Abstract
Macrophages participate in the pathogenesis of ankylosing spondylitis (AS) by producing inflammatory cytokines. Extracellular adenosine triphosphate (eATP), released during cell stress, acts through purinergic receptors (P2XR and P2YR) and induces inflammatory responses. We investigated the effect of 2ʹ(3ʹ)-O-(4-benzoyl benzoyl) ATP (BzATP) (a prototypic agonist of P2X7R) on the production of inflammatory cytokines in both monocyte-generated (M2-like) and M1 macrophages from patients and controls. Macrophages were differentiated from isolated periphery-monocytes (n = 14 in each group) by macrophage colony-stimulating factor (M-CSF). Using LPS and IFN-γ, macrophages were skewed toward M1 type and were treated with BzATP. Gene expression and protein release of IL-1β, IL-23, and TNF-α were evaluated by real-time PCR and ELISA methods respectively before and after treatment. BzATP significantly increased the protein release of TNF-α and the expression of TNFA and IL1B in monocyte-generated macrophages. Besides, BzATP treatment significantly upregulated IL1B expression, reduced TNFA and IL23A expression, and TNF-α release in M1 macrophages from both groups. Monocyte-generated and M1 macrophages from AS patients released higher TNF-α and expressed more IL1B in response to the same concentration of BzATP treatment respectively. Based on our results, AS macrophages were more sensitive to BzATP treatment and responded more intensively. Besides, the diverse effects of BzATP on monocyte-derived and M1 macrophages in our study may represent the differed inflammatory properties of these two groups of macrophages in response to eATP in the body.
Similar content being viewed by others
Introduction
Under the normal physiological state, adenosine triphosphate (ATP) exists at a low level in the extracellular space, however, under cellular stress or damage, it is released in large amounts to the outside milieu1,2. Extracellular nucleotides and their derived nucleosides (such as adenosine) participate in purinergic signaling which is an evolutionarily conserved signaling pathway and is involved in various pathophysiological events including neuromodulation, cell differentiation, migration, immune responses, and inflammation3. During inflammation, ATP is released from activated leukocytes by passive leakage or active release through Pannexin 1 channels4. Extracellular ATP acts through type 2 purinergic receptors (P2R) including P2X ligand-gated ion channels and G protein-coupled receptors5. P2X7 is the most studied P2R in the innate and adaptive immunological pathways and is thought to be responsible for the NLR family pyrin domain-containing (NLRP)-3 inflammasome activation and the maturation and secretion of inflammatory cytokines such as interleukin (IL)-1β and IL-18 of immune cells especially macrophages6. In addition, extracellular ATP-dependent P2X7 activation can induce cellular death and apoptosis via caspases activation7.
Macrophages as the effector cells of innate immunity have a fundamental role in the induction and resolution of inflammation. Macrophages have been classified into two groups, M1 (classically activated) that produce inflammatory cytokines and participate in autoimmune responses, and M2 (alternatively activated) which participate mainly in tissue remodeling8,9. The role of extracellular ATP in the macrophages' activation and function has been demonstrated in the different in vitro and in vivo studies10,11,12. Macrophage treatment with lipopolysaccharides (LPS) and ATP resulted in the NLRP3 inflammasome activation and production of IL-1β and IL-18 in cell culture13.
Ankylosing spondylitis (AS) is a type of axial inflammatory arthritis called spondyloarthritis (SpA)14. The disease involves mainly the spine and sacroiliac joints and is characterized by long-term inflammation, inflammatory destruction, and post-new bone formation of spinal vertebrae15. Environmental and genetic factors including human leukocyte antigene (HLA)-B27 positivity are contributed to the increased risk of disease16,17,18. Studies demonstrated a central role of the tumor necrosis factor (TNF)-α and IL-23/IL17 axis in the inflammation and pathogenesis of AS19. Macrophages participate in the AS pathogenesis by producing a large amount of inflammatory cytokines and are one of the most infiltrated cells in the AS patients’ lesions20,21. It is also demonstrated that HLA-B27 misfolding induces endoplasmic reticulum (ER) stress and increases IL-23 production in macrophages and immune dysregulation22.
During the pathological condition, the level of extracellular ATP is increased and ATP-dependent P2X7R activation induces the inflammatory responses in macrophages. Concerning the importance of macrophages in the inflammatory responses of AS disease, here we investigated the effect of a prototypic agonist of P2X7R (BzATP) on the production of inflammatory cytokine in both monocyte-generated (M2-like) and M1 macrophages from AS patients in contrast to macrophages from healthy subjects.
Results
Extracellular ATP in the supernatant of monocyte-generated macrophages
The level of eATP in the supernatant of M2-like macrophages from AS patients and controls was 2.6 ± 1.6 μM and 3.3 ± 2.1 μM respectively (Supplementary Fig. S1) and there was not a notable difference between patients and controls.
The gene expression and protein secretion of inflammatory cytokines in monocyte-derived and M1 macrophages
First, we measured the level of mRNA expression and protein secretion of IL-23, IL-1β, and TNF-α pro-inflammatory factors in monocyte-generated macrophages and M1 macrophages from AS and healthy individuals. As we reported earlier, M2-like macrophages of patients produced higher IL-23 compared to control macrophages23. We did not find remarkable differences in the gene expression and protein production of TNF-α and IL-1β in M2-like macrophages between controls and patients.
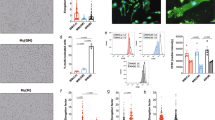
The gene expression of IL1B, TNFA, and IL23A and the protein release of IL-23 and TNF-α were notably elevated in macrophages after their differentiation to M1 type in both AS patients and controls (P-value < 0.01 for all). However, only macrophages from AS patients secreted a significantly elevated level of IL-1β after differentiation to M1 type (P-value < 0.01). M1 macrophages from AS patients significantly expressed an elevated level of IL1B gene (P-value < 0.05) compared to controls. Although the secretion level of IL-1β was also upregulated in AS M1 macrophages compared to controls, the difference was not significant. Besides, we did not find notable differences in the expression and production level of IL-23 and TNF-α between M1 macrophages from AS patients and controls. The relative gene expression and protein secretion levels of studied pro-inflammatory cytokines in monocyte-derived and M1 macrophages of patients and controls are shown in Table 1 and Fig. 1.
The mRNA expression and the protein production of pro-inflammatory cytokines in monocyte-generated and M1 macrophages from AS and healthy individuals. M1 macrophages from AS patients significantly expressed an elevated level of IL1B gene. The gene expression levels of IL1B, TNFA, and IL23A and the protein production levels of IL-23 and TNF-α were significantly elevated in macrophages after their differentiation to M1 type. However, only macrophages from AS patients produced a significantly elevated level of IL-1β after differentiation to M1 type. The data are presented as the mean ± SEM (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
The gene expression and protein secretion of inflammatory cytokines in monocyte-generated macrophages after BzATP treatment
The effect of 200 μM BzATP treatment on the secretion of pro-inflammatory cytokines in monocyte-derived macrophages from healthy individuals and patients was examined. BzATP significantly elevated the gene expression level of TNFA in AS and healthy macrophages (P-value < 0.01 and P-value < 0.05 respectively). The secretion level of TNF-α was also notably upregulated after BzATP treatment in both groups (P-value < 0.01 and P-value < 0.001 respectively), however, monocyte-generated macrophages from AS patients significantly released a higher amount of TNF-α after treatment compared to control ones (P-value < 0.05). Although BzATP significantly elevated the expression level of IL1B in monocyte-generated macrophages from both patients and controls (P-value < 0.05 and P-value < 0.01 respectively), the elevated protein secretion of IL-1β after treatment did not reach statistical significance. We also did not find any remarkable differences in the expression and release of IL-23 after BzATP stimulation. The relative gene expression and protein secretion levels of studied pro-inflammatory cytokines in monocyte-generated macrophages before and after BzATP treatment are shown in Table 2 and Fig. 2.
The effect of BzATP treatment on the expression and production of pro-inflammatory cytokines in monocyte-generated macrophages from patients and controls. BzATP significantly increased the mRNA expression level of TNFA in AS and healthy macrophages. The production level of TNF-α was also significantly upregulated after BzATP treatment in both groups and monocyte-generated macrophages from AS patients significantly produced a higher amount of TNF-α after treatment compared to control ones. Besides, BzATP significantly elevated the expression level of IL1B in monocyte-derived macrophages from both patients and controls. The data are presented as the mean ± SEM (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
The gene expression and protein secretion of inflammatory cytokines in M1 macrophages after BzATP treatment
We also assessed the effect of 200 μM BzATP on the secretion of the pro-inflammatory cytokines in AS and healthy M1 macrophages. BzATP treatment significantly upregulated IL1B gene expression in M1 macrophages from both groups (P-value < 0.01 for both). It was also found that AS M1 macrophages significantly expressed higher IL1B mRNA in response to BzATP treatment compared to controls (P-value < 0.05). The effect of BzATP on the secretion of IL-1β in M1 macrophages was not significant. Interestingly, BzATP significantly reduced the expression and secretion of TNF-α in both healthy and AS M1 macrophages (P-value < 0.01 for all). It was also found that BzATP meaningfully reduced IL23A gene expression in M1 macrophages from controls and patients groups (P-value < 0.01 for both) but the change in IL-23 secretion was not significant. The relative gene expression and protein secretion levels of studied pro-inflammatory cytokines in M1 macrophages before and after BzATP treatment are shown in Table 3 and Fig. 3.
The effect of BzATP treatment on the production of pro-inflammatory cytokines in M1 macrophages from patients and controls. BzATP treatment significantly upregulated IL1B gene expression in M1 macrophages from both groups of macrophages. AS M1 macrophages significantly expressed higher IL1B mRNA in response to BzATP treatment compared to controls. Interestingly, BzATP significantly diminished the expression and production of TNF-α and the expression of IL23A in both healthy and AS M1 macrophages. The data are presented as the mean ± SEM (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
The cellular death rate of BzATP treated monocyte-generated and M1 macrophages
The cellular death rate of monocyte-derived and M1 macrophages treated with 200 µM BzATP was also investigated and is shown in Supplementary Figure S2. The cellular death rate of M2-like macrophages after BzATP treatment was 17.6% and the cellular death rate of M1 macrophages before and after treatment was 12.6% and 32.5% (P < 0.05) respectively.
Discussion
Macrophages play a substantial role in the pathogenesis of rheumatic diseases and are predominant in SpAs inflammatory lesions21. They participate in the induction of autoimmunity and autoinflammatory responses of AS24,25. While M2 macrophages are involved in tissue remodeling and destruction and resolving the inflammation, M1 macrophages are known for producing a high amount of inflammatory mediators and can induce Th1 immunity26. Previous research showed that M1 macrophage polarization with LPS alone or in combination with IFN-γ leads to the release of an elevated amount of inflammatory cytokines including TNF-α, IL-23, IL-12, and IL-1β27,28,29. In line with previous research, in our study, the level of gene expression and protein secretion of TNF-α and IL-23 was significantly upregulated in both AS and healthy macrophages by polarization toward M1 type. However, we found that only M1 macrophages from AS patients and not healthy macrophages produce an increased amount of IL-1β after polarization. We also compared the level of inflammatory cytokines between patients' and controls' macrophages. Although M1 macrophages from AS patients expressed and produced higher levels of IL-23, IL-1β, and TNF-α compared to healthy M1 macrophages, the increase did not reach statistical significance except for IL1B expression. We also did not find a significant difference in TNF-α production between AS and healthy macrophages. Nevertheless, different studies reported increased production levels of TNF-α from peripheral blood mononuclear cells (PBMCs) and M1 macrophages of AS patients25,30. Regarding our results, it seems that IL-1β expression and secretion are dysregulated in M1 macrophages of AS patients. In line with our results, Gu et al. found that IL1B was highly expressed in PBMCs from SpA patients compared to healthy ones31. Besides in a meta-analysis study of microarray datasets from AS/SpA patients, it was demonstrated that the expression of IL1B was elevated in the blood samples of patients32. According to the increased IL-23 production in monocyte-derived macrophages and elevated IL1B expression in M1 macrophages of AS patients, it seems that macrophages from AS patients display an upregulated inflammatory phenotype compare to healthy ones.
It has been demonstrated that extracellular ATP acts as a danger signaling molecule through activation of purinergic receptors on the cell surface and can trigger inflammatory cascades in macrophages10,12. Therefore, we compared the level of extracellular ATP between AS macrophages and healthy ones. We did not find a remarkable difference. The concentration of extracellular ATP in the macrophages supernatant was approximately 3 μM in our study which is higher than the concentration reported in previous studies which are about 10 nM under physiological conditions33. To the best of our knowledge, it is the first study that assessed the concentration of eATP in primary macrophages environment and more investigations are needed.
Previous reports have suggested a critical role for P2X7 purinergic receptor on the activation, polarization, and inflammatory cytokines' production of macrophages34. P2X7R activation induces ATP release to the cell-extracellular environment through activation of pannexin 1 (PANX1) hemichannels in a positive feedback loop and amplifies inflammatory signaling pathways35. Previously we reported that the expression of PANX1 and P2RX7 did not significantly differ between M2-like macrophages from patients and controls36. So Due to the role of macrophages and their inflammatory cytokines in AS pathogenesis, we aimed to consider the effects of BzATP as a prototypic P2X7R agonist on the expression and release of inflammatory cytokines in monocyte-derived (M2-like) and LPS and IFN-γ-primed M1 macrophages from AS patients.
It has been suggested that LPS from gram-negative bacteria induces the biosynthesis of pro-IL-1β in macrophages and eATP via P2X7R induces the NLRP3 inflammasome assembly, activates caspase-1, and releases IL-1β37,38. In our study, BzATP induced the expression of IL1B in both monocyte-derived and M1 macrophages of AS and control individuals. Although the treatment induced IL-1β release in M2-like and M1 macrophages, an increase was not significant in neither groups. This may be due to our limited sample size and the small amount of released IL-1β. Besides, in the current study, BzATP showed bi-functional effects on TNF-α expression and release from macrophages. It increased the gene expression and protein release of TNF-α in monocyte-generated macrophages of both AS and healthy groups. However, the treatment diminished TNF-α release in LPS and IFN-γ-primed M1 macrophages of AS patients and controls. Our results are different from the previous studies showing that P2X7R activation by eATP induces TNF-α release by LPS-primed macrophages39,40. In line with our study, Sala et al. realized that eATP in a dose-dependently manner inhibits LPS-induced TNF-α production of dendritic cells (DCs)41. Kucher et al. also demonstrated that BzATP (100 or 1000 µM) or a high dose of eATP attenuates TNF-α release from rat LPS-primed cortical astrocytes42. Although they did not see any effect of BzATP and LPS treatment on cell viability of astrocytes, in our study BzATP in combination with LPS and IFN-γ significantly reduced cell viability of primary macrophages.
The effect of eATP on the production of IL-23 and IL-12 family members has not been deeply studied. It is suggested that eATP inhibits IL-12 production41 and IL-27 expression by DCs43. Schnurr et al. found that eATP enhances IL-23 expression in DCs or LPS-primed DCs43. Besides, Paustian et al. found that eATP and activation of toll-like receptor 2 (TLR2) induce the release of IL-23 from cultured monocytes44. In a study by Diaz-Perez et al., intradermal injection of BzATP into mice did not induce notable inflammatory responses, though BzATP in combination with POM1, an inhibitor of ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDase), resulted in a significant increase in IL-23 production45. In our study, BzATP treatment did not influence the expression and secretion of IL-23 in monocyte-generated macrophages. Besides, LPS-IFN-γ primed M1 macrophages from controls and AS patients expressed reduced IL23A gene in response to BzATP treatment. Although most of the studies reportd the effect of BzATP on LPS-primed macrophage at protein release level, here we showed that BzATP alone or in combination with LPS and IFN-γ can affect TNF-α, IL-1β and IL-23 at gene expression level on macrophages.
Previous research found that eATP in combination with LPS may induce cell death in macrophages by activating caspase 144,46. Extracellular ATP has been shown to have growth inhibitory and systemic cytotoxic activity on different cell types47,48,49. The effect of eATP depends on the cell types, its concentration, and the time of exposure50. Due to its cytotoxicity, it was considered as an alternative therapeutic option for the treatment of malignancies51. In our study, it was shown that BzATP treatment, along with LPS and IFN-γ significantly increases the death rate in macrophages. While the expression of IL1B was increased in this group, IL-23 and TNFα expression and production level were diminished, this may be due to the increased death rate of treated cells. It seems that the use of eATP can induce cell death and diminish inflammatory cytokine production in classically activated macrophages, however considering the systemic cytotoxic effects of eATP, discovering new specific analogs with a less cytotoxic activity which could be applied in future systemic treatments.
Results from the current study showed that monocyte-derived and M1 macrophages of AS patients released higher TNF-α and expressed more IL1B in response to the same concentration of BzATP treatment respectively. It seems that macrophages from AS patients display elevated inflammatory reactions in response to BzATP. In the literature, there are no previous studies regarding P2X7R activation and extracellular ATP in AS patients and there is only a recent publication on P2X7R genetic susceptibility in AS. In a study by Pan et al., it is found that rs7958311 A allele in the P2RX7 gene shows a protective effect on AS incidence in females and is associated with higher disease activity in male patients52. However, some studies evaluated the effect of eATP in other rheumatic diseases. Li et al. reported that BzATP stimulation of CD4+ PBMCs from rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) patients results in higher Ca2 + influx compared to controls53. Castrichini et al. also found that LPS-primed monocytes from patients with Behcet disease (BD), release elevated IL-1β in response to BzATP than controls54. Monaco et al. investigated the effect of eATP on the fibroblast of patients with systemic sclerosis (SSc). They found that patient’s fibroblasts are at a pre-activated state and eATP treatment induces IL-6 release even in the absence of LPS priming. Moreover, both Ca2+ influx and release occurred at lower ATP concentrations in SSc fibroblasts55. Gentile et al. also reported higher Ca2+ permeability in response to eATP in SSc fibroblasts than in controls56. Based on the results, it seems that fibroblasts and immune cells from various rheumatic diseases are more sensitive to the presence of eATP and inflammatory environments compared to healthy ones. Therefore, controlling the level of eATP and targeting its signaling pathway can be a new therapeutic option for treating these patients.
Conclusion
In conclusion, diverse observed effects of BzATP on monocyte-derived and M1 classically activated macrophages in our study, especially regarding TNF-α and IL-23, may represent the differed inflammatory properties of these two groups of macrophages in response to environmental ATP in the body. Besides, it seems that the cytotoxicity activity of BzATP on classically activated macrophages may affect their production of inflammatory cytokines. In addition, according to our results, AS macrophages were more sensitive to BzATP treatment, responded more intensively and expressed an increased level of IL1B, and released elevated TNF-α in response to the same concentration of treatment. Therefore, it is expected that alternation in this signaling pathway may contribute to the pathogenesis of AS disease, and targeting this pathway can consider as a therapeutic option in the future. There are no other reports about the effects of eATP and activation of purinergic signaling on macrophages from AS patients. Due to the obtained results in this research, more research with a bigger sample size on different types of immune cells is needed for discovering the exact pathogenic role of this signaling pathway in AS disease.
Methods and patients
Study participants
In this research, 30 ml of venous blood were drawn from AS patients and sex/age-matched healthy volunteers (11 males and 3 females). Patients were recruited from the outpatient rheumatology clinic, Shariati Hospital, Tehran, Iran. Patients were included based on the modified New York classification criteria57 and had Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) more than four. Due to the significant influence of different biological therapy on the production of inflammatory cytokines, treatment-naive patients were chosen for this study. Besides, healthy volunteer participants did not have a previous familial or personal history of rheumatic disorders or any other autoimmune diseases. The clinical and demographical details of patients and healthy contributors are summarized in Table 4. This research was conducted based on the Declaration of Helsinki guidelines and was approved by the Ethics Committee of Tehran University of Medical Sciences (Approval No: IR.TUMS.REC.1394.1504). All participants provided signed written informed consent before their contribution to the study.
Monocyte separation, macrophage differentiation, and culture
Ethylenediaminetetraacetic acid (EDTA) containing tubes were used to collect venous blood. Collected samples were processed in less than 4 h. The PBMCs were separated from 1:2 diluted blood with phosphate-buffered saline (PBS; GIBCO Invitrogen) using Ficoll density gradient centrifugation. To separate the monocytes, extracted PBMCs were washed by PBS solution and incubated with CD14 microbeads. Positive selection for CD14 marker undertook using magnetic-activated cell sorting (MACS) methods (Miltenyi Biotec). The purity of isolated monocytes was analyzed by flow cytometry using anti-CD14 antibodies (phycoerythrin (PE)-conjugated; BD bioscience) and 92–95% of isolated cells were CD14 positive58. To differentiate monocytes toward macrophages, the separated CD14+ monocytes were seeded on 24-well plates at a concentration of 5 × 10 ^5 cells per well in Roswell Park Memorial Institute (RPMI) medium supplemented with recombinant macrophage-colony stimulating factor (50 ng/ml M-CSF; eBioscience) for 1 week. The culture media also included L-glutamine (2 mM, Biosera), penicillin (100 U/ml, Sigma), streptomycin (100 μg/ml, Sigma), and 10% fetal bovine serum (FBS; Gibco BRL). CD206 and CD163 macrophages' surface markers were assessed by flow cytometry with appropriate antibodies and isotype controls and the purity was 95 and 97% respectively58.
M1 macrophage polarization
Using LPS (100 ng/ml; Sigma) and interferon-gamma (1000 units/ml IFN-γ; R&D systems, MN) for 24 h, monocyte-generated (M2-like) macrophages were polarized toward M1 type. Polarization was confirmed by analysis of the transcriptional phenotype of M2 and M1 macrophages by SYBR green real-time polymerase chain reaction (PCR). After 24 h of incubation with LPS and IFN-γ, the gene expression of M1 macrophage-certain markers (CXCL11, CCR7, and IDO) and M2 macrophage-specific ones (MRC1 and CD36) was notably increased and decreased respectively (Supplementary Fig. S3).
Extracellular ATP evaluation
Following 7 days of monocyte-incubation with M-CSF, monocyte-generated macrophages from both patients and healthy individuals were seeded on 24-well plates for 24 h. The concentration of secreted ATP in the cell-free supernatant was measured by colorimetric ATP assay kit (Abcam, ab83355) according to the manufacturer’s structure and the absorbance was read at 450 nm with a microplate reader.
BzATP stimulation and measurement of the expression level of the inflammatory cytokines
M1 and monocyte-generated macrophages from AS patients and healthy individuals were treated with 200 µM of 2ʹ(3ʹ)-O-(4-benzoyl benzoyl) adenosine-5ʹ-triphosphate (BzATP, Sigma-Aldrich), a potent P2X7R agonist. Samples were incubated with or without BzATP for 24 h and compared. Using the High Pure RNA Isolation Kit (Roche), the total RNA was extracted from BzATP treated and non-treated monocyte-derived and M1 macrophages. The complementary DNA (cDNA) was synthesized from an equal amount of total RNA by CellAmp direct RNA prep kit (Takara bio) for real-time PCR. The relative gene expression level of TNF-α (TNFA), IL-23 subunit alpha (IL23A, P19), IL-1β (IL1B), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, as an internal control gene) was measured by SYBR green master mix (Ampliqon) using StepOnePlus™ real-time PCR system (Applied Biosystems). The experiment was done with specific primer sequences for IL1B (Forward: 5′ATGGCTTATTACAGTGGCAATGAG3′, Reverse: 5′GTAGTGGTGGTCGGAGATTCG3′), TNFA (Forward: 5′CCTGCCCCAATCCCTTTATT3′, Reverse: 5′CCCTAAGCCCCCAATTCTCT3′), IL23A (Forward: 5′GGGACAACAGTCAGTTCTGCTT3′, Reverse: 5′TGGGACTGAGGCTTGGAATC3′), and GAPDH (Forward: 5′GAGTCAACGGATTTGGTCGT3′, Reverse: 5′GACAAGCTTCCCGTTCTCAG3′). The comparative CT method (2−ΔΔCT) was utilized to compare the expression level of cytokines' genes between the BzATP-incubated and control groups.
Evaluation of cytokine secretion level in macrophages
The secretion level of TNF-α, IL-1β, and IL-23 inflammatory cytokines was investigated by the ELISA method in the supernatant of M2-like and M1 macrophages from healthy individuals and patients before and after BzATP treatment according to manufacturer's protocol (Mabtech).
Macrophages cell death assessment
To evaluate the effect of BzATP on the cellular death rate of macrophages, the colorimetric MTT assay was performed. The viability of the 200 µM BzATP treated and non-treated monocyte-derived and M1 macrophages was measured after 24 h.
Statistical analysis
The normality of all data was evaluated by the Kolmogorov–Smirnov normality test. To compare the relative mRNA expression and cytokines secretion between parallel-group, the independent t-test, and paired samples T-test were used for normal distributions and the Mann Whitney test and Wilcoxon tests were used for non-normal distributions. P-values less than 0.05 were considered statistically significant. All of the statistical analyses were performed by SPSS version 22 and graphs were prepared by GraphPad Prism 6.
Ethics approval
This study was performed based on the Declaration of Helsinki guidelines and was approved by the Ethics Committee of Tehran University of Medical Sciences (Approval No: IR.TUMS.REC.1394.1504).
Consent to participate
The written informed consent was signed by all participants before enrolling in the study.
Data availability
All data generated or analyzed during this study are available upon request.
References
Khakh, B. S. & Burnstock, G. The double life of ATP. Sci Am. 301, 84–92 (2009).
Eltzschig, H. K., Sitkovsky, M. V. & Robson, S. C. Purinergic signaling during inflammation. N Engl J Med. 367, 2322–2333 (2012).
Burnstock, G. Introduction to purinergic signaling. Methods Mol Biol. 2041, 1–15 (2020).
Idzko, M., Ferrari, D. & Eltzschig, H. K. Nucleotide signalling during inflammation. Nature 509, 310–317 (2014).
Velasquez, S. & Eugenin, E. Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Front. Physiol. 5, 96 (2014).
Surprenant, A. & North, R. A. Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359 (2009).
Schulze-Lohoff, E. et al. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am. J. Physiol. 275, F962-971 (1998).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000 Reports. 6, 13–13 (2014).
Tarique, A. A. et al. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 53, 676–688 (2015).
Hanley, P. J. et al. Extracellular ATP induces oscillations of intracellular Ca2 and membrane potential and promotes transcription of IL-6 in macrophages. Proc. Natl. Acad. Sci. USA 101, 9479–9484 (2004).
Cruz, C. M. et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282, 2871–2879 (2007).
Kawamura, H., Kawamura, T., Kanda, Y., Kobayashi, T. & Abo, T. Extracellular ATP-stimulated macrophages produce macrophage inflammatory protein-2 which is important for neutrophil migration. Immunology 136, 448–458 (2012).
Le Feuvre, R. A., Brough, D., Iwakura, Y., Takeda, K. & Rothwell, N. J. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J Biol Chem. 277, 3210–3218 (2002).
Keat, A. Ankylosing spondylitis. Medicine (Baltimore) 38, 185–189 (2010).
Tam, L. S., Gu, J. & Yu, D. Pathogenesis of ankylosing spondylitis. Nat Rev Rheumatol. 6, 399–405 (2010).
Brewerton, D. A. et al. Ankylosing spondylitis and HL-A 27. Lancet 1, 904–907 (1973).
Mathieu, A. et al. The interplay between the geographic distribution of HLA-B27 alleles and their role in infectious and autoimmune diseases: a unifying hypothesis. Autoimmun. Rev. 8, 420–425 (2009).
Benjamin, M. & McGonagle, D. Basic concepts of enthesis biology and immunology. J Rheumatol Suppl. 83, 12–13 (2009).
Coates, L. C., Marzo-Ortega, H., Bennett, A. N. & Emery, P. Anti-TNF therapy in ankylosing spondylitis: insights for the clinician. Ther Adv Musculoskelet Dis. 2, 37–43 (2010).
Cunnane, G., Bresnihan, B. & FitzGerald, O. Immunohistologic analysis of peripheral joint disease in ankylosing spondylitis. Arthritis Rheum. 41, 180–182 (1998).
Bollow, M. et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis- cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann. Rheum. Dis. 59, 135–140 (2000).
DeLay, M. L. et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 60, 2633–2643 (2009).
Akhtari, M. et al. Activation of adenosine A2A receptor induced interleukin-23 mRNA expression in macrophages of ankylosing spondylitis patients. Cytokine 128, 154997 (2020).
Ambarus, C. A., Yeremenko, N. & Baeten, D. L. Altered cytokine expression by macrophages from HLA-B27-positive spondyloarthritis patients without evidence of endoplasmic reticulum stress. Rheumatol. Adv. Pract. 2, rky014 (2018).
Zeng, L., Lindstrom, M. J. & Smith, J. A. Ankylosing spondylitis macrophage production of higher levels of interleukin-23 in response to lipopolysaccharide without induction of a significant unfolded protein response. Arthritis Rheum. 63, 3807–3817 (2011).
Laria, A. et al. The macrophages in rheumatic diseases. J Inflamm Res. 9, 1–11 (2016).
Goerdt, S. et al. Alternative versus classical activation of macrophages. Pathobiology 67, 222–226 (1999).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Mosser, D. M. The many faces of macrophage activation. J Leukoc Biol. 73, 209–212 (2003).
Asadbeik, M. et al. Gene expression profile of proinflammatory cytokines in Iranian patients with ankylosing spondylitis. Rheumatol. Res. 2, 31–38 (2017).
Gu, J. et al. A 588-gene microarray analysis of the peripheral blood mononuclear cells of spondyloarthropathy patients. Rheumatology (Oxford) 41, 759–766 (2002).
Robin, P. et al. Gene expression profile in patients with axial spondyloarthritis: meta-analysis of publicly accessible microarray datasets. J. Rheum. Dis. 23, 363–372 (2016).
Trautmann, A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci. Signal. 2, pe6 (2009).
Lemaire, I., Falzoni, S., Zhang, B., Pellegatti, P. & Di Virgilio, F. The P2X7 receptor and Pannexin-1 are both required for the promotion of multinucleated macrophages by the inflammatory cytokine GM-CSF. J. Immunol. 187, 3878–3887 (2011).
Pelegrin, P. & Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082 (2006).
Akhtari, M. et al. P2 receptors mRNA expression profiles in macrophages from ankylosing spondylitis patients and healthy individuals. Int. J. Rheum. Dis. 23, 350–357 (2020).
Gross, O., Thomas, C. J., Guarda, G. & Tschopp, J. The inflammasome: an integrated view. Immunol. Rev. 243, 136–151 (2011).
Rathinam, V. A., Vanaja, S. K. & Fitzgerald, K. A. Regulation of inflammasome signaling. Nat. Immunol. 13, 333–342 (2012).
Barberà-Cremades, M. et al. P2X7 receptor induces tumor necrosis factor-α converting enzyme activation and release to boost TNF-α production. Front. Immunol. 8, 862–862 (2017).
de Torre-Minguela, C., Barberà-Cremades, M., Gómez, A. I., Martín-Sánchez, F. & Pelegrín, P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci. Rep. 6, 22586–22586 (2016).
la Sala, A. et al. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 166, 1611–1617 (2001).
Kucher, B. M. & Neary, J. T. Bi-functional effects of ATP/P2 receptor activation on tumor necrosis factor-alpha release in lipopolysaccharide-stimulated astrocytes. J. Neurochem. 92, 525–535 (2005).
Schnurr, M. et al. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood 105, 1582–1589 (2005).
Paustian, C. et al. Extracellular ATP and Toll-like receptor 2 agonists trigger in human monocytes an activation program that favors T helper 17. PLoS ONE 8, e54804 (2013).
Diaz-Perez, J. A. et al. Extracellular ATP and IL-23 form a local inflammatory circuit leading to the development of a neutrophil-dependent psoriasiform dermatitis. J. Invest. Dermatol. 138, 2595–2605 (2018).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Chahwala, S. B. & Cantley, L. C. Extracellular ATP induces ion fluxes and inhibits growth of Friend erythroleukemia cells. J. Biol. Chem. 259, 13717–13722 (1984).
Weisman, G. A. et al. Growth inhibition of transformed mouse fibroblasts by adenine nucleotides occurs via generation of extracellular adenosine. J Biol Chem. 263, 12367–12372 (1988).
Spranzi, E., Djeu, J. Y., Hoffman, S. L., Epling-Burnette, P. K. & Blanchard, D. K. Lysis of human monocytic leukemia cells by extracellular adenosine triphosphate: mechanism and characterization of the adenosine triphosphate receptor. Blood 82, 1578–1585 (1993).
Schneider, C., Wiendl, H. & Ogilvie, A. Biphasic cytotoxic mechanism of extracellular ATP on U-937 human histiocytic leukemia cells: involvement of adenosine generation. Biochim. Biophys. Acta. 1538, 190–205 (2001).
Rapaport, E. Utilization of ATP administration for the treatment of cancer and AIDS. Expert Opin. Investig. Drugs 3, 379–389 (1994).
Pan, Z. et al. Genetic variation of rs7958311 in P2X7R gene is associated with the susceptibility and disease activity of ankylosing spondylitis. Postgrad. Med. J. 95, 251–257 (2019).
Li, M. et al. The expression of P2X7 receptor on Th1, Th17, and regulatory T cells in patients with systemic lupus erythematosus or rheumatoid arthritis and its correlations with active disease. J. Immunol. 205, 1752–1762 (2020).
Castrichini, M. et al. The purinergic P2×7 receptor is expressed on monocytes in Behçet’s disease and is modulated by TNF-α. Eur J Immunol. 44, 227–238 (2014).
Lo Monaco, A. et al. Increased sensitivity to extracellular ATP of fibroblasts from patients affected by systemic sclerosis. Ann. Rheum. Dis. 66, 1124–1125 (2007).
Gentile, D. et al. Searching novel therapeutic targets for scleroderma: P2X7-receptor is up-regulated and promotes a fibrogenic phenotype in systemic sclerosis fibroblasts. Front. Pharmacol. 8, 638 (2017).
van der Linden, S., Valkenburg, H. A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
Akhtari, M. et al. Ankylosing spondylitis monocyte-derived macrophages express increased level of A2A adenosine receptor and decreased level of ectonucleoside triphosphate diphosphohydrolase-1 (CD39), A1 and A2B adenosine receptors. Clin. Rheumatol. 37, 1589–1595 (2018).
Funding
This study was supported by a grant from the Deputy of Research, Tehran University of Medical Sciences (Grant No. 94-02-41-28991).
Author information
Authors and Affiliations
Contributions
MA, MV: Acquisition of data, drafting the article, analysis and interpretation of data, final approval of the article. SJZ, AJ, MM: The conception and design of the study, revising the article critically, interpretation of data, final approval of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akhtari, M., Zargar, S.J., Vojdanian, M. et al. Monocyte-derived and M1 macrophages from ankylosing spondylitis patients released higher TNF-α and expressed more IL1B in response to BzATP than macrophages from healthy subjects. Sci Rep 11, 17842 (2021). https://doi.org/10.1038/s41598-021-96262-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96262-2
This article is cited by
-
Molecular mechanisms of AMPK/YAP/NLRP3 signaling pathway affecting the occurrence and development of ankylosing spondylitis
Journal of Orthopaedic Surgery and Research (2023)
-
Ankylosing spondylitis PET imaging and quantifications via P2X7 receptor-targeting radioligand [18F]GSK1482160
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
How Has Molecular Biology Enhanced Our Undertaking of axSpA and Its Management
Current Rheumatology Reports (2023)
-
A2A adenosine receptor agonist reduced MMP8 expression in healthy M2-like macrophages but not in macrophages from ankylosing spondylitis patients
BMC Musculoskeletal Disorders (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.