Abstract
Recent evidence indicates that our understanding of the relationship between cardiac function and ischemic stroke remains incomplete. The Cardiovascular Health Study enrolled community-dwelling adults ≥ 65 years old. We included participants with speckle-tracking data from digitized baseline study echocardiograms. Exposures were left atrial reservoir strain (primary), left ventricular longitudinal strain, left ventricular early diastolic strain rate, septal e’ velocity, and lateral e’ velocity. The primary outcome was incident ischemic stroke. Cox proportional hazards models were adjusted for demographics, image quality, and risk factors including left ventricular ejection fraction and incident atrial fibrillation. Among 4,000 participants in our analysis, lower (worse) left atrial reservoir strain was associated with incident ischemic stroke (HR per SD absolute decrease, 1.14; 95% CI 1.04–25). All secondary exposure variables were significantly associated with the outcome. Left atrial reservoir strain was associated with cardioembolic stroke (HR per SD absolute decrease, 1.42; 95% CI 1.21–1.67) and cardioembolic stroke related to incident atrial fibrillation (HR per SD absolute decrease, 1.60; 1.32–1.95). Myocardial dysfunction that can ultimately lead to stroke may be identifiable at an early stage. This highlights opportunities to identify cerebrovascular risk earlier and improve stroke prevention via therapies for early myocardial dysfunction.
Similar content being viewed by others
Strokes account for 10% of deaths worldwide1, and two-thirds of strokes are ischemic strokes. A substantial and increasing proportion of ischemic strokes result from cardiac embolism2. Cardioembolic strokes cause more disability than other types of ischemic stroke3, making cardiac disease an especially important target for stroke prevention efforts. Several cardiac conditions have been firmly established as stroke risk factors, most notably atrial fibrillation (AF), heart failure with reduced ejection fraction, acute myocardial infarction (MI), and severe valvular disease4. Recent studies suggest that our understanding of the relationships between cardiac structure and function and ischemic stroke remains incomplete4,5,6,7,8. At the same time, approximately one-fifth of ischemic strokes do not have a specific identifiable cause9, and most of these cryptogenic strokes are likely caused by emboli from currently unrecognized sources in the heart, aorta, or large cerebral vessels10. An improved understanding of the relationship between cardiac disease and ischemic stroke may thus shed light on currently unrecognized sources of stroke and lead to better therapy for stroke prevention.
Speckle tracking is a validated technique that allows for quantification of myocardial tissue deformation using two-dimensional echocardiographic images11,12. With this technique, subtle cardiac dysfunction can be detected before the appearance of overt structural abnormalities or clinical manifestations13. Myocardial strain indices have been associated with incident AF14,15,16,17, markers of left atrial thromboembolism18,19, and stroke in patients with AF20,21 or acute MI. Few data exist on the association between myocardial strain and incident ischemic stroke in the general population. Since speckle-tracking echocardiography illuminates aspects of cardiac function that are not readily detectable by conventional echocardiography, elucidating the relationship between myocardial strain and stroke may fill gaps in knowledge about cardioembolic stroke. Using data from participants in a prospective, longitudinal cohort study, we tested the hypothesis that indices of cardiac mechanics, especially those related to atrial function, are independently associated with subsequent ischemic stroke.
Methods
Design
The Cardiovascular Health Study (CHS) prospectively enrolled and follows a community-dwelling cohort of individuals ≥ 65 years of age. CHS study centers recruited a first cohort of 5,201 participants in 1989–1990 and a second, predominantly African-American, cohort of 687 participants in 1992–1993. These 5,888 participants were selected from a random sample of people on Medicare eligibility lists in four counties, one each in California, Maryland, North Carolina, and Pennsylvania22. Participants returned for in-person study visits annually until 1998–1999 and again in 2005–2006. Throughout follow-up, participants were contacted via semiannual telephone calls to ascertain events, and follow-up data were also linked with Medicare claims. Institutional review boards at the University of Washington and each field center approved this study, and all participants provided written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Participants
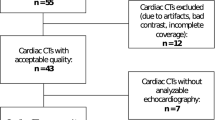
CHS excluded individuals who were younger than 65 years of age, could not give consent or answer questions without a surrogate, resided in an institutional setting, were wheelchair dependent, or were receiving active treatment for cancer. For this study, we included participants with available speckle-tracking data from their baseline echocardiogram. We excluded participants who had experienced a stroke (ischemic or hemorrhagic) or were diagnosed with AF at or prior to their baseline echocardiogram.
Measurements
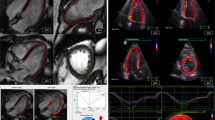
Comprehensive 2-dimensional, M-mode, and Doppler echocardiograms were obtained in 1989–1990 and again in 1994–1995, and they served as the baseline scans for the original and supplemental cohorts, respectively23. The echocardiograms were performed using a standardized protocol and were interpreted at core reading centers. At the four field centers, echocardiograms were recorded onto Super VHS tapes using Toshiba SSH-160A cardiac ultrasound machines. Videotapes were sent to the CHS Echocardiography Reading Center (Irvine, CA, USA, for the 1989–1990 echocardiograms, and Washington, DC, USA for the 1994–1995 echocardiograms), where images were digitized and initial measurements were made using M-mode, 2-D, and Doppler images. From 2016 to 2018, archived CHS echocardiograms were digitized using the TIMS 2000 DICOM system (Foresight Imaging, Chelmsford, Massachusetts, USA), using methods developed by our group for a similar analysis done in the Hypertension Genetic Epidemiology Study (HyperGEN Study)13. Cine loops of 2–4 cardiac cycles from the apical 4-chamber24 view were digitized at a frame rate of 30 frames per second and stored offline in DICOM format (Northwestern University, Chicago, IL, USA). Digitized cine loops of the apical 4-chamber view from the highest-quality cardiac cycle were analyzed using 2-dimensional wall-motion tracking software (2D Cardiac Performance Analysis, TomTec v4.5, Unterschleisshein, Germany).
Our echocardiographic exposure variables were prespecified based on an evolving conceptual understanding of the relationship between myocardial disease and thromboembolism, which posits a central role for a thrombogenic atrial myopathy resulting from ventricular dysfunction, primary atrial dysfunction, and/or systemic inflammation4,5,6,7,8,25,26,27,28. The primary exposure variable was left atrial reservoir strain, which is a hallmark of atrial myopathy and represents the degree of deformation as the left atrium fills and stretches during ventricular systole26. Secondary exposure variables were related to measures of left ventricular function: left ventricular average longitudinal strain, early diastolic strain rate, septal e’ velocity, and lateral e’ velocity.
For both the left ventricle and left atrium, 6 segmental strain curves were measured for each chamber in the apical 4-chamber view using electrocardiographic R-to-R wave gating. Speckle-tracking analysis was only performed in the apical 4-chamber view and not in all 3 apical views for feasibility purposes and based on our prior finding that, in population-based studies, strain parameters from all 3 apical views are highly correlated13. For left ventricular longitudinal strain, average longitudinal strain was the average of the 6 segmental strain curves at ventricular end-systole. Early diastolic (e’) velocities measured by speckle-tracking are lower than tissue Doppler velocities used clinically because they represent the average and not peak e’ velocity at the septal and lateral mitral annulus13. Experienced operators blinded to all other variables performed the speckle-tracking analysis and assessed chamber-specific tracking quality using a standard 4-point scale13. This method of measuring cardiac mechanics from archived echocardiograms has been validated against native digital speckle tracking on modern echocardiography machines13. Inter- and intra-observer reproducibility demonstrated high reliability and relatively low bias (Online Table I).
The primary outcome was incident ischemic stroke. Methods for identifying and adjudicating strokes in CHS have been previously published29. Stroke was defined as the rapid onset of a neurological deficit lasting > 24 h or until death, or a lesion on computed tomography or magnetic resonance imaging if symptoms lasted < 24 h, and no evidence that the symptoms were due to brain trauma, tumor, or infection. To be classified as an ischemic stroke, a stroke also had to involve: (1) a focal neurological deficit without evidence of intracranial hemorrhage on computed tomography, magnetic resonance imaging, or cerebrospinal fluid analysis, or (2) imaging evidence of brain ischemia in a location compatible with the presenting symptoms.
Potential confounders included in multivariable models were age, sex, race (African-American versus other race), education level (< high school level versus high school or more), body mass index, coronary heart disease, heart failure, diabetes, anti-hypertensive medications and systolic blood pressure, high- and low-density lipoprotein and triglyceride levels, smoking status (never, past, or current), left ventricular ejection fraction (≥ 55%, 45–54%, or < 45%), CHS site, speckle-tracking analyst, chamber-specific echocardiogram image quality (using a standard 4-point scale13), and a time-varying variable for incident AF during follow-up. Cholesterol and triglyceride levels were from 2 years prior to the baseline echocardiogram for the second cohort; all other covariates were defined at the time of the baseline echocardiogram. Incident AF was defined through 2014 from study visit ECGs, hospital discharge diagnoses including those ascertained from inpatient Medicare claims, and diagnoses from outpatient or physician service claims from Medicare data. AF occurring only during a hospitalization for valve surgery or coronary artery bypass grafting was not considered an AF event.
Statistical analysis
Due to the timing of echocardiographic assessments, the baseline for analyses was 1989–1990 for the first (original) cohort and 1994–1995 (2 years after study baseline) for the second cohort. Baseline data were summarized separately for CHS participants with and without speckle-tracking echocardiography data who otherwise met inclusion criteria for analysis. Baseline characteristics were reported as mean and standard deviation (SD) for continuous variables and number and percent for categorical variables. Strain and tissue velocity measurements were converted to absolute values, with lower absolute values representing worse strain and tissue velocities.
After verification of the proportional hazards assumption, Cox proportional hazards analysis was used to model the association between the exposure variables and incident ischemic stroke. Participants were censored at the time of hemorrhagic stroke or stroke of unknown type, last follow-up, or administrative censoring (June 31, 2015) or death if they occurred prior to ischemic stroke. We determined the functional form of exposure variables with penalized cubic splines before including these variables in the Cox model. As the associations were reasonably linear, we modeled exposure variables without transformation or the use of splines in the following main models. Model 1 was adjusted for age, race, sex, CHS site, speckle-tracking analyst, and chamber-specific image quality. Model 2 was additionally adjusted for education, body mass index, coronary heart disease, heart failure, diabetes, systolic blood pressure, anti-hypertension medication, high-density lipoprotein level, low-density lipoprotein level, triglyceride level, smoking status, and left ventricular ejection fraction. Model 3 was additionally adjusted for AF as a time-varying covariate. Model 4 was applied only to our secondary exposure variables, and, in addition to the variables in Model 2, was also adjusted for our primary exposure variable of left atrial reservoir strain as well as left atrial image quality, with the goal of exploring whether any relationships between left ventricular mechanics and stroke were mediated by left atrial mechanics.
We performed several sensitivity analyses. First, we included only participants in the first cohort who underwent echocardiography at the same time as the study baseline. Second, we included participants with prevalent AF and instead adjusted for this baseline covariate. Third, we excluded participants with prevalent coronary heart disease or heart failure. Fourth, we additionally adjusted for left atrial volume, which was measured on 2-dimensional echocardiogram images by tracing the left atrium at ventricular end-systole in the apical 4-chamber view, with the method of discs used to calculate left atrial volume. Fifth, in an analysis of our primary exposure of left atrial reservoir strain, we additionally adjusted for our secondary exposure variables, with the goal of exploring whether the relationship between left atrial mechanics and stroke was mediated by left ventricular mechanics.
We also performed post-hoc exploratory analyses of associations of our exposure variables with the specific ischemic stroke subtypes of cardioembolic stroke and AF-related cardioembolic stroke. Cardioembolic stroke was adjudicated by the CHS adjudication committee using standard definitions29. We defined AF-related cardioembolic stroke as the occurrence of a cardioembolic stroke in a participant who also had a diagnosis of incident AF before or at the same time as the cardioembolic stroke. All statistical tests were 2-tailed and the threshold of statistical significance was set at alpha = 0.05. Statistical analyses were performed using Stata 12.1 (StataCorp, College Station, TX, USA).
Results
Of the 5,888 participants in the overall CHS cohort, 275 were excluded because of prevalent stroke at baseline, 1,304 because of missing speckle-tracking echocardiogram data, 127 because of prevalent AF at baseline, and 182 because of other missing covariates, resulting in 4,000 participants who were eligible for our analysis (Online Figure I). Of the 1,304 participants with missing speckle-tracking data, 1,029 otherwise met our inclusion criteria. These 1,029 otherwise-eligible participants who were excluded because of missing speckle-tracking data had broadly similar baseline characteristics to the 4,000 included participants except for race and location of recruitment (Table 1); these differences were due to the relative availability of echocardiogram data from the first versus second CHS cohorts. Among the 4,000 included participants, the mean left atrial reservoir strain was in the normal range30 (40.8% ± 15.4%; n = 3,892) and the mean left ventricular longitudinal strain was in the borderline range (16.7% ± 4.3%; n = 3,987) (Table 1)31,32.
During a median 12.9 years of follow-up, 651 ischemic strokes occurred among the 4,000 CHS participants included in this analysis (16.3%). After adjustment for demographics and echocardiogram image quality, worse left atrial reservoir strain was associated with incident ischemic stroke (hazard ratio [HR] per standard deviation [SD] absolute decrease, 1.23; 95% confidence interval [CI] 1.13–1.34) (Table 2). This association was attenuated but persisted after further adjustment for left ventricular ejection fraction and vascular risk factors including a time-varying covariate for incident AF (HR per SD absolute decrease, 1.14; 95% CI 1.04–1.25). All secondary exposure variables were also significantly associated with incident ischemic stroke (Table 2). The associations between measures of left ventricular mechanics and stroke persisted after additional adjustment for left atrial reservoir strain (Table 2, Model 4); similarly, the association between left atrial reservoir strain and stroke persisted after adjustment for measures of left ventricular mechanics (Table 3, Sensitivity Analysis 5). The associations between measures of myocardial mechanics and ischemic stroke were not substantially different in sensitivity analyses that included only participants with echocardiograms at the time of study baseline, included participants with prevalent AF, excluded participants with prevalent coronary heart disease or heart failure, or additionally adjusted for left atrial volume (Table 3). Of the 651 ischemic strokes, 238 were classified as cardioembolic. In post-hoc exploratory analyses, all exposure variables with the exception of LV lateral e’ velocity were significantly associated with incident cardioembolic stroke (Table 4). In an analysis of 167 AF-related cardioembolic strokes, significant associations were present with left atrial reservoir strain and several secondary exposure variables (Table 4).
Discussion
In a community-based, longitudinal cohort study, we found that echocardiographic evidence of myocardial mechanical dysfunction was associated with subsequent ischemic stroke. Associations between myocardial strain indices and stroke were not substantially attenuated after adjustment for vascular risk factors, left ventricular ejection fraction, left atrial volume, and clinically apparent AF. These associations persisted after excluding participants with clinically apparent coronary heart disease or heart failure. Left atrial mechanics and left ventricular mechanics remained associated with ischemic stroke independently of each other.
Previous studies have examined associations between echocardiographic measures of myocardial strain and incident AF14,15,16, markers of left atrial thromboembolism18,19, and stroke in patients with AF20,21 or acute MI33. Several studies have examined the association between myocardial strain and a composite of major adverse cardiovascular endpoints, but these involved single-center cohorts34,35 or lacked power to assess ischemic stroke specifically36,37. Single-center case–control studies found worse left atrial reservoir strain in patients with cardioembolic38 or cryptogenic stroke compared to controls39,40. An analysis of data from a prospective, longitudinal cohort study found a non-significant association between left atrial global longitudinal strain on cardiac magnetic resonance imaging and cerebrovascular events41. In this context, our study provides novel findings that early myocardial mechanical dysfunction, including in the absence of clinically overt coronary heart disease or heart failure, is associated with subsequent ischemic stroke in the general population.
Our finding of an association between early left ventricular dysfunction and ischemic stroke, independent of left atrial dysfunction and of similar magnitude with both cardioembolic and non-cardioembolic stroke subtypes, suggests that measurement of cardiac mechanics may allow detection of early end-organ damage from systemic microvascular and microvascular disease affecting both the heart and brain. Furthermore, our findings may have implications for the evolving understanding of the substrate necessary for cardiac embolism. Common cardiac conditions that have long been established as cardioembolic sources, such as clinically apparent AF and clinically apparent MI, may be phenotypically broader than previously recognized. Isolated episodes of subclinical AF42 and markers of left atrial remodeling43,44 have been associated with a higher risk of ischemic stroke independent of clinically apparent AF. Beyond the acute period of stroke risk traditionally associated with clinically apparent MI, chronic myocardial scar from temporally remote or silent MI may also be a risk factor for cardiac embolism45,46. Similarly, while gross structural abnormalities such as chamber dilatation47,48 and frank cardiac mechanical dysfunction, as manifested by reduced ejection fraction41,49 and emptying velocity50, have long been recognized as sources of thromboembolism, our results raise the possibility that cardiac embolism might occur even earlier along the spectrum of myocardial dysfunction. Such a possibility is supported by the common occurrence of strokes that lack a cardioembolic source per traditional criteria but nevertheless appear to have arisen from a central embolic source9. At a minimum, our findings imply that the currently recognized substrate for cardiac embolism is heralded by more subtle evidence of cardiac dysfunction. Although a single echocardiographic assessment is unlikely to provide robust risk prediction for outcomes many years later, our findings suggest opportunities to test whether serial cardiac assessments, in the form of heart-rhythm monitoring, biomarker assays, echocardiography, and other diagnostic modalities, can identify cardioembolic risk earlier than current practice, in which many ischemic stroke patients present with previously undiagnosed cardioembolic sources such as AF51 that may not have resulted in stroke if recognized and treated earlier. Our findings also imply opportunities to test whether stroke risk can be reduced by early recognition of myocardial dysfunction and institution of intensive upstream therapies to arrest or reverse its progression52,53,54, as well as early use of tailored antithrombotic therapy in high-risk patients in sinus rhythm55, in addition to the current approach of managing downstream complications such as AF with anticoagulation.
Our study has several limitations. First, participants did not undergo continuous heart-rhythm monitoring or systematic serial echocardiographic assessments throughout follow-up. Therefore, we could not examine the degree to which associations between indices of cardiac mechanics and stroke were mediated by interim development of subclinical AF or grossly abnormal systolic function. Second, participants with available speckle-tracking echocardiogram data differed from the overall CHS cohort, especially in regard to race. There were fewer African-American participants in the study population analyzed than in the overall CHS cohort, and future studies should strive to include more African-American individuals and other understudied populations in studies of myocardial strain and stroke risk. Third, myocardial strain was measured from digitized versions of analog echocardiogram images. This method substantially differs from contemporary clinical and research protocols involving direct digital acquisition and strain measurement, most notably in regards to a slower frame rate than currently used. On the other hand, our approach enabled more prolonged longitudinal follow-up for outcomes than would be possible with more contemporary cardiac imaging protocols. In population-based studies, our methodology has been useful in showing associations of cardiac strain measures with disease phenotypes and prediction of outcomes, supporting the validity of our methods to identify important relationships between myocardial function and disease that can be expected to apply to contemporary patients13,56. Moreover, any imprecision of our methods would have decreased our ability to find meaningful associations, suggesting that our findings may actually understate the association of cardiac strain measures with incident ischemic stroke. Fourth, given the long follow-up period, there was a substantial rate of mortality in the study population. This may affect calculations of cumulative rates of stroke, although it should not affect our estimates of cause-specific HRs57.
Based on our findings, it appears that subtle cardiac dysfunction that can ultimately lead to stroke may be identifiable at an early stage. These findings highlight an opportunity to identify cardioembolic risk earlier and institute preventive measures including upstream therapeutic strategies directed at preventing further progression of thrombogenic cardiac dysfunction. Such a strategy may be a promising avenue for reducing the burden of cardioembolic stroke, a severe and increasingly common form of a disease that is the second-leading cause of death1 worldwide.
Data availability
The data used for this study is available upon reasonable request to the corresponding author.
References
Feigin, V. L. et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 383, 245–254 (2014).
Bogiatzi, C., Hackam, D. G., McLeod, A. I. & Spence, J. D. Secular trends in ischemic stroke subtypes and stroke risk factors. Stroke 45, 3208–3213. https://doi.org/10.1161/STROKEAHA.114.006536 (2014).
Lin, H. J. et al. Stroke severity in atrial fibrillation. The Framingham study. Stroke 27, 1760–1764 (1996).
Kamel, H. & Healey, J. S. Cardioembolic stroke. Circ. Res. 120, 514–526. https://doi.org/10.1161/CIRCRESAHA.116.308407 (2017).
Goldberger, J. J. et al. Evaluating the atrial myopathy underlying atrial fibrillation: Identifying the arrhythmogenic and thrombogenic substrate. Circulation 132, 278–291. https://doi.org/10.1161/CIRCULATIONAHA.115.016795 (2015).
Kamel, H., Okin, P. M., Elkind, M. S. & Iadecola, C. Atrial fibrillation and mechanisms of stroke: Time for a new model. Stroke J. Cerebral Circul. 47, 895–900. https://doi.org/10.1161/STROKEAHA.115.012004 (2016).
Brambatti, M. et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 129, 2094–2099. https://doi.org/10.1161/CIRCULATIONAHA.113.007825 (2014).
Freedman, B. et al. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat Rev Cardiol 14, 701–714. https://doi.org/10.1038/nrcardio.2017.94 (2017).
Hart, R. G., Catanese, L., Perera, K. S., Ntaios, G. & Connolly, S. J. Embolic stroke of undetermined source: A systematic review and clinical update. Stroke 48, 867–872. https://doi.org/10.1161/STROKEAHA.116.016414 (2017).
Hart, R. G. et al. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 13, 429–438. https://doi.org/10.1016/S1474-4422(13)70310-7 (2014).
Edvardsen, T. et al. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: Validation against three-dimensional tagged magnetic resonance imaging. Circulation 106, 50–56 (2002).
Amundsen, B. H. et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance imaging. J. Am. Coll. Cardiol. 47, 789–793. https://doi.org/10.1016/j.jacc.2005.10.040 (2006).
Aguilar, F. G. et al. Archeological echocardiography: Digitization and speckle tracking analysis of archival echocardiograms in the HyperGEN study. Echocardiography 33, 386–397. https://doi.org/10.1111/echo.13095 (2016).
Russo, C. et al. Left ventricular systolic dysfunction by longitudinal strain is an independent predictor of incident atrial fibrillation: A community-based cohort study. Circ. Cardiovasc. Imaging 8, e003520. https://doi.org/10.1161/CIRCIMAGING.115.003520 (2015).
Tsai, W. C. et al. Association of left atrial strain and strain rate assessed by speckle tracking echocardiography with paroxysmal atrial fibrillation. Echocardiography 26, 1188–1194. https://doi.org/10.1111/j.1540-8175.2009.00954.x (2009).
Kim, D. et al. Incremental value of left atrial global longitudinal strain for prediction of post stroke atrial fibrillation in patients with acute ischemic stroke. J. Cardiovasc. Ultrasound 24, 20–27. https://doi.org/10.4250/jcu.2016.24.1.20 (2016).
Pagola, J. et al. Left atria strain is a surrogate marker for detection of atrial fibrillation in cryptogenic strokes. Stroke J. Cerebral Circul. 45, e164-166. https://doi.org/10.1161/strokeaha.114.005540 (2014).
Kim, D. et al. Clinical implications and determinants of left atrial mechanical dysfunction in patients with stroke. Stroke 47, 1444–1451. https://doi.org/10.1161/STROKEAHA.115.011656 (2016).
Karabay, C. Y. et al. Left atrial deformation parameters predict left atrial appendage function and thrombus in patients in sinus rhythm with suspected cardioembolic stroke: A speckle tracking and transesophageal echocardiography study. Echocardiography 30, 572–581. https://doi.org/10.1111/echo.12089 (2013).
Shih, J. Y. et al. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J. Am. Soc. Echocardiogr. 24, 513–519. https://doi.org/10.1016/j.echo.2011.01.016 (2011).
Azemi, T., Rabdiya, V. M., Ayirala, S. R., McCullough, L. D. & Silverman, D. I. Left atrial strain is reduced in patients with atrial fibrillation, stroke or TIA, and low risk CHADS(2) scores. J. Am. Soc. Echocardiogr. 25, 1327–1332. https://doi.org/10.1016/j.echo.2012.09.004 (2012).
Fried, L. P. et al. The cardiovascular health study: Design and rationale. Ann. Epidemiol. 1, 263–276 (1991).
Manolio, T. A., Gottdiener, J. S., Tsang, T. S., Gardin, J. M. & Cardiovascular Health Study Collaborative Research, G. Left atrial dimensions determined by M-mode echocardiography in black and white older (> or =65 years) adults (The Cardiovascular Health Study). Am. J. Cardiol. 90, 983–987 (2002).
Badano, L. P. et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 19, 591–600. https://doi.org/10.1093/ehjci/jey042 (2018).
Packer, M. HFpEF is the substrate for stroke in obesity and diabetes independent of atrial fibrillation. JACC Heart Failure 8, 35–42. https://doi.org/10.1016/j.jchf.2019.09.002 (2020).
Patel, R. B. & Shah, S. J. Therapeutic targeting of left atrial myopathy in atrial fibrillation and heart failure with preserved ejection fraction. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.0136 (2020).
Smietana, J., Plitt, A. & Halperin, J. L. Thromboembolism in the absence of atrial fibrillation. Am. J. Cardiol. 124, 303–311. https://doi.org/10.1016/j.amjcard.2019.04.027 (2019).
Sajeev, J. K., Kalman, J. M., Dewey, H., Cooke, J. C. & Teh, A. W. The atrium and embolic stroke: Myopathy not atrial fibrillation as the requisite determinant?. JACC Clin. Electrophysiol. 6, 251–261. https://doi.org/10.1016/j.jacep.2019.12.013 (2020).
Longstreth, W. T. Jr. et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology 56, 368–375 (2001).
Pathan, F., D’Elia, N., Nolan, M. T., Marwick, T. H. & Negishi, K. Normal ranges of left atrial strain by speckle-tracking echocardiography: A systematic review and meta-analysis. J. Am. Soc. Echocardiogr. 30, 59-70 e58. https://doi.org/10.1016/j.echo.2016.09.007 (2017).
Marwick, T. H., Shah, S. J. & Thomas, J. D. Myocardial strain in the assessment of patients with heart failure: A review. JAMA Cardiol. 4, 287–294. https://doi.org/10.1001/jamacardio.2019.0052 (2019).
Yingchoncharoen, T., Agarwal, S., Popovic, Z. B. & Marwick, T. H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. 26, 185–191. https://doi.org/10.1016/j.echo.2012.10.008 (2013).
Ersbøll, M. et al. Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: A two-dimensional speckle-tracking study. Eur. Heart J. 35, 648–656. https://doi.org/10.1093/eurheartj/eht179 (2014).
Sulemane, S. et al. Subclinical markers of cardiovascular disease predict adverse outcomes in chronic kidney disease patients with normal left ventricular ejection fraction. Int. J. Cardiovasc. Imaging 33, 687–698. https://doi.org/10.1007/s10554-016-1059-x (2017).
Minamisawa, M. et al. Prognostic impact of diastolic wall strain in patients at risk for heart failure. Int. Heart J. 58, 250–256. https://doi.org/10.1536/ihj.16-315 (2017).
Russo, C. et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur. J. Heart Fail. 16, 1301–1309. https://doi.org/10.1002/ejhf.154 (2014).
Blomstrand, P. et al. Left ventricular diastolic function, assessed by echocardiography and tissue Doppler imaging, is a strong predictor of cardiovascular events, superior to global left ventricular longitudinal strain, in patients with type 2 diabetes. Eur. Heart J. Cardiovasc. Imaging 16, 1000–1007. https://doi.org/10.1093/ehjci/jev027 (2015).
Johansen, M. C., Doria de Vasconcellos, H., Nazarian, S., Lima, J. A. C. & Gottesman, R. F. The investigation of left atrial structure and stroke etiology: The I-LASER study. J. Am. Heart Assoc. 10, e018766. https://doi.org/10.1161/JAHA.120.018766 (2021).
Leong, D. P. et al. Left atrial dysfunction in the pathogenesis of cryptogenic stroke: novel insights from speckle-tracking echocardiography. J. Am. Soc. Echocardiogr. 30, 71-79.e71. https://doi.org/10.1016/j.echo.2016.09.013 (2017).
Sanchis, L. et al. Left atrial function is impaired in some patients with stroke of undetermined etiology: potential implications for evaluation and therapy. Revista Espanola de Cardiologia (English ed.) 69, 650–656. https://doi.org/10.1016/j.rec.2015.11.033 (2016).
Habibi, M. et al. Left atrial mechanical function and incident ischemic cerebrovascular events independent of AF: Insights from the MESA study. JACC Cardiovasc. Imaging 12, 2417–2427. https://doi.org/10.1016/j.jcmg.2019.02.021 (2019).
Healey, J. S. et al. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 366, 120–129. https://doi.org/10.1056/NEJMoa1105575 (2012).
He, J. et al. P-wave indices and risk of ischemic stroke: A systematic review and meta-analysis. Stroke J Cerebral Circul 48, 2066–2072. https://doi.org/10.1161/strokeaha.117.017293 (2017).
Daccarett, M. et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J. Am. Coll. Cardiol. 57, 831–838. https://doi.org/10.1016/j.jacc.2010.09.049 (2011).
Merkler, A. E. et al. Association between unrecognized myocardial infarction and cerebral infarction on magnetic resonance imaging. JAMA Neurol. 76, 956–961. https://doi.org/10.1001/jamaneurol.2019.1226 (2019).
Merkler, A. E. et al. Duration of heightened ischemic stroke risk after acute myocardial infarction. J. Am. Heart Assoc. 7, e010782. https://doi.org/10.1161/jaha.118.010782 (2018).
SPAF: Stroke Prevention in Atrial Fibrillation Investigators. Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann. Intern. Med. 116, 6–12 (1992).
Benjamin, E. J., D’Agostino, R. B., Belanger, A. J., Wolf, P. A. & Levy, D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation 92, 835–841 (1995).
Witt, B. J. et al. Ischemic stroke after heart failure: A community-based study. Am. Heart J. 152, 102–109. https://doi.org/10.1016/j.ahj.2005.10.018 (2006).
Goldman, M. E. et al. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study). J. Am. Soc. Echocardiogr. 12, 1080–1087. https://doi.org/10.1016/s0894-7317(99)70105-7 (1999).
Sposato, L. A. et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 14, 377–387. https://doi.org/10.1016/s1474-4422(15)70027-x (2015).
Rienstra, M. et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: Results of the RACE 3 trial. Eur. Heart J. 39, 2987–2996. https://doi.org/10.1093/eurheartj/ehx739 (2018).
Pathak, R. K. et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: The ARREST-AF cohort study. J. Am. Coll. Cardiol. 64, 2222–2231. https://doi.org/10.1016/j.jacc.2014.09.028 (2014).
Edelmann, F. et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J. Am. Coll. Cardiol. 58, 1780–1791. https://doi.org/10.1016/j.jacc.2011.06.054 (2011).
Kamel, H. et al. The AtRial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: Rationale and methods. Int. J. stroke 14, 207–214. https://doi.org/10.1177/1747493018799981 (2019).
Patel, R. B. et al. Characterization of cardiac mechanics and incident atrial fibrillation in participants of the Cardiovascular Health Study. JCI Insight https://doi.org/10.1172/jci.insight.141656 (2020).
Austin, P. C., Lee, D. S. & Fine, J. P. Introduction to the analysis of survival data in the presence of competing risks. Circulation 133, 601–609. https://doi.org/10.1161/CIRCULATIONAHA.115.017719 (2016).
Acknowledgements
None.
Funding
This study was supported by a research Grant from the National Institutes of Health (R01HL107577 to S.J.S. and R01NS097443 to H.K.). Dr. Shah is additionally supported by NIH Grants R01HL127028, R01HL140731, and R01HL149423. Dr. Kim is supported by NIH Grant K23HL140092. This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, N01HC35129, 75N92021D00006, and Grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Dr. H.K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: All authors. Acquisition of data: W.T.L., J.G., J.R.K., J.M.G., S.S. Analysis and interpretation of data: All authors. Drafting of the manuscript: H.K. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: T.M.B. Administrative, technical, or material support: W.T.L., S.S. Study supervision: S.S.
Corresponding author
Ethics declarations
Competing interests
Drs. Elkind, Kamel, and Longstreth serve as PIs for the NIH-funded ARCADIA trial which receives in-kind study drug from the BMS-Pfizer Alliance and ancillary study support from Roche Diagnostics. Dr. Kamel serves as a steering committee member of Medtronic’s Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition. Dr. Elkind receives royalties from UpToDate for chapters related to cryptogenic stroke and other topics. Dr. Shah has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapeutics, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardiora, CVRx, Cytokinetics, Edwards, Eisai, GSK, Ionis, Ironwood, Imara, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Regeneron, Sanofi, Shifamed, Tenax, and United Therapeutics. The other authors report no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamel, H., Bartz, T.M., Longstreth, W.T. et al. Cardiac mechanics and incident ischemic stroke: the Cardiovascular Health Study. Sci Rep 11, 17358 (2021). https://doi.org/10.1038/s41598-021-96702-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96702-z
This article is cited by
-
Atrial cardiomyopathy and incident ischemic stroke risk: a systematic review and meta-analysis
Journal of Neurology (2023)
-
Utility of speckle-tracking echocardiography for predicting atrial fibrillation following ischemic stroke: a systematic review and meta-analysis
The International Journal of Cardiovascular Imaging (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.