Abstract
Defining the hemodynamic response to volume therapy is integral to managing critically ill patients with acute circulatory failure, especially in the absence of cardiac index (CI) measurement. This study aimed at investigating whether changes in central venous-to-arterial CO2 difference (Δ-ΔPCO2) and central venous oxygen saturation (ΔScvO2) induced by volume expansion (VE) are reliable parameters to define fluid responsiveness in sedated and mechanically ventilated septic patients. We prospectively studied 49 critically ill septic patients in whom VE was indicated because of circulatory failure and clinical indices. CI, ΔPCO2, ScvO2, and oxygen consumption (VO2) were measured before and after VE. Responders were defined as patients with a > 10% increase in CI (transpulmonary thermodilution) after VE. We calculated areas under the receiver operating characteristic curves (AUCs) for Δ-ΔPCO2, ΔScvO2, and changes in CI (ΔCI) after VE in the whole population and in the subgroup of patients with an increase in VO2 (ΔVO2) ≤ 10% after VE (oxygen-supply independency). Twenty-five patients were fluid responders. In the whole population, Δ-ΔPCO2 and ΔScvO2 were significantly correlated with ΔCI after VE (r = − 0.30, p = 0.03 and r = 0.42, p = 0.003, respectively). The AUCs for Δ-ΔPCO2 and ΔScvO2 to define fluid responsiveness (increase in CI > 10% after VE) were 0.76 (p < 0.001) and 0.68 (p = 0.02), respectively. In patients with ΔVO2 ≤ 10% (n = 36) after VE, the correlation between ΔScvO2 and ΔCI was 0.62 (p < 0.001), and between Δ-ΔPCO2 and ΔCI was − 0.47 (p = 0.004). The AUCs for Δ-ΔPCO2 and ΔScvO2 were 0.83 (p < 0.001) and 0.73 (p = 0.006), respectively. In these patients, Δ-ΔPCO2 ≤ -37.5% after VE allowed the categorization between responders and non-responders with a positive predictive value of 100% and a negative predictive value of 60%. In sedated and mechanically ventilated septic patients with no signs of tissue hypoxia (oxygen-supply independency), Δ-ΔPCO2 is a reliable parameter to define fluid responsiveness.
Similar content being viewed by others
Introduction
Hemodynamic optimization through fluid resuscitation is commonly used in critically ill patients with tissue hypoperfusion. The goal of volume expansion (VE) is to raise cardiac output and oxygen supply to improve tissue oxygenation. Recognizing patients who would benefit from VE remains challenging1. Identifying such patients is often dependent on measuring cardiac output2,3. Echocardiography is a skill that has made strides but not fully penetrated all critical care areas; it is limited by ultrasound availability and poor echogenicity, especially in mechanically ventilated patients. Passive leg raising test and end-expiratory occlusion methods also necessitate cardiac output measurement to asses fluid responsiveness4,5. Other measurements such as pulse pressure and stroke volume variations require specific technologies6 or are restricted to certain patient populations7. With these limitations, defining fluid responsiveness without cardiac output measurement would be of great help to the clinician at the bedside.
Venous-to-arterial CO2 tension difference reflects the balance between CO2 production and CO2 delivery to the lungs, a surrogate of the cardiac output8,9. Opposing changes over time in central venous-to-arterial CO2 tension difference (ΔPCO2) and cardiac output were reported in septic shock patients10,11,12. In post-cardiac surgery sedated and mechanically ventilated patients, Yazigi et al. observed a significant inverse correlation between changes in ΔPCO2 (Δ-ΔPCO2) and changes in cardiac index (ΔCI) induced by VE13. Moreover, in the same population, changes in central venous oxygen saturation (ΔScvO2) after VE were a reliable parameter to define fluid responsiveness14. However, data in the septic population is lacking.
In situations with tissue hypoxia, the increase in cardiac output and oxygen delivery (DO2) after VE would result in an increase in CO2 production (VCO2) and oxygen consumption (VO2) (oxygen supply dependency). These metabolic changes might confound fluctuations in arteriovenous O2 and CO2 parameters attributed solely to circulatory changes. This might reduce the changes in ΔPCO2 and ScvO2 induced by VE. Clinical studies have shown that the ratio of ΔPCO2 over the arterial-to venous oxygen content (ΔPCO2/ΔContO2) was a good indicator of oxygen supply dependency (tissue hypoxia) in critically ill patients15,16. This indicator could perhaps be used to identify such patients and guide the usage of CO2 and oxygen gaps.
Therefore, our study aimed to investigate: (1) if Δ-ΔPCO2 and ΔScvO2 are reliable parameters to identify fluid responsiveness in overall sedated and mechanically ventilated septic patients; (2) if the reliability of these parameters would be better in the sub-group of patients with no tissue hypoxia, defined as the absence of an increase in VO2 induced by a rise in DO2 after VE (oxygen supply independency); (3) if baseline ΔPCO2 /ΔContO2 ratio is a good predictor of tissue hypoxia. Such measurements are readily available in these patients with central venous and arterial catheters.
Materials and methods
This prospective and observational study was conducted in a single, mixed medical and surgical adult intensive care unit (ICU) between April and December 2017. The study was approved by the local institutional ethics committee (Comité d’Ethique du centre hospitalier du Dr. Shaffner de Lens). Informed consent was obtained from the next of kin of each patient. All experiments were performed in accordance with relevant guidelines and regulations.
Patients
We studied mechanically ventilated patients with sepsis17 for whom the attending physician decided to give VE due to the presence of at least one clinical sign of tissue hypoperfusion17 as previously described15: (a) systolic arterial pressure < 90 mmHg, mean arterial pressure < 65 mmHg, or the requirement for vasopressor administration; (b) skin mottling; (c) lactate levels > 2 mmo/l; or urinary output < 0.5 ml/kg/h for ≥ 2 h. Also, patients had to have a PiCCO device (PiCCO, Pulsion Medical System, Munich, Germany) as part of routine management of persistent signs of inadequate tissue perfusion in our ICU. Exclusion criteria were: pregnancy, age < 18 years old, moribund, and risk of fluid loading-induced pulmonary edema.
Measurements
Demographic data, acute circulatory failure etiology, the Simplified Acute Physiology Score (SAPS) II, and the Sequential Organ Failure Assessment (SOFA) scores were obtained on the day of enrollment. CI was obtained with the PiCCO device by central venous injections of 20 ml of iced 0.9% saline solution and recorded as the average of the three measurements.
Arterial and central venous blood gases were measured using the GEM Premier 4000 (Instrumentation Laboratory Co, Paris, France). The central venous blood was collected from a central venous catheter with the tip confirmed to be in the superior vena cava, near or at the right atrium, by radiograph as previously described15. ∆PCO2 was calculated as the difference between the central venous carbon dioxide tension and the arterial carbon dioxide tension. The arterial oxygen content was calculated as CaO2 (ml) = 1.34 × Hb (g/dl) × SaO2 + 0.003 × PaO2 (mmHg), where SaO2 is the oxygen saturation of arterial blood, Hb the hemoglobin concentration, and PaO2 the arterial oxygen tension. The central venous oxygen content was calculated as CcvO2 (ml) = 1.34 × Hb (g/dl) × ScvO2 + 0.003 × PcvO2 (mmHg), where PcvO2 is the central venous oxygen tension. ΔContO2 (ml) was calculated as CaO2 − CcvO2. DO2 (ml/min/m2) was calculated as CaO2 × CI × 10. VO2 (ml/m2) was calculated as CI × ΔContO2 × 10. Oxygen extraction was defined as OE = VO2/DO2. We also calculated the ∆PCO2/ΔContO2 ratio.
Study protocol
A first set of hemodynamic and oxygen-CO2 derived variables measurements was performed at baseline, including heart rate (HR), systemic arterial pressure, CI (thermodilution), DO2, VO2, ScvO2, arterial lactate level, and ∆PCO2. A 500 ml of colloid solution (4% Human serum albumin, Vialebex®; LFB) was administered to the patient over 15 min via a specific venous line. The same set of measurements was repeated immediately after the end of VE infusion. Ventilation parameters and infusions of norepinephrine and sedation drugs were remained unchanged during the VE.
Changes in hemodynamic and oxygenation variables were expressed as relative changes (([parameter after volume expansion − parameter before volume expansion]/parameter before volume expansion) × 100).
Statistical analysis
Patients in whom 500-ml VE increased thermodilution-derived CI > 10% were defined as responders and the remaining ones as non-responders. Also, patients were divided into two subgroups according to their increases in VO2 (≤ or > 10%) induced by VE. All data are expressed as mean ± SD, or as median [25–75%, interquartile range, (IQR)], as appropriate. The normality of data distribution was assessed using the Shapiro–Wilk test. Comparisons of values between responders and non-responders were performed by two-tailed Student’s t test, or Wilcoxon rank-sum test, as appropriate. Pairwise comparisons between different study times were assessed using paired Student’s t test or Wilcoxon signed-rank test, as appropriate. Analysis of categorical data was performed using the Chi2 and Fisher’s exact tests. Linear correlations were tested by using the Pearson or the Spearman test, as appropriate.
Receiver operating characteristic (ROC) curves were constructed to evaluate the ability of each parameter to predict fluid responsiveness after fluid challenge. The AUCs were compared using the nonparametric technique described by DeLong et al.18. Previously, we have shown that the upper 95% confidence interval values of the least significant changes (LSC), which are the minimum changes that needed to be measured by a laboratory analyzer in order to recognize a real change of measurement, for ΔPCO2 and ScvO2 were 36.5% and 5.0% respectively19. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+), negative likelihood ratio (LR−), and their 95% confidence intervals were calculated for Δ-ΔPCO2 and ΔScvO2.
Variables are usually considered of good clinical tool (having good discriminative property tests) when the inferior limits of the 95% confidence interval of their AUC are more than 0.7520. For this purpose, 43 patients would be sufficient for a power of 80% and an alpha risk of 0.05. Statistical analysis was performed using STATA 14.0 (StataCorp LP, College Station, Texas, USA). p < 0.05 was considered statistically significant. All reported p values are 2-sided.
Results
We studied 49 patients whose characteristics are summarized in Table 1. Twenty-five of the 49 patients (51%) were defined as responders because thermodilution CI increased by > 10% after VE of 500-ml.
There were no significant differences in patient characteristics, SOFA score, and norepinephrine between responders and non-responders (Table 1).
Effect of volume expansion on hemodynamic variables
At baseline, all the tested hemodynamic variables were similar between the two groups (Table 2). VE significantly increased arterial pressures, CVP, and intrathoracic blood volume, and decreased hemoglobin in both groups. CI and stroke volume index increased significantly only in responders after VE, whereas HR and extravascular lung water did not change (Table 2). Arterial and venous pH were not significantly different between responders and non-responders’ groups at baseline, and did not change significantly after VE (Table 2).
Effect of volume expansion on oxygenation and CO2-derived variables
At baseline, there were no significant differences between the responders and non-responders’ groups regarding all the oxygenation and CO2-derived variable (Table 3). DO2 and ScvO2 increased significantly, and OE decreased only in responders’ group after VE. VE significantly reduced ΔPCO2 and lactate levels only in the responders’ group (Table 3). VO2 and ΔPCO2/ΔContO2 ratio remained unchanged in both groups after VE.
We observed significant correlations between ΔScvO2 and ΔCI (r = 0.42, p = 0.003) and between Δ-ΔPCO2 and ΔCI (r = − 0.30, p = 0.03) after VE.
In patients with an increase in VO2 ≤ 10% (n = 36) after VE, the correlation between ΔScvO2 and ΔCI was of 0.62 (p < 0.001). Also, in these patients, Δ-ΔPCO2 was significantly correlated with ΔCI (r = − 0.47, p = 0.004).
Ability of ΔScvO2 and Δ-ΔPCO2 to define fluid responsiveness (increase in CI > 10% after VE)
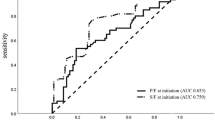
The AUC for ΔScvO2 was 0.68 (95% CI: 0.53–0.83) (p = 0.02) and for Δ-ΔPCO2 was 0.76 (95% CI: 0.63–0.89) (p < 0.001) (Fig. 1). There were no significant differences between the AUCs for ΔScvO2 and Δ-ΔPCO2 (p = 0.41).
The best cutoff value (according to Youden index) for ΔScvO2 was ≥ 3.5% (sensitivity = 64% [95% CI: 42–82%], specificity = 65% [95% CI: 43–84%]), which was lower than its LSC (5%). Taking into account the repeatability (LSC), the best cutoff value was ≥ 7.4% (sensitivity = 56% [95% CI: 35–76%], specificity = 71% [95% CI: 49–87%], PPV = 63% [95% CI: 39–83%], NPV = 61% [95% CI: 41–78%], LR+ = 1.8 [95% CI: 0.9–3.7], and LR− = 0.7 [95% CI: 0.4–1.1]).
The best cutoff value (according to Youden index) for Δ-ΔPCO2 was ≤ -23.5% (sensitivity = 52% [95% CI: 31–72%], specificity = 87% [95% CI: 68–97%], which was lower than its LSC (36.5%). Taking into account the repeatability (LSC), the best cutoff value was ≤ -37.5% (sensitivity = 32% [95% CI:15–53%], specificity = 92% [95% CI: 73–99%], PPV = 79% [95% CI: 42–97%], NPV = 58% [95% CI: 42–74%], LR+ = 3.8 [95% CI: 0.9–16.3], and LR− = 0.7 [95% CI:0.6–1.0]).
In patients with an increase in VO2 ≤ 10%, the AUC for ΔScvO2 was 0.73 (95% CI: 0.57–0.90) (p = 0.006) and for Δ-ΔPCO2 was 0.83 (95% CI: 0.69–0.97) (p < 0.001) (Fig. 2). There was no significant difference between the AUCs for ΔScvO2 and Δ-ΔPCO2 (p = 0.41).
Receiver operating characteristic (ROC) curves showing the ability of the changes in ΔPCO2 (Δ-ΔPCO2) (green curve), ScvO2 (ΔScvO2) (blue curve) between before and after 500 mL of volume expansion to define fluid responsiveness (increase in cardiac index > 10% after volume expansion) in the subgroup of patients with an increase in oxygen consumption ≤ 10%.
The best cutoff value (according to Youden index) for ΔScvO2 was ≥ 8.1% (sensitivity = 65% [95% CI: 38–86%], specificity = 74% [95% CI: 49–91%]), PPV = 73% [95% CI: 47–91%], NPV = 65% [95% CI: 40–85%], LR+ = 2.5 [95% CI: 1.1–5.6], and LR− = 0.5 [95% CI: 0.2–1.0]).
The best cutoff value (according to Youden index) for Δ-ΔPCO2 was ≤ -25% (sensitivity = 59% [95% CI: 33–82%], specificity = 89% [95% CI: 65–99%], which was lower than its LSC (36.5%). Taking into account the repeatability (LSC), the best cutoff value was ≤ − 37.5% (sensitivity = 41% [95% CI: 18–67%], specificity = 100% [95% CI: 59–100%], PPV = 100% [95% CI: 62–100%], NPV = 60% [95% CI: 40–78%], LR+ = ∞, and LR− = 0.60 [95% CI: 0.4–0.9]).
Characteristics of patients with tissue hypoxia (ΔVO2 > 10% after VE)
At baseline, ΔPCO2/ΔContO2 ratio was significantly higher in patients with ΔVO2 > 10% (tissue hypoxia) induced by volume expansion compared to patients with ΔVO2 ≤ 10% (2.03 [1.73–2.27] vs. 1.39 [1.00–1.71] mmHg/mL, p = 0.03, respectively). We did not observe significant differences between patients with ΔVO2 > 10% and ΔVO2 ≤ 10% regarding baseline lactate levels (2.35 [1.02–3.97] vs. 1.70 [1.40–3.10] mmol/L, p = 0.89, respectively) and baseline ScvO2 levels (60 ± 17% vs. 59 ± 13%, p = 0.78, respectively).
The AUCs of baseline lactate and ScvO2 values to predict ΔVO2 > 10% were 0.48 (95% CI: 0.28–0.69) (p = 0.89) and 0.54 (95% CI: 0.33–0.74) (p = 0.72), respectively. The AUC of baseline ΔPCO2/ΔContO2 ratio to predict ΔVO2 > 10% after volume expansion was 0.84 (95% CI: 0.71–0.96) (p < 0.001) (Fig. 3). The best cutoff value (according to Youden index) for baseline ΔPCO2/ΔContO2 ratio was > 1.70 (sensitivity = 77% [95% CI: 46–95%], specificity = 77% [95% CI: 60–90%]), PPV = 90% [95% CI: 73–98%], NPV = 55% [95% CI :31–78%], LR+ = 3.4 [95% CI: 1.7–6.6], and LR− = 0.3 [95% CI: 0.1–0.8]).
Discussion
The main findings of our study are: (1) in the whole population Δ-ΔPCO2 induced by VE has an acceptable ability to define fluid responsiveness, but not ΔScvO2; (2) The abilities of Δ-ΔPCO2 and ΔScvO2 to define fluid responsiveness improved when we considered only patients in whom changes in VO2 were minimal (ΔVO2 ≤ 10%) after VE, i.e., patients without tissue hypoxia; 3) Baseline ΔPCO2/ΔContO2 ratio has a good ability to predict the presence of tissue hypoxia (increases in VO2 > 10% after VE).
Applying the modified Fick method to CO2, venous-to-arterial PCO2 difference reflects cardiac output. Several experimental studies have demonstrated the primary role of decreased tissue blood flow in the increased venous-to-arterial PCO2 gap8,21,22. Similarly, a mathematical model analysis has confirmed that blood flow represents the major determinant in the elevation of venous-to-arterial PCO2 gap23. A rise in mixed venous-to-arterial PCO2 gap that was directly linked to a decrease in cardiac output has been observed in different types of circulatory failure including septic shock24,25. Mecher et al. found a significant negative correlation between the changes in cardiac output and mixed venous-to-arterial PCO2 gap after fluid resuscitation in septic shock patients (r = − 0.42, p < 0.01)24. In post-cardiac surgery sedated and mechanically ventilated patients with cardiac index < 2.5 L/min/m2, ΔCI induced by VE (500-mL bolus of crystalloid given over 30 min) was found to be significantly correlated with Δ-ΔPCO2 (r = − 0.53, p = 0.001)13. However, the AUC for Δ-ΔPCO2 to define fluid responsiveness was not determined in that study.
The correlation between Δ-ΔPCO2 and ΔCI induced by VE was weaker in our septic patients than what was observed in post-cardiac surgery patients13. Also, the ability of Δ-ΔPCO2 to define fluid responsiveness was not robust (AUC = 0.76). These findings could be explained by several factors. First, the relationship between ΔPCO2 and CI is curvilinear9, which means that the magnitude of changes in ΔPCO2 is more pronounced at low CI than at normal or high CI. Second, the relationship between CO2 content and PCO2, which is curvilinear rather than linear, is influenced by many factors such as the degree of metabolic acidosis, hematocrit, and oxygen saturation (Haldane effect)9,26. However, we believe that this factor is unlikely to have occurred in our patients. Although base excess and hemoglobin significantly decreased in both groups (responders and non-responders) and ScvO2 increased only in the responders’ group (Tables 2, 3) after VE, it is unlikely that these clinically irrelevant changes could have affected the PCO2/CO2 content relationship. If these changes had affected the PCO2/CO2 content relationship, it would have resulted in an increase in ΔPCO2 in both groups. Third, in situations of tissue hypoxia with VO2/DO2 dependency phenomenon and anaerobic CO2 production, the rise in CI would increase VO2 and VCO2. This would attenuate the decrease in ΔPCO2 related to the increase in blood flow9,27. We believe that this factor may have contributed to the reduction in the performance of Δ-ΔPCO2 (AUC = 0.76) in defining an increase in CI > 10% induced by VE (fluid responsiveness) in the overall population. When we excluded patients with tissue hypoxia, patients with an increase in VO2 ≤ 10% (VO2/DO2 independency), we observed an improvement in the ability of Δ-ΔPCO2 to define fluid responsiveness with a very good AUC of 0.83 (Fig. 2). A decrease in Δ-ΔPCO2 of more or equal than 37.5% induced by VE allowed discrimination between responders and non-responders with a PPV of 100%. Also, the correlation between Δ-ΔPCO2 and ΔCI was higher than in the overall population.
Venous oxygen saturation is a global marker of adequacy between oxygen consumption and oxygen supply28. Therefore, changes in venous oxygen saturation reflect changes in the balance between VO2 and DO2 and indicate tissue oxygenation. Giraud et al. observed, in 30 cardiogenic shock or postoperative cardiac surgery patients, that ΔScvO2 was significantly correlated with ΔCI induced by a bolus of 500 mL of normal saline administered over 10-min (r = 0.67, p < 0.001)14. Also, ΔScvO2 had an excellent ability to define an increase in CI ≥ 15% after VE (fluid responsiveness) with an AUC of 0.90. Our findings are different from those reported by Giraud et al.14. We observed in our whole septic population a weaker correlation between ΔScvO2 and ΔCI (r = 0.42), and ΔScvO2 had a poor ability to discriminate between responders and non-responders. In the subgroup of patients without tissue hypoxia (no significant increase in VO2, or VO2/DO2 independency), even though the correlation between ΔScvO2 and ΔCI improved, the ability of ΔScvO2 to characterize fluid responsiveness was not good. The main explanation of the discrepancies between our findings and those of Giraud et al.14 is that the patient populations are different. As has been previously described, venous oxygen saturation may not be a good indicator of tissue oxygenation in the setting of sepsis, due to microcirculatory shunting and mitochondrial dysfunction that can result in oxygen extraction abnormalities29.
It has been suggested that ΔPCO2/ΔContO2 ratio, considered as a surrogate of the respiratory quotient, can be used as a marker of global tissue hypoxia in critically ill patients15,16,30. In our study, we found that baseline ΔPCO2/ΔContO2 value was significantly higher in patients with global tissue hypoxia (defined as an increase in VO2 > 10% after VE) than those without global tissue hypoxia. Also, baseline ΔPCO2/ΔContO2 ratio had a very good ability to predict the presence of VO2/DO2 dependency (global tissue hypoxia). Our results confirmed our previous findings15 and those reported by Monnet et al.16, who observed excellent predictability of baseline ΔPCO2/ΔContO2 value for tissue hypoxia (AUCs = 0.96 and 0.94, respectively). Baseline lactate and ScvO2 levels had poor ability to predict VO2/DO2 dependency (global tissue hypoxia) in our study. This finding is in line of what we observed previously in septic shock patients15.
To the best of our knowledge, our study is the first to investigate the role of Δ-ΔPCO2 and ΔScvO2 in defining fluid responsiveness in septic patients. Our findings are valuable as they can be integrated in a clinical algorithm and used by the bedside provider as part of the assessment of fluid responsiveness. These values are readily available as patients in septic shock usually have arterial and central venous catheters inserted. After measuring O2 content and CO2 partial pressures, the ΔPCO2/ΔContO2 ratio can help the provider recognize patients without tissue hypoxia. In these patients, ΔPCO2 can be measured before and after VE and be used to appreciate if the latter has resulted in a significant increase in CI and to guide further fluid resuscitation when cardiac output monitoring is not available.
Our study presents several limitations. First, it is a single-center study, so the results might not universally apply. Second, we used central venous samples instead of mixed venous to assess oxygen and CO2-derived variables, limiting its accuracy. However, we were interested in the changes in these variables induced by fluid challenge rather than their absolute values. Moreover, it has been shown that calculating the oxygen and CO2-derived variables from the central venous blood permitted the detection of global tissue hypoxia in critically ill patients15,16. Third, our patients were sedated and mechanically ventilated with stable oxygen consumption; thus, our findings might not apply to spontaneously breathing patients with varying oxygen demands. Finally, our study was not sufficiently powered for subgroup analyses; thus, our findings need to be replicated in a future study with larger sample size.
Conclusions
In sedated and mechanically ventilated septic patients with no signs of tissue hypoxia, Δ-ΔPCO2 is a reliable parameter to define fluid responsiveness and can be used in the absence of CI measurement. Baseline ΔPCO2/ΔContO2 ratio could help the physician recognize the presence of tissue hypoxia.
Abbreviations
- CO2 :
-
Carbon dioxide
- VO2 :
-
Oxygen consumption
- DO2 :
-
Oxygen delivery
- ∆PCO2 :
-
Venous-to-arterial carbon dioxide tension difference
- ΔContO2 :
-
Arterial-to-venous oxygen content difference
- PaCO2 :
-
Partial arterial carbon dioxide tension
- PcvCO2 :
-
Central venous carbon dioxide tension
- ScvO2 :
-
Central venous oxygen saturation
- SaO2 :
-
Arterial oxygen saturation
- PaO2 :
-
Partial arterial oxygen tension
- PvO2 :
-
Partial venous oxygen tension
- Hb:
-
Hemoglobin
- CI:
-
Cardiac index
- LSC:
-
Least significant change
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- LR:
-
Likelihood ratio
- VE:
-
Volume expansion
- HR:
-
Heart rate
- ICU:
-
Intensive care unit
References
Michard, F. & Teboul, J. L. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121, 2000–2008 (2002).
Vincent, J. L. & Weil, M. H. Fluid challenge revisited. Crit. Care Med. 34, 1333–1337 (2006).
Cecconi, M., Parsons, A. K. & Rhodes, A. What is a fluid challenge?. Curr. Opin. Crit. Care 17, 290–295 (2011).
Monnet, X. et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit. Care Med. 34, 1402–1407 (2006).
Monnet, X. et al. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit. Care Med. 37, 951–956 (2009).
Mallat, J. et al. Decrease in pulse pressure and stroke volume variations after mini-fluid challenge accurately predicts fluid responsiveness. Br. J. Anaesth. 115, 449–456 (2015).
Mahjoub, Y. et al. Evaluation of pulse pressure variation validity criteria in critically ill patients: a prospective observational multicentre point-prevalence study. Br. J. Anaesth. 112, 681–685 (2014).
Vallet, B., Teboul, J. L., Cain, S. & Curtis, S. Venoarterial CO(2) difference during regional ischemic or hypoxic hypoxia. J. Appl. Physiol. 89, 1317–1321 (2000).
Mallat, J., Lemyze, M., Tronchon, L., Vallet, B. & Thevenin, D. Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J. Crit. Care Med. 5, 47–56 (2016).
Mallat, J. et al. Central venous-to-arterial carbon dioxide partial pressure difference in early resuscitation from septic shock: a prospective observational study. Eur. J. Anaesthesiol. 31, 371–380 (2014).
Vallée, F. et al. Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock?. Intensive Care Med. 34, 2218–2225 (2008).
Cuschieri, J. et al. Central venous-arterial carbon dioxide difference as an indicator of cardiac index. Intensive Care Med. 31, 818–822 (2005).
Yazigi, A. et al. Correlation between central venous-arterial carbon dioxide tension gradient and cardiac index changes following fluid therapy. Ann. Card Anaesth. 13, 269–271 (2010).
Giraud, R. et al. ScvO(2) as a marker to define fluid responsiveness. J Trauma 70(4), 802–807 (2011).
Mallat, J. et al. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann. Intensive Care. 6, 10 (2016).
Monnet, X. et al. Lactate and venoarterial carbon dioxide difference/arterial–venous oxy-gen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit. Care Med. 41, 1412–1420 (2013).
Dellinger, R. P. et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 41, 580–637 (2013).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988).
Mallat, J. et al. Repeatability of blood gas parameters, PCO2 gap, and PCO2 gap to arterial-to-venous oxygen content difference in critically ill adult patients. Medicine (Baltimore) 94, e415 (2015).
Ray, P., Le Manach, Y., Riou, B. & Houle, T. T. Statistical evaluation of a biomarker. Anesthesiology 112, 1023–1040 (2010).
Nevière, R., Chagnon, J. L., Teboul, J. L., Vallet, B. & Wattel, F. Small intestine intramucosal PCO(2) and microvascular blood flow during hypoxic and ischemic hypoxia. Crit. Care Med. 30, 379–384 (2002).
Dubin, A. et al. Intramucosal-arterial PCO2 gap fails to reflect intestinal dysoxia in hypoxic hypoxia. Crit. Care 6, 514–520 (2002).
Gutierrez, G. A mathematical model of tissue-blood carbon dioxide exchange during hypoxia. Am. J. Respir. Crit. Care Med. 169, 525–533 (2004).
Mecher, C. E., Rackow, E. C., Astiz, M. E. & Weil, M. H. Venous hypercarbia associated with severe sepsis and systemic hypoperfusion. Crit. Care Med. 18, 585–589 (1990).
Bakker, J. et al. Veno-arterial carbon dioxide gradient in human septic shock. Chest 101, 509–515 (1992).
Teboul, J. L. & Scheeren, T. Understanding the Haldane effect. Intensive Care Med. 43, 91–93 (2017).
Lamia, B., Monnet, X. & Teboul, J. L. Meaning of arterio-venous PCO2 difference in circulatory shock. Minerva Anestesiol. 72, 597–604 (2006).
Squara, P. Central venous oxygenation: when physiology explains apparent discrepancies. Crit. Care 18, 579 (2014).
Vallet, B., Pinsky, M. R. & Cecconi, M. Resuscitation of patients with septic shock: please “mind the gap”!. Intensive Care Med. 39, 1653–1655 (2013).
Mekontso-Dessap, A. et al. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. 28, 272–277 (2002).
Acknowledgements
The authors thank the research nurses of the intensive care unit at Lens hospital. Without their participations, this work would not have been possible.
Author information
Authors and Affiliations
Contributions
J.M., B.N., and M.B. designed the study. J.M. conducted statistical analyses. N.V., G.G., F.P., and J.T. collected data. J.M., B.N., L.T., and D.T. participated in manuscript writing and reviewing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nassar, B., Badr, M., Van Grunderbeeck, N. et al. Central venous-to-arterial PCO2 difference as a marker to identify fluid responsiveness in septic shock. Sci Rep 11, 17256 (2021). https://doi.org/10.1038/s41598-021-96806-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96806-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.