Abstract
Stress responses are highly plastic and vary across physiological states. The female estrous cycle is associated with a number of physiological changes including changes in stress responses, however, the mechanisms driving these changes are poorly understood. Corticotropin-releasing hormone (CRH) neurons are the primary neural population controlling the hypothalamic–pituitary–adrenal (HPA) axis and stress-evoked corticosterone secretion. Here we show that CRH neuron intrinsic excitability is regulated over the estrous cycle with a peak in proestrus and a nadir in estrus. Fast inactivating voltage-gated potassium channel (IA) currents showed the opposite relationship, with current density being lowest in proestrus compared to other cycle stages. Blocking IA currents equalized excitability across cycle stages revealing a role for IA in mediating plasticity in stress circuit function over the female estrous cycle.
Similar content being viewed by others
Introduction
The female estrous cycle is associated with multiple changes in physiology and behaviour, including marked changes in stress responses1,2. The hypothalamic–pituitary–adrenal (HPA) axis is most active during the proestrus stage of the estrous cycle. Basal circulating levels of corticosterone (CORT) are highest on proestrus3,4,5,6, as are stress-evoked secretion of both CORT and ACTH7. There are also notable changes in stress and anxiety associated behaviours over the estrous cycle1,2,8,9,10. The mechanisms which drive these estrus cycle dependent changes in stress responses are currently unclear.
Hypothalamic corticotropin-releasing hormone (CRH) neurons control both the HPA axis and behavioral responses to stress11,12,13,14,15,16,17. CRH neuron excitability and stress responses are highly plastic, thus allowing organisms to mount appropriate stress responses in different behavioral or physiological states. Adaptation of CRH neuron responses is thought to be mediated by synaptic plasticity18, however, recent evidence suggests that plasticity of intrinsic excitability also plays an important role19,20,21. Plasticity of intrinsic excitability is mediated by changing levels of expression or function of ion channels in the cell membrane22,23,24. In CRH neurons, plasticity of intrinsic excitability can be mediated via voltage-gated potassium channels21. In other neurons, expression of voltage-gated potassium channels has also been shown to be regulated over the estrous cycle25,26. Hence, we speculated that changes to potassium channels influences intrinsic excitability of CRH neurons over the estrous cycle.
Results
CRH neuron intrinsic excitability changes over the estrous cycle
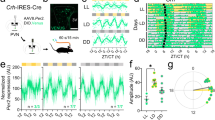
To assay CRH neuron intrinsic excitability, neurons were held around − 60 mV in current clamp before injecting a family of current steps from 0 pA to + 50 pA in 5 pA increments (Fig. 1C). This protocol was performed on CRH neurons from female animals in proestrus, estrus or diestrus stages of the estrous cycle. A Two-way RM ANOVA revealed that there was a significant effect of estrous cycle stage on CRH neuron spiking responses (F(2,86) = 4.387, P = 0.0153, Fig. 1C, Table 1), a significant effect of current step (F(10,860) = 470, P < 0.0001, supplementary Table 1) and a significant interaction (F(20,860) = 3.158, P < 0.0001, supplementary Table 1). Post hoc tests showed that at multiple current steps excitability in proestrus was significantly higher than both estrus (P < 0.05) and diestrus (P < 0.05, Fig. 1B). Peak firing frequency (frequency at the 50 pA step) of CRH neurons from proestrus animals was significantly higher than estrus and diestrus (F(2,86) = 6.62. P = 0.002, Fig. 1C insert). Additionally, the slopes of the F/I curves for each group were significantly different (F(2,79) = 5.49, P = 0.006) (see Table 1 for values and Supplementary Table 1 for full statistics), with the highest gain in proestrus compared to estrus (P = 0.0044). The slope of the F/I curve was not significantly different between proestrus and diestrus (P = 0.1493). The total number of action potentials (APs) fired over all current steps was also significantly different (one-way ANOVA, F(2,83 ) = 5.35, P = 0.006; Table 1) with post hoc tests showing higher numbers of spikes in proestrus compared to the estrus group (P = 0.005). Interestingly, analysis of the AP parameters showed no differences between the groups. AP amplitude, rise time, half width and decay time were not significantly different (Table 1). There were also no significant differences in capacitance or input resistance between groups (one-way ANOVA, F(2,104), P = 0.59 and F(2,101) = 1.05, P = 0.35 respectively, Table 1). We next measured first spike latency (FSL) to determine if this was different across the estrous cycle. A Two-way RM ANOVA showed that there was a significant effect of estrous cycle stage on FSL (F(2,47) = 3.57, P = 0.036), a significant effect of current step (F(8,376) = 143.2, P < 0.0001) and a significant interaction (F(16,376) = 2.16, P = 0.006). Post hoc tests revealed that CRH neurons from proestrus animals exhibited a significantly shorter latency to fire an action potential (AP) at the 10 and 15 pA current steps compared to estrus (P < 0.05) and diestrus groups (P < 0.05, Fig. 1D).
CRH neuron intrinsic excitability varies across the female estrous cycle. (A) Image of left hemisphere PVN showing expression of tdTomato (red) in CRH neurons. White cells are tdTomato positive CRH neurons that have been filled with neurobiotin via the whole cell recording pipette. (B) Representative responses of CRH neurons to 0 pA, 10 pA, 30 pA and 50 pA current steps. Proestrus in red, estrus in blue and diestrus in purple. (C) Summary data for the F/I curve. Cells from proestrus animals (n = 29 cells) show a significantly higher firing frequency compared to those from estrus (n = 38) and diestrus (n = 22). Symbols denote significance determined by Tukey’s post hoc multiple comparisons test (Two-way ANOVA results in Supplementary Table 1), blue: proestrus versus estrus, purple: proestrus versus diestrus. Insert shows total number of APs fired over all current steps for each group. (D) Graph of first spike latency (FSL) for each current step. Cells from proestrus animals had a shorter FSL at 10 and 15 pA steps. Stars are results of post hoc multiple comparisons, as in B. N = 9, 15 and 10 mice for diestrus, estrus, and proestrus groups respectively. P values: * ≤ 0.05, ** ≤ 0.01, *** ≤ 0.001, **** ≤ 0.0001.
Taken together these data show that CRH neuron excitability fluctuates over the estrous cycle with higher levels of excitability during proestrus compared to estrus and diestrus stages. These findings align well with previous reports showing higher levels of HPA axis activity in proestrus7,27.
Changes in IA potassium current across the estrous cycle
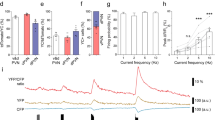
Changes in intrinsic excitability are commonly due to changes in voltage-gated ion channel density or function. IA currents contribute to FSL in CRH neurons21 and expression of potassium channel subunits which contribute to IA current change over the estrous cycle in other neurons25. As FSL was found to vary across the estrous cycle we subsequently used a voltage clamp protocol (see “Methods”) to determine if IA currents exhibited similar changes. A Two-way RM ANOVA revealed that there was a significant effect of estrous cycle on IA current density (F(2,21) = 9.59, P = 0.001; Fig. 2A,B), a significant effect of voltage step (F(14,294) = 239.8, P < 0.0001) and a significant interaction (F(28,295) = 10.5, P < 0.0001). Post hoc tests showed that current densities at multiple voltage steps were lowest in proestrus animals compared to estrus (P < 0.05) and diestrus animals (P < 0.05). Peak amplitude of the current at the maximum voltage step (+ 30 mV) was also significantly different between groups (One-way ANOVA, F(2, 23) = 10.93, P = 0.0005, Fig. 2C). There was no significant difference in the decay tau of the IA currents between the groups (Tau measured at + 30 mV step, One-way ANOVA, F(2,30) = 0.51, P = 0.603, data not shown). Voltage dependence of inactivation and time course of recovery from inactivation were also not significantly different between the groups (Two-way ANOVA, F(2,8) = 1.92, P = 0.201, and F(2,7) = 0.44, P = 0.66, respectively; data not shown).
IA currents vary across the estrous cycle influencing FSL. (A) Example IA currents evoked from individual cells during each cycle stage; proestrus (red), estrus (blue) and diestrus (purple). (B) IA current densities plotted for each 10 mV voltage step. Cells from proestrus (n = 8) animals had significantly smaller IA currents compared to those from estrus (n = 9) and diestrus (n = 7) animals. (C) Peak amplitude IA currents (not normalized to capacitance) evoked by a + 30 mV step. Peak IA currents during proestrus were significantly smaller than during other cycle stages. (D) Example traces showing CRH neuron spiking response to a one second current ramp protocol (bottom right). Grey traces are example traces from each group in the presence of 4-AP. (E) Bar graph of first spike latency, measured from the onset of the ramp to AP threshold, for each group under control conditions and in the presence of 4-AP (grey bars). 4-AP had a significant effect on FSL in cells from estrus and diestrus animals but did not affect FSL in proestrus animals. Results of one and two-way ANOVAs reported in Supplementary Table 1. Star symbols denote significance by Tukey’s multiple comparisons test, purple: diestrus versus proestrus, blue: estrus versus proestrus. There were no significant differences between estrus and diestrus groups. N = 5–8 mice for all groups. P values: * ≤ 0.05, ** ≤ 0.01, *** ≤ 0.001, **** ≤ 0.0001.
Role of IA in estrous cycle changes in CRH neuron excitability
To determine if changes in IA currents contributed to the differences in intrinsic excitability observed, we measured FSL with a ramp protocol (0 to 40 pA ramp over one second, Fig. 2D). We then applied 2 mM 4-AP to inhibit IA currents and repeated the ramp protocol. Two-way ANOVA revealed a significant effect of 4-AP on FSL (F(1,19) = 19.2, P = 0.0003, Fig. 2E), no significant effect of estrous cycle stage (F(2,19) = 0.7432, P = 0.4889), but a significant interaction between estrous cycle stage and 4-AP (F(2,19) = 6.06, P = 0.009). While FSL was significantly shorter in the proestrus group compared to the estrus group in control (P = 0.039), there was no significant difference between the groups in the presence of 4-AP. Consistent with this, 4-AP significantly reduced FSL in the estrus (P < 0.0001) and diestrus (P = 0.03) groups, but not in the proestrus group (P = 0.999, Fig. 2D,E).
Discussion
There are marked changes in activity of the HPA axis across the female reproductive cycle. Basal circulating levels of CORT are highest on proestrus3,4,5,6, as are stress evoked secretion of both CORT and ACTH7. Here we show that CRH neuron intrinsic excitability is also regulated across the estrous cycle with a similar pattern. Specifically, CRH neuron spiking excitability and gain were found to be highest in proestrus and lowest in estrous. FSL and IA current density were lowest in proestrus. Differences in FSL across estrous cycle stages were abolished when IA currents were inhibited with 4-AP.
Expression of voltage-gated potassium channel subunits have previously been shown to vary over the estrous cycle in GnRH neurons25. In these same neurons, estradiol can also regulate voltage-gated potassium channel function28. It is tempting to suggest that the increases in CRH neuron excitability at proestrus are also mediated directly by estradiol since estradiol levels peak at this stage of the estrous cycle29. However, several studies have shown that when delivered in vivo to ovariectomized rats, estradiol suppresses stress evoked CRH neuron activity assessed by cfos30,31,32. Exogenous estradiol can however, enhance corticosterone secretion via direct actions on the adrenal gland30,33,34. Therefore, it is worth considering that when estradiol levels are artificially elevated in ovariectomized animals, changes in CRH neuron excitability may also be influenced by changing levels of corticosterone negative feedback. It is also important to note that CRH neurons do not appear to possess estrogen receptor (ER) alpha35 and have little ERbeta36. This has led to the idea that any estradiol actions on CRH neurons may either be mediated by membrane associated estrogen receptors37 or be mediated indirectly through the regulation of afferent neural circuits31,38,39. Since progesterone levels also vary over the estrous cycle, it too may play a role in regulating CRH neuron excitability. While the role of estradiol and progesterone could be addressed by giving steroid implants in ovariectomized animals, these experiments do not mimic the natural fluctuations in these hormones and may therefore lead to erroneous conclusions as to their normal effects on CRH neuron function.
Overall, our findings demonstrate plasticity of CRH neuron intrinsic excitability over the course of the natural estrous cycle which may in turn underlie changes in HPA axis output. In addition to controlling adrenal corticosteroid secretion, CRH neurons also play an important role in regulating stress associated behaviours14,15,17,40. Hence, changes in CRH neuron excitability may also contribute to estrous cycle changes in behavioural stress responses.
Methods
Animals
All experiments were carried out in adult female (2–6 months old) Crh-IRES-Cre;Ai14 (tdTomato) mice. These mice faithfully label CRH neurons in the PVN (Fig. 1A)41,42,43. Animals had a 12 h light/dark cycle (7 a.m.–7 p.m. lights on) with food and water available ad libitum. A small amount of male bedding was put in the cages each week to promote normal estrous cycling. To establish estrous cycle stage, animals were vaginally smeared post mortem44. Animals in metestrus and diestrus were combined into one group and referred to as diestrus. All protocols and procedures were approved by the University of Otago Animal Ethics Committee and carried out in accordance with the New Zealand Animal Welfare Act. The study was carried out in compliance with the ARRIVE guidelines.
Slice preparation
Mice were killed by cervical dislocation between 9 and 11 a.m., their brain quickly removed and placed in ice-cold oxygenated (95% O2, 5% CO2) slicing solution containing (in mM); 87 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 6 MgCl2, 25 d-Glucose, 75 sucrose, pH 7.2–7.4. A vibratome (VT1200S, Lecia Microsystems) was used to cut 200 µm-thick coronal slices of the PVN, which were then incubated in oxygenated artificial cerebrospinal fluid (aCSF) containing in (mM); 126 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.5 MgCl2, 10 d-Glucose at 30 °C for at least 1 h before recording. For recording, slices were transferred to a recording chamber and continuously perfused with 30 °C aCSF at 1.5 ml min−1. CRH neurons within the PVN were visualized using a 40× objective and epifluorescence to excite tdTomato.
Whole-cell electrophysiology recordings
Electrophysiological recordings were collected with a Multiclamp 700B amplifier (Molecular Devices), filtered at 2 kHz, and digitized using the Digidata 1440a (Molecular Devices). Data were analysed with Clampfit 10.7 (Molecular Devices).
For whole-cell recordings, borosilicate glass pipettes (tip resistance: 2–5 MΩ) were filled with an internal solution containing (in mM): 120 K‐gluconate, 15 KCl, 0.5 Na2EGTA, 2 Mg2ATP, 0.4 Na2GTP, 10 HEPES, 5 Na2‐phosphocreatine and 0.25% Neurobiotin (adjusted to pH 7.2 with KOH; adjusted to ≈ 290 mOsm with sucrose). All current clamp experiments were performed in the presence of 10 μM cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione) (CNQX) and picrotoxin (50 μM). Each cell was held at approximately − 60 mV. The liquid junction potential was calculated to be approximately − 14.1 mV and was not compensated for. Cells were not recorded from if input resistance was below 0.7 GΩ or access resistance was above 30 MΩ and both input and access resistance were monitored throughout to ensure stable recording. We used a current step protocol to determine spike output and first spike latency (FSL) for Fig. 1. The step protocol consisted of 300 ms square steps from 0 to + 50 pA in 5 pA increments. Spikes were detected using a threshold search in Clampfit and were analysed for rise time, decay time, amplitude and half width. FSL was calculated from the time of the depolarizing step initiation to the action potential (AP) threshold for the first spike evoked at steps equal or greater than 10 pA. AP threshold was defined as the voltage at which the AP first derivative crossed 10 mV/ms. The same analysis criteria were used to identify FSL and AP threshold for a 1 s, + 40 pA/s ramp protocol (Fig. 2). Slopes of the F-I curve for individual cells were calculated using linear regression.
For all voltage clamp recordings neurons were clamped at − 60 mV, input resistance, access resistance and capacitance were monitored periodically throughout recordings. IA current recordings were performed in the presence of CNQX (10 μM), picrotoxin (50 μM), TTX (0.5 μM), TEA (30 mM) (or XE991, 40 μM) and nifedipine (100 μM). To evoke IA currents, neurons were hyperpolarized from − 60 to − 110 mV for 500 ms before a family of depolarizing steps were delivered in 10 mV steps from − 100 to + 30 mV. Peak IA amplitude for each voltage step was measured and normalized to capacitance to give the current densities (pA/pF). Voltage dependence of inactivation and time course of recovery from inactivation protocols are the same as used in Sonner and Stern45. Four mM 4-Aminopyridine (4-AP) blocked peak IA current amplitude by 90.7 ± 3.83% (n = 3).
Immunohistochemistry and confocal microscopy
Slices containing neurobiotin filled cells were fixed with 4% paraformaldehyde in phosphate buffered solution overnight at 4 °C. Similar to the protocol reported in46, slices then underwent immunohistochemical staining using Cy5-Streptavidin secondary antibody (1:30 dilution in TBS, Invitrogen). Slices were mounted on slides, air dried and coverslipped using mounting media (Vectorshield, Vector Laboratories).
Confocal images were then acquired on a Nikon A1R confocal microscope with a 10× objective. tdTomato was excited with a 560 nm laser and Cy5 was excited with a 635 nm laser, images were acquired at 1024 × 1024 pixels.
Analysis
Statistical analysis was performed using GraphPad Prism 8. All reported values are the mean ± SEM. Comparisons between groups were carried out using either One or Two-way ANOVA where appropriate, with Tukeys or sidak’s post hoc multiple comparison tests. All n-values represent neuron number, all groups had N > 3 animals. P < 0.05 was considered statistically significant. Full ANOVA results are reported in Supplementary Table 1. P values reported on figures are for post hoc multiple comparison tests.
References
Oyola, M. G. & Handa, R. J. Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: Sex differences in regulation of stress responsivity. Stress 20, 476–494 (2017).
Ter Horst, J. P., de Kloet, E. R., Schächinger, H. & Oitzl, M. Relevance of stress and female sex hormones for emotion and cognition. Cell. Mol. Neurobiol. 32, 725–735 (2012).
Critchlow, V., Liebelt, R. A., Bar-Sela, M., Mountcastle, W. & Lipscomb, H. Sex difference in resting pituitary-adrenal function in the rat. Am. J. Physiol.-Legacy Content 205, 807–815 (1963).
Raps, D., Barthe, P. & Desaulles, P. Plasma and adrenal corticosterone levels during the different phases of the sexual cycle in normal female rats. Experientia 27, 339–340 (1971).
Buckingham, J. C., Dohler, K.-D. & Wilson, C. A. Activity of the pituitary-adrenocortical system and thyroid gland during the oestrous cycle of the rat. J. Endocrinol. 78, 359–366 (1978).
Carey, M., Deterd, C., De Koning, J., Helmerhorst, F. & De Kloet, E. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J. Endocrinol. 144, 311–321 (1995).
Viau, V. & Meaney, M. J. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology 129, 2503–2511 (1991).
Miller, C. K., Halbing, A. A., Patisaul, H. B. & Meitzen, J. Interactions of the estrous cycle, novelty, and light on female and male rat open field locomotor and anxiety-related behaviors. Physiol. Behav. 228, 113203 (2020).
van Doeselaar, L. et al. Chronic social defeat stress in female mice leads to sex-specific behavioral and neuroendocrine effects. Stress 24, 168–180 (2021).
Wiersielis, K. R. et al. Sex differences in corticotropin releasing factor-evoked behavior and activated networks. Psychoneuroendocrinology 73, 204–216 (2016).
Herman, J. P. & Cullinan, W. E. Neurocircuitry of stress: Central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 20, 78–84 (1997).
Ulrich-Lai, Y. M. & Herman, J. P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409 (2009).
Kim, J. S., Han, S. Y. & Iremonger, K. J. Stress experience and hormone feedback tune distinct components of hypothalamic CRH neuron activity. Nat. Commun. 10, 1–15 (2019).
Füzesi, T., Daviu, N., Cusulin, J. I. W., Bonin, R. P. & Bains, J. S. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat. Commun. 7, 11937 (2016).
Kim, J. et al. Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat. Neurosci. 22, 576–585 (2019).
Sterley, T.-L. et al. Social transmission and buffering of synaptic changes after stress. Nat. Neurosci. 21, 393–403 (2018).
Daviu, N. et al. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat. Neurosci. 23, 398–410 (2020).
Bains, J. S., Cusulin, J. I. W. & Inoue, W. Stress-related synaptic plasticity in the hypothalamus. Nat. Rev. Neurosci. 16, 377–388 (2015).
Kim, J. S. & Iremonger, K. J. Temporally tuned corticosteroid feedback regulation of the stress axis. Trends Endocrinol. Metab. 30, 783–792 (2019).
Matovic, S. et al. Neuronal hypertrophy dampens neuronal intrinsic excitability and stress responsiveness during chronic stress. J. Physiol. 598, 2757–2773 (2020).
Senst, L., Baimoukhametova, D., Sterley, T.-L. & Bains, J. S. Sexually dimorphic neuronal responses to social isolation. Elife 5, e18726 (2016).
Camp, A. Intrinsic neuronal excitability: A role in homeostasis and disease. Front. Neurol. 3, 50 (2012).
Debanne, D., Inglebert, Y. & Russier, M. Plasticity of intrinsic neuronal excitability. Curr. Opin. Neurobiol. 54, 73–82 (2019).
Debanne, D. & Russier, M. The contribution of ion channels in input-output plasticity. Neurobiol. Learn. Memory 166, 107095 (2019).
Arroyo, A., Kim, B. S., Biehl, A., Yeh, J. & Bett, G. C. Expression of kv4. 3 voltage-gated potassium channels in rat gonadotrophin-releasing hormone (GnRH) neurons during the estrous cycle. Reprod. Sci. 18, 136–144 (2011).
Vastagh, C., Solymosi, N., Farkas, I. & Liposits, Z. Proestrus differentially regulates expression of ion channel and calcium homeostasis genes in GnRH neurons of mice. Front. Mol. Neurosci. 12, 137 (2019).
Atkinson, H. C. & Waddell, B. J. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: Sexual dimorphism and changes across the estrous cycle. Endocrinology 138, 3842–3848 (1997).
DeFazio, R. A. & Moenter, S. M. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol. Endocrinol. 16, 2255–2265 (2002).
Nilsson, M. E. et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology 156, 2492–2502 (2015).
Figueiredo, H. F., Ulrich-Lai, Y. M., Choi, D. C. & Herman, J. P. Estrogen potentiates adrenocortical responses to stress in female rats. Am. J. Physiol.-Endocrinol. Metab. 292, E1173–E1182 (2007).
Dayas, C., Xu, Y., Buller, K. & Day, T. Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. J. Neuroendocrinol. 12, 784–794 (2000).
Gerrits, M. et al. Cyclic estradiol replacement attenuates stress-induced c-Fos expression in the PVN of ovariectomized rats. Brain Res. Bull. 67, 147–155 (2005).
Kitay, J. I. Effect of oestradiol in adrenal corticoidogenesis: An additional step in steroid biosynthesis. Nature 209, 808–809 (1966).
Lo, M. J., Chang, L. L. & Wang, P. S. Effects of estradiol on corticosterone secretion in ovariectomized rats. J. Cell. Biochem. 77, 560–568 (2000).
Shughrue, P. J., Lane, M. V. & Merchenthaler, I. Comparative distribution of estrogen receptor-α and-β mRNA in the rat central nervous system. J. Compar. Neurol. 388, 507–525 (1997).
Isgor, C., Shieh, K.-R., Akil, H. & Watson, S. J. Colocalization of estrogen β-receptor messenger RNA with orphanin FQ, vasopressin and oxytocin in the rat hypothalamic paraventricular and supraoptic nuclei. Anat. Embryol. 206, 461–469 (2003).
Hu, P. et al. Gq protein-coupled membrane-initiated estrogen signaling rapidly excites corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus in female mice. Endocrinology 157, 3604–3620 (2016).
Kelly, M. J., Qiu, J. & Rønnekleiv, O. K. Estrogen signaling in the hypothalamus. Vitam. Horm. 71, 123–145 (2005).
Weiser, M. & Handa, R. J. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic–pituitary–adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 159, 883–895 (2009).
Ono, D. et al. The mammalian circadian pacemaker regulates wakefulness via CRF neurons in the paraventricular nucleus of the hypothalamus. Sci. Adv. 6, eabd0384 (2020).
Chen, Y., Molet, J., Gunn, B. G., Ressler, K. & Baram, T. Z. Diversity of reporter expression patterns in transgenic mouse lines targeting corticotropin-releasing hormone-expressing neurons. Endocrinology 156, 4769–4780 (2015).
Cusulin, J. I. W., Füzesi, T., Watts, A. G. & Bains, J. S. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS ONE 8, e64943 (2013).
Jamieson, B., Nair, B. & Iremonger, K. Regulation of hypothalamic corticotropin-releasing hormone neurone excitability by oxytocin. J. Neuroendocrinol. 29, e12532 (2017).
Cora, M. C., Kooistra, L. & Travlos, G. Vaginal cytology of the laboratory rat and mouse: Review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol. Pathol. 43, 776–793 (2015).
Sonner, P. M. & Stern, J. E. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J. Physiol. 582, 1219–1238 (2007).
Bittar, T. P. et al. Corticosterone mediated functional and structural plasticity in corticotropin-releasing hormone neurons. Neuropharmacology 154, 79–86 (2019).
Acknowledgements
This work was supported by a Royal Society of New Zealand Marsden Grant. The authors would like to thank Dr Dharshini Ganeshan for technical assistance and Prof Colin Brown and Dr Michel Herde for helpful discussions and feedback on earlier versions of the manuscript.
Funding
Funding was provided by Marsden Fund Grant No. UOO1701.
Author information
Authors and Affiliations
Contributions
E.P. performed the experimental work and prepared the figures and tables. K.I. and E.P. wrote the main manuscript text. Both authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Power, E.M., Iremonger, K.J. Plasticity of intrinsic excitability across the estrous cycle in hypothalamic CRH neurons. Sci Rep 11, 16700 (2021). https://doi.org/10.1038/s41598-021-96341-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96341-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.