiol20,21,25, because estradiol can inhibit isolated xanthine oxidase (uric acid-generating enzyme)24 and it can also pharmacologically decrease circulating uric acid25. Our results did not support this speculation. Firstly, during adolescence (12–19 years) when the sex difference in circulating uric acid was established, the significant increase in estradiol over time in girls was accompanied by a relatively unchanged circulating level of uric acid. The establishment of sex difference in uric acid was a result of a dramatic increase in uric acid in adolescent boys. Secondly, when sex difference in uric acid was established during adolescence, estradiol was not inversely associated with uric acid in either boys or girls. Instead, all three fractions of estradiol (free, bioavailable, and total) were positively associated with uric acid in adolescent boys and girls after full adjustment. These results indicate that the establishment of sex difference in uric acid was not a result of the suppressing effect of estradiol on uric acid.
On the other hand, during adolescence when sex difference in uric acid was established, all three fractions of testosterone were positively associated with serum uric acid in adolescent boys. These observations are in agreement with literature reports: testosterone has been reported to stimulate isolated xanthine oxidase26, and it can also increase circulating uric acid pharmacologically25,27. All these results suggest that testosterone may contribute to the establishment of sex difference in circulating uric acid. However, reversal causality cannot be ruled out.
We also found that SHBG was inversely associated with uric acid in adolescent boys and girls. This result is consistent with a study with a small sample size (N = 205) of diabetic men28 that reported a similar finding. The mechanism underlying the observed negative association was not clear. This could be due to that SHBG binds to testosterone thus decreasing the levels of free and bioavailable testosterone. It could also be due to some unknown mechanisms. The establishment of sex difference in circulating uric acid coincided with a significant decrease in SHBG in boys, suggesting that SHBG may play a role in the establishment of such a sex difference. On the other hand, the possibility that uric acid affects the homeostasis of SHBG cannot be ruled out.
Associations of sex hormones with uric acid are more complex and less clear in adult men. For example, uric acid levels decreased slowly and gradually over decades of life in this sub-cohort. Total and free testosterone were inversely associated with uric acid, whereas there lacked an association between bioavailable testosterone with uric acid. These observations contradict the results from adolescent boys where all three types of testosterone were positively associated with uric acid. It is worthwhile to note that the gradual decline in total and free testosterone over decades of adult life was not companied by a gradual increase in uric acid in adult men over time, suggesting that testosterone was less likely to contribute to the gradual decrease in uric acid in men over decades of adult life.
In adult men, all three fractions of estradiol, similar to the findings in adolescent boys and girls, were positively associated with uric acid, suggesting that estradiol was less likely to suppress uric acid in adult men under physiological conditions. The gradual decline in uric acid over time in adult men was companied by a gradual decrease in free and bioavailable estradiol (but not total estradiol), suggesting that lower levels of free and bioavailable estradiol might lead to lower uric acid in adult men, although available reports are against this speculation24,25. Again, the possibility that lower uric acid leads to lower estradiol cannot be ruled out in adult men.
The gradual decline in uric acid over time in adult men was also companied by a gradual increase in SHBG, and SHBG was inversely associated with uric acid in this sub-cohort. These findings suggest that SHBG may play a role in regulating uric acid in adult men.
Similar to the situation in men, associations of sex hormones with uric acid are more complex and less clear in adult women. For example, total estradiol, but not free nor bioavailable estradiol, was inversely associated with uric acid in adult women or perimenopausal women after full adjustment. The gradual decrease in estradiol during perimenopause was accompanied by an increase in uric acid, suggesting that total estradiol may suppress uric acid in adult women and contribute to the rise in uric acid later in women’s life. However, it is unknown why there lacked a significant association between biologically active fractions of estradiol and uric acid in adult women, and why this possible suppressing effect of total estradiol on uric acid was only presented in adult women but not adult men nor adolescents.
Consistent with results from adolescents, all three fractions of testosterone in adult women were positively associated with uric acid and so were free and bioavailable testosterone in perimenopausal women, but total testosterone was not associated with uric acid in the latter sub-cohort. In addition, the gradual decrease in testosterone in adult women from their twenties to early forties was companies by a gradual decrease in uric acid during this period, and the increase in uric acid in perimenopausal women seemed accompanied by an overall increase in bioavailable testosterone. These results suggest that bioavailable testosterone may increase uric acid in adult women.
High circulating uric acid, high testosterone, and low SHBG are all associated with some health problems, e.g. hypertension11,29,30, diabetes12,30,31, and cancer18,30,32. Whether uric acid is a mediator linking high testosterone- or low SHBG with certain diseases needs to be investigated in the future.
A strength of this study is the large sample size which is representative of the US population. Another strength is that the associations of sex hormones with serum uric acid were adjusted for a wide range of known or perceived confounders. One weakness of this study is that it is based on cross-sectional data; therefore, any causal relationship between testosterone or SHBG and uric acid cannot be established. Another weakness is that data on luteinizing hormone and follicle-stimulating hormone were not available.
In conclusion, in contrast to the experts’ speculation, biologically active estradiol (free and bioavailable estradiol) was not inversely associated with uric acid in adolescents nor adults from a general US population. The sex difference in circulating uric acid was established during adolescence due to a dramatic increase in uric acid in boys and this establishment was associated with higher testosterone and lower SHBG in adolescent boys.
Methods
Study participants
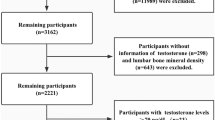
NHANES provides data from a representative sample of noninstitutionalized US population. From 2013 to 2016, a total of 13,104 participants had total estradiol, total testosterone, and SHBG data. The following 3632 participants were sequentially excluded: those without albumin (N = 1697) or uric acid (N = 3) or creatinine (N = 1) or body mass index data (N = 115), those pregnant (N = 57), those undergoing hysterectomy or ovary removal (N = 966), those taking sex hormone medication including testosterone, progesterone, estrogen, or unspecified sex hormones (N = 170), and those with kidney disease (estimated glomerular filtration rate < 60 mL/min per 1.73 m2, N = 623). Albumin data were needed to calculate free and bioavailable estradiol or testosterone concentrations33 and creatinine data were needed to calculate estimated glomerular filtration rate34. The resulting 9472 subjects were included in the final analysis of the current study.
Free and bioavailable estradiol and testosterone
Serum sex hormones were measured at the National Center for Environmental Health. SHBG was quantified based on the reaction of SHBG with immuno-antibodies and chemo-luminescence measurements of the reaction products by a photomultiplier tube35. Total testosterone and estradiol were measured using an isotope dilution-high performance liquid chromatography-tandem mass spectrometry36.
Estradiol and testosterone mainly exist in three fractions: free, albumin-bound, or SHBG bound. The fraction that is bound to SHBG is biologically inactive. Bioavailable estradiol or testosterone is the biologically active fraction of estradiol or testosterone, and it includes both free and albumin-bound fractions of each hormone. Free and bioavailable estradiol and testosterone were calculated according to the method provided by Vermeulen and colleagues33 based on serum concentrations of total estradiol or testosterone, SHBG, and albumin.
Uric acid
Uric acid was measured using the uricase peroxidase method37. In brief, uric acid was oxidized by uricase to produce hydrogen peroxide and the latter produced a colored product in the presence of peroxidase. The absorbance of the colored product, being measured at 520 nm, was directly proportional to the concentration of uric acid in the sample.
eGFR calculation and definition of kidney disease
eGFR was calculated by the CKD-EPI equation34: eGFR = 141 × min(Scr/κ, 1)α × max(Scr/κ, 1)− 1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is − 0.329 for females and − 0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1. Kidney disease38 was defined as eGFR < 60 mL/min per 1.73 m2 and people with kidney disease were excluded from this study because impaired kidney function significantly increases uric acid levels in the circulation6,10.
Covariates
Confounding covariates included age, ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or other), body mass index (continuous variable), eGFR (continuous variable, an indicator of kidney function), and self-reported health status (excellent, very good, good, fair, poor, or unknown). Lifestyle confounders included smoking status (never, former, current, or unknown), alcohol consumption (non-drinker, former drinker, current drinker, or unknown), and leisure-time physical activity (0, 1–149, 150–299, and ≥ 300 min per week, or unknown)39,40. Leisure-time physical activity was calculated using the following formula40: leisure-time physical activity (min/week) = 2 × vigorous activity frequency × vigorous activity duration + moderate activity frequency × moderate activity duration. Self-reported comorbidities included self-reported diagnoses of hypertension (yes, no, or unknown), diabetes (yes, no, borderline, or unknown), hypercholesterolemia (yes, no, or unknown), coronary heart disease (yes, no, or unknown), stroke (yes, no, or unknown), sleep disorder (yes, no, or unknown), gout (yes, no, or unknown), and cancer (yes, no, or unknown).
Statistical analysis
Data from the two NHANES cycles (2013–2014 and 2015–2016) were combined using the appropriate weighting methods41. Four-year weights were calculated by dividing the two-year weights by two42 and used in all analyses to adjust for unequal selection probability and non-response bias following NHANES analytical guidelines41. Population means, medians, and proportions were estimated and reported. Uric acid, age, and eGFR were approximately normally distributed. Estradiol, testosterone, SHBG, and body mass index were not normally distributed, and these variables were log-transformed before regression analyses were conducted. Descriptive statistics were presented as median and interquartile range (non-normally distributed continuous data), or mean and SD (approximately normally distributed continuous data), or percentages (categorical data). The association analyses were conducted using weighted least squares regression43 with or without adjusting for covariates. Sub-analyses were conducted in the following sub-cohorts: participants aged 12–19 years or 20–80 years or perimenopausal women aged 47–56 years. All tests were two-sided and a P value of < 0.05 was regarded as statistically significant. All statistical analyses were performed using SPSS version 27.0 (IBM SPSS Statistics for Windows, Armonk, NY, International Business Machines Corporation).
Ethical considerations
The National Center for Health Statistics Research Ethics Review Board (ERB) approved all study protocols (ERB No. NHANES Protocol #2011-17). All research procedures were performed following the relevant guidelines and regulations. Written informed consent was obtained from all participants. For participants who were under 18 years old and were 12 years old or older (the youngest age of this study was 12 years), written informed consent was obtained from both the participant and a parent or guardian of the participant; for emancipated minors who were 16 or 17 years old, the written informed consent was obtained from the participant only. The participants’ records were anonymized before being accessed by the author.
Data availability
All data in the current analysis are publicly available on the NHANES website.
References
Wu, X. W., Lee, C. C., Muzny, D. M. & Caskey, C. T. Urate oxidase: Primary structure and evolutionary implications. Proc. Natl. Acad. Sci. U S A 86, 9412–9416 (1989).
Mandal, A. K. & Mount, D. B. The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 77, 323–345 (2015).
Tsushima, Y. et al. Uric acid secretion from adipose tissue and its increase in obesity. J. Biol. Chem. 288, 27138–27149 (2013).
Qian, T. et al. Hyperuricemia is independently associated with hypertension in men under 60 years in a general Chinese population. J. Hum. Hypertens. https://doi.org/10.1038/s41371-020-00455-7 (2020).
Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 63, 3136–3141 (2011).
Qiu, L. et al. Prevalence of hyperuricemia and its related risk factors in healthy adults from Northern and Northeastern Chinese provinces. BMC Public Health 13, 664–664 (2013).
Zeng, J. et al. Association between serum uric acid and obesity in Chinese adults: A 9-year longitudinal data analysis. BMJ Open 11, e041919 (2021).
Ni, Q., Lu, X., Chen, C., Du, H. & Zhang, R. Risk factors for the development of hyperuricemia: A STROBE-compliant cross-sectional and longitudinal study. Medicine 98, e17597 (2019).
Raja, S. et al. Frequency of hyperuricemia and its risk factors in the adult population. Cureus 11, e4198–e4198 (2019).
Wang, Y. et al. Reduced renal function may explain the higher prevalence of hyperuricemia in older people. Sci. Rep. 11, 1302 (2021).
Cheng, W. et al. The association between serum uric acid and blood pressure in different age groups in a healthy Chinese cohort. Medicine 96, e8953 (2017).
Lv, Q. et al. High serum uric acid and increased risk of type 2 diabetes: A systemic review and meta-analysis of prospective cohort studies. PLoS ONE 8, e56864 (2013).
Kuwabara, M. et al. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: A five-year cohort study in Japan. Int. J. Cardiol. 261, 183–188 (2018).
Brand, F. N., McGee, D. L., Kannel, W. B., Stokes, J. 3rd. & Castelli, W. P. Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am. J. Epidemiol. 121, 11–18 (1985).
Lehto, S., Niskanen, L., Rönnemaa, T. & Laakso, M. Serum uric acid is a strong predictor of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke 29, 635–639 (1998).
Zheng, C. et al. Serum uric acid is independently associated with risk of obstructive sleep apnea-hypopnea syndrome in Chinese patients with type 2 diabetes. Dis. Mark. 2019, 4578327 (2019).
Shiozawa, A., Szabo, S. M., Bolzani, A., Cheung, A. & Choi, H. K. Serum uric acid and the risk of incident and recurrent gout: A systematic review. J. Rheumatol. 44, 388–396 (2017).
Dovell, F. & Boffetta, P. Serum uric acid and cancer mortality and incidence: A systematic review and meta-analysis. Eur. J. Cancer Prev. 27, 399–405 (2018).
Braga, T. T. et al. Soluble uric acid activates the NLRP3 inflammasome. Sci. Rep. 7, 39884 (2017).
Guo, L. Interpretation of the Chinese expert consensus: Recommendations for diagnosis and treatment of asymptomatic hyperuricemia complicated with cardiovascular diseases. J. Transl. Int. Med. 2, 93–96 (2014).
Feig, D. I., Kang, D. H. & Johnson, R. J. Uric acid and cardiovascular risk. N. Engl. J. Med. 359, 1811–1821 (2008).
Multidisciplinary Expert Task Force on Hyperuricemia and Related Diseases. Chinese multidisciplinary expert consensus on the diagnosis and treatment of hyperuricemia and related diseases. Chin. Med. J. (Engl) 130, 2473–2488 (2017).
Liu, R. et al. Prevalence of hyperuricemia and gout in Mainland China from 2000 to 2014: A systematic review and meta-analysis. Biomed. Res. Int. 2015, 762820 (2015).
Huh, K., Shin, U. S., Choi, J. W. & Lee, S. I. Effect of sex hormones on lipid peroxidation in rat liver. Arch. Pharmacol. Res. 17, 109–114 (1994).
Yahyaoui, R. et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J. Clin. Endocrinol. Metab. 93, 2230–2233 (2008).
Olatunji, L. A., Areola, E. D. & Badmus, O. O. Endoglin inhibition by sodium acetate and flutamide ameliorates cardiac defective G6PD-dependent antioxidant defense in gestational testosterone-exposed rats. Biomed. Pharmacother. 107, 1641–1647 (2018).
Kurahashi, H. et al. Testosterone replacement elevates the serum uric acid levels in patients with female to male gender identity disorder. Endocr. J. 60, 1321–1327 (2013).
Cao, W. et al. Association between sex hormone and blood uric acid in male patients with type 2 diabetes. Int. J. Endocrinol. 2017, 4375253 (2017).
Huisman, H. W. et al. The influence of testosterone on blood pressure and risk factors for cardiovascular disease in a black South African population. Ethn. Dis. 16, 693–698 (2006).
Wang, Y. Definition, prevalence, and risk factors of low sex hormone-binding globulin in US adults. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgab416 (2021).
Ding, E. L., Song, Y., Malik, V. S. & Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 295, 1288–1299 (2006).
Ruth, K. S. et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 26, 252–258 (2020).
Vermeulen, A., Verdonck, L. & Kaufman, J. M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 84, 3666–3672 (1999).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
National Center for Environmental Health. Method 1031: Sex Hormone-Binding Globulin Immunoassay. Laboratory Procedure Manual (2016).
National Center for Environmental Health. Method 1033: Simultaneous Measurement of Estradiol and Testosterone in Human Serum by ID LC-MS/MS. Laboratory Procedure Manual (2016).
Collaborative Laboratory Services. Uric acid NHANES 2015–2016. Laboratory Procedure Manual (2017).
Bauer, C., Melamed, M. L. & Hostetter, T. H. Staging of chronic kidney disease: Time for a course correction. J. Am. Soc. Nephrol. 19, 844–846 (2008).
Mehta, N. & Preston, S. Continued increases in the relative risk of death from smoking. Am. J. Public Health 102, 2181–2186 (2012).
Wang, Y., Nie, J., Ferrari, G., Rey-Lopez, J. P. & Rezende, L. F. M. Association of physical activity intensity with mortality: A National Cohort Study of 403,681 US adults. JAMA Intern. Med. 181, 203–211 (2021).
Chen, T. C. et al. National Health and Nutrition Examination Survey: Estimation procedures, 2011–2014. National Center for Health Statistics. Vital Health Stat. 177, 1–26 (2018).
Chen, T. C., Clark, J., Riddles, M. K., Mohadjer, L. K. & Fakhouri, T. H. National Health and Nutrition Examination Survey, 2015–2018: Sample design and estimation procedures. Vital Health Stat. 2(184), 1–35 (2020).
Taylor, M. K., Mahnken, J. D. & Sullivan, D. K. NHANES 2011–2014 reveals cognition of US older adults may benefit from better adaptation to the Mediterranean diet. Nutrients 12, 1929 (2020).
Acknowledgements
Yutang Wang is supported by a grant from the National Health and Medical Research Council of Australia (1062671).
Author information
Authors and Affiliations
Contributions
Y.W.: conceptualization, data analysis, writing—original draft preparation, and funding acquisition; F.J.C.: writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Charchar, F.J. Establishment of sex difference in circulating uric acid is associated with higher testosterone and lower sex hormone-binding globulin in adolescent boys. Sci Rep 11, 17323 (2021). https://doi.org/10.1038/s41598-021-96959-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96959-4
This article is cited by
-
New perspectives on ‘Breathomics’: metabolomic profiling of non-volatile organic compounds in exhaled breath using DI-FT-ICR-MS
Communications Biology (2024)
-
Association between elevated serum uric acid levels and high estimated glomerular filtration rate with reduced risk of low muscle strength in older people: a retrospective cohort study
BMC Geriatrics (2023)
-
The association between serum uric acid and creatine phosphokinase in the general population: NHANES 2015–2018
BMC Cardiovascular Disorders (2023)
-
Dietary fatty acids and mortality risk from heart disease in US adults: an analysis based on NHANES
Scientific Reports (2023)
-
Association between serum uric acid and triglyceride-glucose index in children and adolescents with short stature
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.