Abstract
Growing evidence indicates that testosterone is a conspicuous marker for assessing male bone mineral density (BMD). However, research regarding testosterone levels and BMD is sparse and controversial for females. Hence, we aimed to investigate the association between testosterone levels and BMD among adult females aged 40–60 years in the United States. In this cross-sectional study, all participants were part of the National Health and Nutrition Examination Survey (2011–2016). A weighted general linear model was used to estimate the association between testosterone levels and lumbar BMD. Age, race, income level, education level, body mass index (BMI), blood urea nitrogen (BUN) level, serum uric acid (UA) level, serum calcium (Ca) level, serum phosphorus (P) level, the use of oral contraceptive pills, the use of hormone replacement therapy (HRT), smoking status, drinking status, and the use of corticosteroids were adjusted using a weighted multiple regression model. Subgroup analyses were performed using the same regression model. We included 2198 female participants in the study, and testosterone levels were positively associated with lumbar BMD after adjusting for all the covariates (β = 1.12, 95% CI 0.31, 1.93). In subgroup analyses, the associations in the fourth quartile of testosterone levels were stronger for the participants aged 40–50 years old (quartile 4, β = 42.92, 95% CI 7.53, 78.30 vs. quartile 1) and 50 to 60-year-old (quartile 4, β = 32.41, 95% CI 0.14, 64.69 vs. quartile 1). Similar results were found in other subgroups, including subgroups for race (Non-Hispanic Black, Other), income level (income ≤ 1.3, income > 3.5), education level (college or higher), BMI > 25 kg/m2, BUN levels ≤ 20 mg/dL, UA levels ≤ 6 mg/dL, Ca levels ≤ 10.1 mg/dL, P levels ≤ 5 mg/dL, drinking status, never smoker, never taking birth control pills, and HRT user. There was no interaction among the covariates in the association between lumbar BMD and testosterone levels (P for interaction > 0.05). In US adult females aged 40–60 years, the testosterone level was a positive predictor of the lumbar BMD after adjusting for covariates.
Similar content being viewed by others
Introduction
Along with the global social structure of population ageing, the trend of ageing causes an increase in the overall incidence and prevalence of osteoporosis1. A survey showed that more than half of women aged 60–70 years will suffer from postmenopausal osteoporosis2. Therefore, advancing the age threshold for research, observation and intervention is helpful to prevent and treat osteoporosis. Females aged 40–60 years are experiencing a special period in which females gradually transition from the childbearing period to menopause; in this period, ovarian function declines, hormone secretion changes3, the balance between bone absorption and bone formation is destroyed, bone loss accelerates, and the incidence of osteoporosis increases4. The decrease in testosterone may be closely related to this process5.
Osteoporosis is a disease of increased bone fragility owing to decreases in bone density and the destruction of bone microarchitecture. Osteoporosis is one of the most important causes of vertebral fractures in middle-aged and older people, seriously affecting patients’ quality of life and increasing socio-economic burden2,6. Lumbar BMD is a vital sign of bone quality, reflecting the degree of osteoporosis and predicting the risk of vertebral fracture. The lumbar spine is the site that is favoured for monitoring treatment, while the lumbar BMD test is one of the gold-standard techniques for the diagnosis of osteoporosis. The diagnostic method has been incorporated into several clinical guidelines7,8.
The relevant correlation analyses between testosterone levels and osteoporosis are limited to the male population9,10; few studies with the female population have been performed, with a lack of high-level evidence, and the study results are contradictory. Previous studies have found that testosterone is positively correlated with cortical BMD in females and is an independent predictor of BMD in healthy young females11,12. Postmenopausal females have lower testosterone and oestradiol (E2) levels than premenopausal females; hence, their BMD is reduced13. Clinical studies have shown that taking testosterone preparations can improve BMD in elderly women with osteoporosis14,15. However, another clinical study found that adding testosterone to oestrogen replacement therapy did not result in a significant increase in BMD16,17. A meta-analysis showed that testosterone substitution or therapy had no significant effect on BMD in females but sufficient evidence of safety was lacking17.
We designed this cross-sectional study based on the current state of research on the relationship between testosterone levels and BMD in females. We examined the associations between testosterone levels and lumbar BMD among US adult females aged 40–60 years using samples from a database of a multiracial population.
Methods
Data source and study population
Data for this study were obtained from the National Health and Nutrition Examination Survey (NHANES) (2011–2016). The National Center for Health Statistics (NCHS) adopted a multistage, complex clustered probability design to select a representative sample from United States civilians. All protocols were approved by the research ethics review board of the NCHS, and informed consent forms were obtained from all participants. The survey data and methodological details about the NHANES are available at http://www.cdc.gov/nchs/nhanes/.
Study variables
The exposure variable was the testosterone level, which has strong androgenic and anabolic effects9. The isotope dilution liquid chromatography-tandem mass spectrometry (ID-LC–MS/MS) method was performed for routine quantitation of serum total testosterone18 based on the National Institute for Standards and Technology’s (NIST) reference method. This method was optimized for higher sample throughput and certified by the CDC Hormone Standardization Program (HoSt). Females with testosterone levels > 70 ng/dL are diagnosed as having hyperandrogenaemia. There are tumour and nontumor reasons for hyperandrogenemia19, including virilizing congenital adrenal hyperplasia, idiopathic hyperandrogenism, virilizing tumours, and polycystic ovary syndrome19,20. Consequently, we could not determine the reasons why the testosterone levels exceeded the normal range. To avoid the impact of these diseases on the results, we excluded participants with testosterone levels > 70 ng/dL18, focusing on the relationship between normal to low testosterone levels and lumbar BMD.
The outcome variable was lumbar BMD. It was quantified using dual-energy X-ray absorptiometry scans acquired on Hologic Discovery Model A densitometers18.
Variables thought to be confounders based on the literature and clinical judgement were included10,21. In this study, the covariates included demographic data (age, race, income level, education level, BMI), laboratory examinations (BUN, UA, Ca, P), and data from questionnaires (smoking status, having at least 12 alcohol drinks in the past year, ever taking birth control pills, ever using female hormones, ever taking prednisone or cortisone daily). The data acquisition process for testosterone levels, lumbar BMD, and the covariates can be found at the following URL: www.cdc.gov/nchs/nhanes/.
Ethics statement
According to the Revised Declaration of Helsinki, the institutional review board (IRB) of the NCHS approved the use of NHANES datasets. Informed consent from all participants was obtained before data collection.
Statistical analyses
Descriptive analysis was applied to all participants’ data. Categorical variables are expressed as proportions (%). As appropriate, continuous variables are expressed as the mean and standard deviation (SD) or median and interquartile range (IQR). Weighted multivariate linear regression models were used to evaluate the association between serum testosterone levels and lumbar BMD to assess differences in clinical characteristics.
β and 95% confidence intervals were calculated using multiple linear regression models for testosterone levels and lumbar BMD. Age, race, income level, education level, BMI, BUN levels, UA levels, P levels, Ca levels, oral contraceptive use, HRT use, smoking status, drinking status, and corticosteroid use were adjusted. A general linear model was used to study the association between testosterone levels and lumbar BMD. A P for trend was assessed to study trends of the association between testosterone levels and lumbar BMD among different testosterone levels. Participants were classified into age subgroups of ≥ 40 years and < 50 years old and ≥ 50 and ≤ 60 years old for the subgroup analyses. Participants were divided into two BMI groups using a cut-off value of 25 kg/m2. Participants were divided into two BUN level groups using a cut-off value of 20 mg/dL. Participants were divided into two UA level groups using a cut-off value of 6 mg/dL. Participants were divided into two Ca level groups using a cut-off value of 10.1 mg/dL. Participants were divided into two P level groups using a cut-off value of 5 mg/dL. These figures are critical values for normal and high values. A P for interaction > 0.05 indicates that no interaction was found. The analyses were performed with the statistical software package R (http://www.R-project.org, The R Foundation), and Free Statistics software version 1.4 (Beijing Free Kelin Medical Technology Co, Ltd.).
Results
Baseline characteristics of the study participants by categories of testosterone
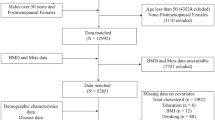
The population for the present analysis consisted of participants enrolled in 2011–2016. Among the 29,902 participants who underwent examinations, we excluded male participants (n = 14,751), those who were under the age of 40 years or over the age of 60 years (n = 11,989), those whose testosterone data (n = 298) or lumbar BMD data (n = 643) were missing and those with testosterone levels over 70 ng/dL (n = 23). Ultimately, 2198 participants were included in this study (Fig. 1).
Among the 2198 participants in the study, the average age was 49.1 years. The mean serum testosterone level was 18.9 ng/dL, and the mean lumbar BMD was 1017 mg/cm2. All variables were significantly different among persons classified into the different quartiles of testosterone. The lowest quartile of testosterone was ≥ 1.05 and < 12.15 ng/dL; the 2nd quartile was ≥ 12.19 and < 17.28 ng/dL; the 3rd quartile was ≥ 17.30 and < 23.25 ng/dL; and the highest quartile was ≥ 23.30 and < 68.20 ng/dL. Compared with participants in quartile 1, those in the other quartiles were younger, had higher lumbar BMD values, education levels, and BMI values, and had lower BUN and P levels. However, the drinking, hormone use rates, corticosteroid use rate, and oral contraceptive pills use rate were higher in quartile 1. The baseline characteristics of all participants are shown in Table 1.
Univariate linear regression analysis and the association between testosterone levels and lumbar BMD
Table 2 shows the results of the univariate linear regression analysis. We found that age, race, income level, education level, BMI, Ca levels, P levels, drinking status, smoking status, oral contraceptive use, and HRT use were significantly associated with lumbar BMD (P < 0.05). Among these, age, Ca levels, and P levels were negatively correlated with lumbar BMD. Income levels, BMI, and education levels were positively correlated with lumbar BMD (P < 0.05). BUN levels, UA levels, and corticosteroid use were not associated with lumbar BMD (P > 0.05). Compared with Mexican American women, Non-Hispanic White, Non-Hispanic Black, and women of other ethnicities aged 40–60 years had a higher lumbar BMD. Compared with never smokers, smokers had a lower lumbar BMD. Compared with drinkers, never drinkers had a higher lumbar BMD. Compared with participants who had never used HRT, those who did use HRT had a higher lumbar BMD. Compared with participants who never used oral contraceptive pills, those who did use oral contraceptive pills had a lower lumbar BMD.
Table 3 shows the β and 95% CI for the association between testosterone levels and lumbar BMD. When analysed in continuous form, there was a significant positive correlation between testosterone levels and lumbar BMD, and this association was found in the unadjusted model (β = 1.56, 95% CI 0.89, 2.23), adjusted Model I (β = 0.94, 95% CI 0.17, 1.71) and adjusted Model II (β = 1.12, 95% CI 0.31, 1.93). When treated as a categorical variable, in the unadjusted model, as the level of testosterone increased, the BMD of the lumbar spine increased (P for trend < 0.001) in Q2 (β = 24.3, 95% CI 5.85, 42.75), Q3 (β = 41.92, 95% CI 23.47, 60.37), and Q4 (β = 46.9, 95% CI 28.51, 65.29). After adjustment for age, race and corticosteroid use, Q2 (β = 33.03, 95% CI 11.31, 54.75), Q3 (β = 37.65, 95% CI 16.18, 59.12), and Q4 (β = 35.90, 95% CI 14.38, 57.41), respectively, were assessed (P for trend < 0.001). There was an obvious positive correlation between testosterone levels and lumbar BMD in Q2, Q3, and Q4. After adjustment for age, race, income level, education level, BMI, BUN levels, UA levels, Ca levels, P levels, smoking status, drinking status, oral contraceptive pills use, HRT use, and corticosteroid use, a positive correlation still existed in Q2 (β = 23.45, 95% CI 0.69, 46.21), Q3 (β = 37.59, 95% CI 15.11, 60.08), and Q4 (β = 37.51, 95% CI 14.68, 60.35) (P for trend < 0.001).
Subgroup analyses of the association between testosterone levels and lumbar BMD
In the stratified analyses, the participants were divided into different levels according to covariates, and then the correlation strength between the covariates and BMD was analysed at each level. In regression, an interaction effect exists when the effect of an independent variable on a dependent variable change, depending on the value(s) of one or more other independent variables. To determine whether the association between testosterone levels and lumbar BMD was stable in different subgroups, we performed stratified analyses and interaction analyses (Table 4). The associations in the fourth quartile of testosterone levels were stronger for the participants aged 40–50 years old (quartile 4, β = 42.92, 95% CI 7.53, 78.30 vs. quartile 1). In addition, the associations between the fourth quartile of the 50 to 60-year-old subgroup and testosterone levels were also stronger (quartile 4, β = 32.41, 95% CI 0.14, 64.69 vs. quartile 1). Similar results were found in other subgroups, including subgroups for race (Non-Hispanic Black, Other), income level (income ≤ 1.3, income > 3.5), education level (college or higher), BMI > 25 kg/m2, BUN levels ≤ 20 mg/dL, UA levels ≤ 6 mg/dL, Ca levels ≤ 10.1 mg/dL, P levels ≤ 5 mg/dL, drinking status, never smoker, never taking birth control pills, and HRT user. There was no interaction among the covariates in the association between lumbar BMD and testosterone levels (P for interaction > 0.05).
Discussion
In this nationally representative cross-sectional study, we combined data from the NHANES 2011–2016, and a total of 2198 females aged 40–60 years were included. In both univariate and multivariate linear regression analysis, our study indicated that testosterone levels were positively associated with lumbar BMD. The positive correlation between testosterone levels and lumbar BMD was still significant after adjusting for all the covariates and remained stable across subgroups. There was no interaction among the covariates in the association between lumbar BMD and testosterone levels.
According to the literature, it is basically clear that testosterone promotes BMD in males22,23, and the results from studies with females are controversial. On the one hand, some studies have found that testosterone levels in women are not related to BMD. A cohort study from California observed 457 females with a mean age of 72.1 years and found that testosterone levels were unrelated to BMD24. In another study, lumbar BMD and sex hormone levels were collected from 16 postmenopausal women. After statistical analysis, it was found that there was no correlation between testosterone levels and lumbar BMD. On the other hand, most studies are consistent with our results. A study from Athens suggested that higher testosterone levels may benefit the maintenance of female bone mass and that women with higher testosterone levels may be protected from the development of osteoporosis and fracture risk later in life25. These conflicting findings may be attributed to the heterogeneity among these studies, including differences in participant selection, study size, study design, and controlled confounders.
The current research focused on postmenopausal osteoporosis. However, the positive correlation between testosterone levels and BMD is not limited to menopausal females; females who are still menstruating may have relative deficiencies in testosterone, with reduced bone densities as a consequence. Bone density begins to decline in women before menopause26. The period of 40–60 years of age is the critical period from the beginning of bone mass reduction to the development of osteoporosis, and large sample clinical data analysis and cross-sectional studies are lacking for this period. Based on previous literature, our study fully considered confounding factors and strictly limited the age of the included population, making the conclusion reliable and filling in the gaps of current research from different perspectives. The mechanism by which testosterone increases BMD is unclear, and several possibilities have been proposed from the following aspects.
In cell culture studies, testosterone is sensitive to osteoblasts and osteoclasts9, and testosterone was shown to regulate osteoclast formation and survival associated with the RANKL pathway27,28,29. This suggests that testosterone or the nonaromatizable androgen dihydrotestosterone (DHT) acts directly on osteoclast progenitors and mature osteoclasts to inhibit osteoclastogenesis and promote osteoclast apoptosis30. Testosterone and DHT also regulate osteoclast activity and reduce bone turnover by inhibiting the production of PGE2 stimulated by parathyroid hormone and IL-131. Another study showed that testosterone signalling through the androgen receptor (AR) expressed in cells of the mesenchymal lineage mediates the protective effects of testosterone on cancellous bone, indirectly decreasing osteoclast numbers and restraining bone resorption in this compartment32. Multiple studies have shown that isolated B lymphocytes can act as osteoclast progenitors33,34,35,36. Testosterone can inhibit the production of B cells and prevent bone loss37,38,39.
The relationship between oestrogen levels and BMD has been widely studied and confirmed9,21,24,40; oestrogen is widely used to prevent and treat osteoporosis. According to our univariate linear regression analysis, we found that female hormone users had higher BMDs. In the ovaries, testosterone is in part aromatized in granulosa cells by cytochrome P450aro (CYP19A1) to form E241. A study found that with increasing age, the fraction of testosterone converted into E2 in the circulation increases42. Testosterone converted into E2 can not only enhance bone density and prevent osteoporosis but also improve menopausal symptoms caused by oestrogen deficiency.
However, the evidence for a positive effect of testosterone on bone health in women is contradictory11,13,43,44. Consequently, we used a large nationally representative sample with participants aged 40–60 years among the US female population, which increased the statistical strength to provide a more reliable result. The current findings have ideal generalizability. It is helpful for clinicians to identify groups at high risk for osteoporosis. There are also some limitations in our study. First, self-reported confounders might be susceptible to self-report bias. Second, limited by the cross-sectional study design, this study had less power regarding the determination of causal relationships between testosterone levels and lumbar BMD. Third, since the study was conducted in a population of middle-aged and elderly participants, the results may not be applicable to other age groups. Fourth, although we controlled for a broad range of lifestyle and health-related factors, correcting for possible confounders remained challenging. As we used questionnaires to estimate health status, residual confounding cannot be excluded. Fifth, we did not include E2 levels as a covariate because we were unable to determine whether the participants had their serum tested during the follicular phase; hence, the E2 value is unstable, and cannot accurately analyse the relationship between testosterone and E2, so the study could not look for an interaction between E2 and testosterone. Finally, due to the limited data, we only analysed the relationship between testosterone levels and lumbar BMD and did not further analyse the effects of androstenedione levels or free testosterone levels on lumbar BMD.
Conclusions
In this cross-sectional study, we found a positive correlation between testosterone levels and lumbar BMD in American females aged 40–60 years. These findings will further deepen our understanding of the pathophysiology of osteoporosis in middle-aged and elderly women, and such a conclusion warrants further prospective studies with intervention trials.
Data availability
The data described in the manuscript, code book, and analytic code are available from the corresponding author upon request.
Abbreviations
- NHANES:
-
The National Health and Nutrition Examination Survey
- BMD:
-
Bone mineral density
- BMI:
-
Body Mass Index
- BUN:
-
Blood urea nitrogen
- UA:
-
Uric acid
- Ca:
-
Calcium
- P:
-
Phosphorus
- HRT:
-
Hormone replacement therapy
- E2 :
-
Oestradiol
- NCHS:
-
National Center for Health Statistics
- ID-LC‒MS/MS:
-
Isotope dilution liquid chromatography-tandem mass spectrometry
- NIST:
-
National Institute for Standards and Technology
- HoSt:
-
Hormone Standardization Program
- IRB:
-
Institutional review board
- SD:
-
Standard deviationor
- IQR:
-
Interquartile range
- DHT:
-
Dihydrotestosterone
- AR:
-
Androgen receptor
References
Alejandro, P. & Constantinescu, F. A review of osteoporosis in the older adult: An update. Rheum. Dis. Clin. N. Am. 44, 437–451. https://doi.org/10.1016/j.rdc.2018.03.004 (2018).
Wu, D. et al. T-cell mediated inflammation in postmenopausal osteoporosis. Front Immunol. 12, 687551. https://doi.org/10.3389/fimmu.2021.687551 (2021).
Zumoff, B., Strain, G. W., Miller, L. K. & Rosner, W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J. Clin. Endocrinol. Metab. 80, 1429–1430. https://doi.org/10.1210/jcem.80.4.7714119 (1995).
Davis, S. R., McCloud, P., Strauss, B. J. & Burger, H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas 21, 227–236. https://doi.org/10.1016/0378-5122(94)00898-h (1995).
Xu, L. et al. The role of sex hormones on bone mineral density, marrow adiposity, and muscle adiposity in middle-aged and older men. Front Endocrinol. (Lausanne) 13, 817418. https://doi.org/10.3389/fendo.2022.817418 (2022).
Kendler, D. L. et al. Vertebral fractures: Clinical importance and management. Am. J. Med. 129(221), e221–e210. https://doi.org/10.1016/j.amjmed.2015.09.020 (2016).
Leib, E. S., Lewiecki, E. M., Binkley, N. & Hamdy, R. C. Official positions of the International Society for Clinical Densitometry. J. Clin. Densitom. 7, 1–6. https://doi.org/10.1385/jcd:7:1:1 (2004).
Binkley, N. et al. Official positions of the International Society for Clinical Densitometry and Executive Summary of the 2005 Position Development Conference. J. Clin. Densitom. 9, 4–14. https://doi.org/10.1016/j.jocd.2006.05.002 (2006).
Mohamad, N. V., Soelaiman, I. N. & Chin, K. Y. A concise review of testosterone and bone health. Clin. Interv. Aging 11, 1317–1324. https://doi.org/10.2147/cia.S115472 (2016).
Wang, N., Wang, L. & Huang, C. Association of total testosterone status with bone mineral density in adults aged 40–60 years. J. Orthop. Surg. Res. 16, 612. https://doi.org/10.1186/s13018-021-02714-w (2021).
Marcus, R. et al. The contribution of testosterone to skeletal development and maintenance: Lessons from the androgen insensitivity syndrome. J. Clin. Endocrinol. Metab. 85, 1032–1037. https://doi.org/10.1210/jcem.85.3.6428 (2000).
Buchanan, J. R., Hospodar, P., Myers, C., Leuenberger, P. & Demers, L. M. Effect of excess endogenous androgens on bone density in young women. J. Clin. Endocrinol. Metab. 67, 937–943. https://doi.org/10.1210/jcem-67-5-937 (1988).
Rariy, C. M. et al. Higher serum free testosterone concentration in older women is associated with greater bone mineral density, lean body mass, and total fat mass: The cardiovascular health study. J. Clin. Endocrinol. Metab. 96, 989–996. https://doi.org/10.1210/jc.2010-0926 (2011).
Need, A. G., Horowitz, M., Bridges, A., Morris, H. A. & Nordin, B. E. Effects of nandrolone decanoate and antiresorptive therapy on vertebral density in osteoporotic postmenopausal women. Arch. Intern. Med. 149, 57–60 (1989).
Watts, N. B. et al. Comparison of oral estrogens and estrogens plus androgen on bone mineral density, menopausal symptoms, and lipid-lipoprotein profiles in surgical menopause. Obstet. Gynecol. 85, 529–537. https://doi.org/10.1016/0029-7844(94)00448-m (1995).
Barrett-Connor, E. et al. A two-year, double-blind comparison of estrogen-androgen and conjugated estrogens in surgically menopausal women: Effects on bone mineral density, symptoms and lipid profiles. J. Reprod. Med. 44, 1012–1020 (1999).
Elraiyah, T. et al. Clinical review: The benefits and harms of systemic testosterone therapy in postmenopausal women with normal adrenal function: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 99, 3543–3550. https://doi.org/10.1210/jc.2014-2262 (2014).
Centers for Disease Control and Prevention (CDC). National Center for health Statistics (NCHS). https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/.
Zaman, A. & Rothman, M. S. Postmenopausal hyperandrogenism: Evaluation and treatment strategies. Endocrinol. Metab. Clin. N. Am. 50, 97–111. https://doi.org/10.1016/j.ecl.2020.12.002 (2021).
Rosenfield, R. L. & Ehrmann, D. A. The pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 37, 467–520. https://doi.org/10.1210/er.2015-1104 (2016).
Zhu, Z., Zhao, J., Fang, Y. & Hua, R. Association between serum estradiol level, sex hormone binding globulin level, and bone mineral density in middle-aged postmenopausal women. J. Orthop. Surg. Res. 16, 648. https://doi.org/10.1186/s13018-021-02799-3 (2021).
Finkelstein, J. S. et al. Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann. Intern. Med. 106, 354–361. https://doi.org/10.7326/0003-4819-106-3- (1987).
Stĕpán, J. J., Lachman, M., Zvĕrina, J., Pacovský, V. & Baylink, D. J. Castrated men exhibit bone loss: Effect of calcitonin treatment on biochemical indices of bone remodeling. J. Clin. Endocrinol. Metab. 69, 523–527. https://doi.org/10.1210/jcem-69-3-523 (1989).
Greendale, G. A., Edelstein, S. & Barrett-Connor, E. Endogenous sex steroids and bone mineral density in older women and men: The Rancho Bernardo Study. J. Bone Miner. Res. 12, 1833–1843. https://doi.org/10.1359/jbmr.1997.12.11.1833 (1997).
Kassanos, D. et al. Augmentation of cortical bone mineral density in women with polycystic ovary syndrome: A peripheral quantitative computed tomography (pQCT) study. Hum. Reprod. 25, 2107–2114. https://doi.org/10.1093/humrep/deq149 (2010).
Steinberg, K. K. et al. Sex steroids and bone density in premenopausal and perimenopausal women. J. Clin. Endocrinol. Metab. 69, 533–539. https://doi.org/10.1210/jcem-69-3-533 (1989).
Hadjidakis, D. J. & Androulakis, I. Bone remodeling. Ann. N. Y. Acad. Sci. 1092, 385–396. https://doi.org/10.1196/annals.1365.035 (2006).
Huber, D. M. et al. Androgens suppress osteoclast formation induced by RANKL and macrophage-colony stimulating factor. Endocrinology 142, 3800–3808. https://doi.org/10.1210/endo.142.9.8402 (2001).
Clarke, B. L. & Khosla, S. Androgens and bone. Steroids 74, 296–305. https://doi.org/10.1016/j.steroids.2008.10.003 (2009).
Miki, Y. et al. Effects of aromatase inhibitors on human osteoblast and osteoblast-like cells: A possible androgenic bone protective effects induced by exemestane. Bone 40, 876–887. https://doi.org/10.1016/j.bone.2006.11.029 (2007).
Pilbeam, C. C. & Raisz, L. G. Effects of androgens on parathyroid hormone and interleukin-1-stimulated prostaglandin production in cultured neonatal mouse calvariae. J. Bone Miner. Res. 5, 1183–1188. https://doi.org/10.1002/jbmr.5650051114 (1990).
Vanderschueren, D. et al. Sex steroid actions in male bone. Endocr. Rev. 35, 906–960. https://doi.org/10.1210/er.2014-1024 (2014).
Katavić, V. et al. The surface antigen CD45R identifies a population of estrogen-regulated murine marrow cells that contain osteoclast precursors. Bone 32, 581–590. https://doi.org/10.1016/s8756-3282(03)00097-8 (2003).
Sato, T., Shibata, T., Ikeda, K. & Watanabe, K. Generation of bone-resorbing osteoclasts from B220+ cells: Its role in accelerated osteoclastogenesis due to estrogen deficiency. J. Bone Miner. Res. 16, 2215–2221. https://doi.org/10.1359/jbmr.2001.16.12.2215 (2001).
Blin-Wakkach, C., Wakkach, A., Rochet, N. & Carle, G. F. Characterization of a novel bipotent hematopoietic progenitor population in normal and osteopetrotic mice. J. Bone Miner. Res. 19, 1137–1143. https://doi.org/10.1359/jbmr.040318 (2004).
Pugliese, L. S. et al. B-1 lymphocytes differentiate into functional osteoclast-like cells. Immunobiology 217, 336–344. https://doi.org/10.1016/j.imbio.2011.07.014 (2012).
Altuwaijri, S. et al. Susceptibility to autoimmunity and B cell resistance to apoptosis in mice lacking androgen receptor in B cells. Mol. Endocrinol. 23, 444–453. https://doi.org/10.1210/me.2008-0106 (2009).
Walters, K. A. & Handelsman, D. J. Role of androgens in the ovary. Mol. Cell Endocrinol. 465, 36–47. https://doi.org/10.1016/j.mce.2017.06.026 (2018).
Henning, P. et al. The effect of estrogen on bone requires ERα in nonhematopoietic cells but is enhanced by ERα in hematopoietic cells. Am. J. Physiol. Endocrinol. Metab. 307, E589-595. https://doi.org/10.1152/ajpendo.00255.2014 (2014).
Riggs, B. L., Khosla, S. & Melton, L. J. 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 23, 279–302. https://doi.org/10.1210/edrv.23.3.0465 (2002).
Kirschner, M. A. & Bardin, C. W. Androgen production and metabolism in normal and virilized women. Metabolism 21, 667–688. https://doi.org/10.1016/0026-0495(72)90090-x (1972).
Hemsell, D. L., Grodin, J. M., Brenner, P. F., Siiteri, P. K. & MacDonald, P. C. Plasma precursors of estrogen: II—Correlation of the extent of conversion of plasma androstenedione to estrone with age. J. Clin. Endocrinol. Metab. 38, 476–479. https://doi.org/10.1210/jcem-38-3-476 (1974).
Bertelloni, S. et al. Androgen-receptor blockade does not impair bone mineral density in adolescent females. Calcif. Tissue Int. 61, 1–5. https://doi.org/10.1007/s002239900282 (1997).
Hughes, I. A. et al. Androgen insensitivity syndrome. Lancet 380, 1419–1428. https://doi.org/10.1016/s0140-6736(12)60071-3 (2012).
Funding
The Special Fund Project of Capital Clinical Characteristic Application Research Project (No. 171100001017104). The Scientific Research Project of the Chinese Society of Ethnic Medicine Project (Nos. 2017KYXM-Z160-20; 2021z2072-340503).
Author information
Authors and Affiliations
Contributions
H.Z. contributed the central idea, and R.M.L., J.N.L., S.F.G., and L.N.M. analysed most of the data. H.Z. wrote the initial draft of the paper. K.M. guided the theory and design of the research and revised the article. All authors contributed to refining the ideas, carrying out additional analyses, and finalizing this paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, H., Ma, K., Li, RM. et al. Association between testosterone levels and bone mineral density in females aged 40–60 years from NHANES 2011–2016. Sci Rep 12, 16426 (2022). https://doi.org/10.1038/s41598-022-21008-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21008-7
This article is cited by
-
Effects of Testosterone in Mediating the Relationship Between Daytime Napping and Osteoporosis in European Populations: A Mendelian Randomization Study
Calcified Tissue International (2024)
-
Androgen deficiency in hypopituitary women: its consequences and management
Reviews in Endocrine and Metabolic Disorders (2024)
-
Mendelian randomization analyses of associations between breast cancer and bone mineral density
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.