Abstract
To reveal the antixenosis potential against the pea aphid Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae) we analyzed the pea aphid survival and probing behavior, and the quantitative and qualitative variation of flavonoids in the leaves of selected soybean Glycine max (L.) Merr (Fabaceae) cultivars ‘Aldana’, ‘Annushka’, ‘Augusta’, ‘Madlen’, ‘Mavka’, ‘Simona’, ‘Violetta’, and ‘Viorica’. Aphid survival was drastically impeded on all cultivars. The electronic monitoring of aphid probing using the Electrical Penetration Graph (EPG) technique revealed that on all soybean cultivars, A. pisum readily probed into leaf tissues but the probes were usually terminated before reaching vascular tissues, which demonstrates the activity of antixenosis mechanisms in peripheral tissues epidermis and/or mesophyll in soybean leaves. The potency of antixenosis factors differed among soybean cultivars, which was reflected in differences in aphid survival and frequency and duration of phloem sap ingestion. Seven flavonoids were found: apigenin, daidzein, genistein, glycitein, isorhamnetin, kaempferol, and rutin, which occurred in different amount and proportion in individual cultivars. The content of apigenin and genistein in all soybean cultivars studied probably made them relatively unacceptable to A. pisum. Kaempferol in ‘Aldana’ might be responsible for the observed strong antixenosis resistance of this cultivar to A. pisum. The results of our survey provide the first detailed data that can be used for future studies.
Similar content being viewed by others
Introduction
Soybean Glycine max (L.) Merr. (Fabaceae) is one of the most important world crops in both the temperate and tropical regions1. In 2019, the world production was over 349.4 million tons from the area of over 128.9 million ha and is still increasing2. In Poland alone, the acreage of soybeans cultivation increased from nearly 0.0 ha in 2015 to 7920 ha in 20192. The growing demand for soybean derives from its multiple uses for human and animal consumption due to high content of protein and oil, industrial application such as biodiesel, and as a nitrogen-fixing ground cover1. Soybean is also a source of biologically active substances for medicinal application. The anti-microbial, anti-inflammatory, antioxidant, and anti-tumor activities of soybean flavonoids and saponins are broadly known3,4.
Within a guild of herbivores associated with soybeans, aphids (Hemiptera: Aphididae) have a special status. Aphids affect plant condition directly due to the removal of nutrients and are able to transmit plant pathogenic viruses. Two legume-associated aphid species: the soybean aphid Aphis glycines Matsumura and the pea aphid Acyrthosiphon pisum (Harris) are crucial in the transmission of major destructive viral pathogens in soybean production worldwide, including the non-persistent transmission of Soybean mosaic virus (SMV) and the persistent transmission of Soybean dwarf virus (SbDV)5,6. A. glycines has gained considerable attention of researchers in the recent years due to its mass occurrence, role in virus transmission, and expansion to all regions of soybeans cultivation7,8,9. The resistance potential in soybeans against A. glycines has been explored9,10,11 and the role of flavonoids in A. glycines—soybean interaction has also been studied in detail12,13. The relationship between G. max and A. pisum remains largely unknown, although the pea aphid occurs abundantly in ecosystems in the vicinity of soybean where its populations are supported by various wild legumes. It must be kept in mind that A. pisum is considered one of the most important pest insects of leguminous plants worldwide and it is able to transmit over 40 plant pathogenic viruses among these plants14,15,16,17. The pea aphid is also a vector of bacterial pathogens such as Pseudomonas syringae, which are excreted in the aphid honeydew18.
The pea aphid comprises at least 11 biotypes, each of which shows a different preference and performs differently on specific host plants19. On less preferred hosts, a given biotype of A. pisum shows a limited ability to ingest sap and reproduce, which may be due to the variation in plant characteristics depending on plant taxonomical position20,21,22,23. At the same time, we demonstrated that the pea aphids of Pisum sativum-derived biotype are able to successfully infest and feed upon various forage and grain legumes that are not the key host plants of this aphid biotype24,25,26. Due to the specificity of behavioral phases in the host-plant selection process, the pea aphid poses a potential threat as a virus vector not only to its optimal host plant but also to second-choice plants or non-hosts. During brief intracellular probes in epidermis and parenchyma that are essential for the recognition of host plants27, aphids transmit non-persistent and semi-persistent viruses and during probing in sieve elements, persistent viruses are transmitted28,29. On highly susceptible plant species or cultivars, the pea aphid probing and feeding activities are not impeded. On moderately susceptible plants, aphids have difficulty to attain the feeding phase. Finally, on resistant plants, the probing time is shortened, non-probing intervals between probes are long, and the success rate in reaching the feeding phase is very low or none24,25,26. A susceptible cultivar may become a reservoir for A. pisum population in agroecosystems and increase the risk of virus spread due to aphid infestation. Thus, the reduction in the duration of stylet penetration and especially the prevention of stylet penetration beyond the epidermis and mesophyll may lower the direct impact of the pea aphid on the yield and contribute to the decrease in the transmission of semi-persistent and persistent viruses28. These goals are often achieved in cultivars that exhibit antixenosis resistance. Antixenosis occurs when plant morphological or chemical factors adversely affect arthropod behavior, leading to delayed acceptance or rejection of a plant as a host30,31,32. Aphid resistance is often significantly correlated with levels of allelochemical antixenosis factors that may act as repellents or feeding deterrents and affect aphid behavior at different phases of stylet penetration activities23,27,28,33,34,35. Flavonoids, which occur abundantly in the soybean, are well-known mediators of plant–insect interactions and represent the major line of constitutive and induced defense against herbivory36. An increase in flavonoid contents has been observed in plants infested by A. pisum37,38,39. Flavonoids are synthesized in the cytosol and stored in vacuoles and apoplast or transported to other tissues via cell-to-cell movement or by phloem vessels40,41,42,43. This means that aphids may come into contact with flavonoids at various stages of stylet penetration, during brief cell punctures in epidermis and mesophyll and during sap ingestion from sieve elements.
In the course of our previous studies on the susceptibility of various species of grain legumes to A. pisum, we discovered strong antixenosis potential against the P. sativum-derived biotype of the pea aphid in soybean cv. ‘Aldana’, which was manifested in a shortened aphid stylet penetration time, long non-probing intervals between probes, and a very low success rate in reaching phloem elements as compared to the most preferred host plants P. sativum and V. faba26. Although G. max is not a favored host for the pea-biotype of the pea aphid, a relatively susceptible soybean cultivar may become a residue for this biotype in the agroecosystem, which may contribute to the risk of virus spread. Significant intraspecific variation in susceptibility to the pea-biotype of A. pisum occurs in scarlet runner bean Phaseolus coccineus L. and string bean P. vulgaris L.; these two species comprise cultivars that are highly resistant and relatively susceptible to A. pisum26. Variation in antibiosis and antixenosis towards A. glycines was also reported among various genotypes of G. max11.
The aim of the present study was to explore the antixenosis potential in a selection of G. max cultivars. We hypothesized that soybean cultivars from different regions of origin in Europe differ in the level of antixenosis towards the pea aphid and that these differences are linked to the content of flavonoids. We monitored the pea aphid choices of soybean cultivars, the ability to survive on these cultivars, and aphid stylet penetration activities in plant tissues. We applied the Electrical Penetration Graph (EPG, known also as electropenetrography) technique, which is crucial in determining the influence of antixenosis factors on individual phases of aphid probing in peripheral as well as in vascular plant tissues23,27,28. In addition, we analyzed the quantitative and qualitative variation in the content of major flavonoids in the leaves of the soybean cultivars.
Results
Free-choice test
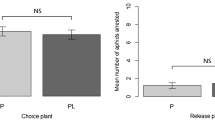
Free-choice test was performed to study the variation in antixenosis-based resistance in soybean cultivars towards the pea-associated biotype of A. pisum (defined as ‘biotype ‘G’19). Twenty-four hours after aphid introduction, each of the eight tested soybean cultivars had statistically fewer aphids compared to susceptible control, P. sativum. At the same time, significantly fewer aphids chose soybean cv. ‘Aldana’ than the seven other cultivars, but no significant differences in the number of aphids were recorded among the other soybean cultivars (Fig. 1).
Number of Acyrthosiphon pisum apterous females (mean ± SD) in free-choice tests for soybean cultivars at 24 h after aphid introduction. For each test (n = 15), 50 A. pisum were introduced initially at the center of the Petri dish containing leaves of soybean cultivars arranged in a circle. Pisum sativum cv. ‘Milwa’ was used as susceptible check for all soybean cultivars. Bars followed by the different letters are significantly different (LSD0.05: 1.759; ANOVA F: 318.85).
Survival of the pea aphid on soybean cultivars
We applied a standard procedure to study aphid life parameters on soybean cultivars, in which an apterous female is transferred from the stock colony to the experimental plant, then the development of the first newborn nymph is monitored until its death as an adult. We recorded that, as expected, all apterous females transferred from peas gave birth to the nymphs. However, no 1st instar nymph molted into 2nd instar on any soybean cultivar. The average survival of 1st instar nymphs of the pea aphid ranged from 1.0 (± 0.0) day on cv. ‘Aldana’ to 2.2 (± 1.4) days on cv. ‘Madlen’. Survival on ‘Simona’ and ‘Violetta’ was 1.5 (± 0.5) and 1.5 (± 0.7) days, respectively, and differed significantly from ‘Madlen’ but was similar to the survival on ‘Aldana’, ‘Annushka’, ‘Augusta’, ‘Mavka’, and ‘Viorica’ (Fig. 2).
Probing behavior of the pea aphid on soybean cultivars
The 8-h EPG monitoring of pea aphid probing on soybean cultivars revealed activities defined as no-probing (= aphid stylets outside plant tissues), pathway phase (= aphid stylets in epidermis and mesophyll), xylem phase (= aphid stylets in xylem vessels), and phloem phase (= aphid stylets in sieve elements) (Table 1). Generally, no-probing predominated over pathway, xylem, and phloem phases on all soybean cultivars during the entire monitoring period (Fig. 3). The highest proportion of no-probing occurred in aphids on ‘Viorica’, ‘Simona’, ‘Aldana’, and ‘Violetta’ (80%, 78%, 75%, and 75% of experimental time, respectively) and the lowest on ‘Madlen’ (61%), while on the remaining cultivars, the proportion of no-probing ranged from 63% on ‘Augusta’ to 71% on ‘Annushka’ and ‘Mavka’ (Table 1, Fig. 3). The activities in non-phloem tissues were divided into pathway stylet penetration in epidermis and mesophyll and sap ingestion from xylem vessels. In mesophyll, short periods of derailed stylet movements (waveform ‘F’) occurred occasionally, irrespective of soybean cultivar. Therefore, in the present analysis all events of ‘F’ were included in the pathway stylet activities. The longest duration of xylem phase occurred on ‘Madlen’ (0.9 ± 1.1 h) and shortest on ‘Viorica’ (0.1 ± 0.3 h). The mean number of probes, the mean duration of probes, and the duration of the first probe were similar in all aphids on all soybean cultivars (Table 1). The shortest time to reach phloem phase from the onset of probing occurred in aphids on ‘Annushka’, and ‘Augusta’ and ‘Violetta’ (4.6 ± 3.4 and 5.1 ± 3.1, and 5.1 ± 3.0 h, respectively) (Table 1). However, on all cultivars except ‘Aldana’ and ‘Mavka’, there were individuals that were able to reach phloem phase as early as during the first hour after access to plants (Fig. 3). The overall success rate in reaching phloem vessels was highest on ‘Augusta’ and ‘Annushka’ (60% and 50% aphids showed phloem phase) and lowest on ‘Aldana’ (12.5%). On ‘Madlen’, ‘Mavka’, ‘Simona’, ‘Violetta’ and ‘Viorica’, similar proportion of aphids reached phloem phase (from 31% on ‘Simona’ to 44% on ‘Viorica’) (Fig. 4). As a result, the proportion of phloem phase in the studied pea aphid population was lowest on ‘Aldana’ (0.12%) and highest on ‘Annushka, Violetta, and Madlen (17.7%, 17.6%, and 14.1%, respectively) (Fig. 5). The number of probes that included the phloem phase was highest in aphids on ‘Augusta’ (1.0 ± 1.1) and lowest on ‘Aldana’ (0.1 ± 0.3) (Table 1). The frequency of phloem phase was low in all aphids on all soybean cultivars. The number of phloem phases per aphid ranged from 0.1 ± 0.3 on ‘Aldana’ to 1.3 ± 1.4 on ‘Augusta’. While on ‘Aldana’ the phloem phase included only the activity associated with salivation into sieve elements, on the remaining soybean cultivars the phloem phase consisted of both salivation and ingestion activities, including the periods of ingestion longer than 10 min (Table 1).
Frequency of phloem phase expressed as the proportion of Acyrthosiphon pisum that reached phloem sieve elements during 8-h access to Glycine max cultivars according to the EPG monitoring of aphid probing. Bars followed by the different letters are significantly different (LSD0.05: 0.337; ANOVA F: 1.41).
Proportion of phloem phase in all probing activities of Acyrthosiphon pisum on Glycine max cultivars recorded during the 8-h EPG monitoring of aphid probing, according to the formula: E/(C + E + G) × 100%, where C = pathway phase, E = phloem phase, G = xylem phase. Bars followed by the different letters are significantly different (LSD0.05: 13.84; ANOVA F: 1.46).
Flavonoids in leaves of soybean cultivars
The total amount of flavonoids analyzed in leaves of soybean cultivars ranged from 1.80 ± 0.21 μg/g dry weight (d.w.) in ‘Annushka’ to 26.14 ± 1.94 μg/g d.w. in ‘Augusta’ (Table 2). These flavonoids included apigenin, daidzein, genistein, glycitein, isorhamnetin, kaempferol, and rutin, which occurred in different amounts and proportions in individual cultivars (Fig. 5). Apigenin and genistein occurred in all cultivars, daidzein occurred in ‘Madlen’ and ‘Violetta’, glycitein occurred in ‘Mavka’, isorhamnetin was detected in ’Augusta’, kaempferol was found in ‘Aldana’ and rutin occurred in ’Aldana’, ‘Augusta’, and ‘Viorica’ (Table 2, Fig. 6).
Correlation analysis
Correlation analysis revealed significant positive correlation between: the total duration of phloem phase and the proportion of phloem phase in total probing (r = 0.854), the number of probes and the total flavonoids (r = 0.790), time from first probe to first phloem phase and the number of probes before first phloem phase (r = 0.753), the total duration of non-probing before first phloem phase and the number of probes before first phloem phase (r = 0.850) as well as apigenin and genistein (r = 0.792). A negative correlation coefficient was observed for the proportion of phloem phase in total probing and time from first probe to first phloem phase (r = − 0.818).
Distribution of soybean cultivars in terms of the first two principal components of nine observed traits of EPG is presented in Fig. 7A. The first two principal components accounted for 84.34% of total multivariate variability between studied cultivars. Distribution of eight soybean cultivars in terms of the first two principal components of three flavonoids traits is presented in Fig. 7B. The first two principal components accounted for 99.85% of total multivariate variability between eight soybean cultivars.
(A) Spatial distribution of eight Glycine max cultivars in terms of the first two principal components of nine observed traits of EPG-monitored probing behavior of Acyrthosiphon pisum. (B) Spatial distribution of eight Glycine max cultivars in terms of the first two principal components of three flavonoid traits.
Discussion
Generally, A. pisum was willing to probe into leaf tissues of all soybean cultivars studied presently. However, the probes were usually terminated within less than four to seven minutes. In consequence, no-probing was the main activity of the pea aphid on soybeans and the success rate in finding the sieve elements was very low or aphids did not reach phloem phase at all within the 8-h period of access to plants. In those rare cases when the pea aphid did find phloem vessels, the phloem phase was very short. The new-born nymphs of the pea aphid did not survive beyond one or two days on all soybean cultivars studied. In the free-choice test, aphids avoided all soybean cultivars in favor of P. sativum. To understand and provide the possible explanation of the pea aphid behavior on soybeans, the analysis of behavioral events that lead to host-plant selection and acceptance must be considered. There are two major phases in this process: (i) the host-plant location in the environment and (ii) the examination of plant features once the prospective host-plant has been traced and approached44. In our study, we concentrated upon the latter phase that comprises the assessment of plant internal traits, mainly of biochemical nature27,45. In contrast to herbivore insects with biting-chewing mouthparts that are equipped with external contact taste receptors, aphids possess sucking-piercing mouthparts that lack such sensory elements27. The essential taste organ is located in the pharynx46, therefore aphids need to take samples of plant sap during probing with their stylets to assess the suitability of the potential host plant27. Consequently, aphid probing behavior reflects the susceptibility of plants to aphid infestation on the one hand and aphid ability to overcome plant defenses on the other27. Generally, aphids respond to the quality of plant sap by either continuing or terminating stylet penetration47,48. The time required by aphid stylets to pass one layer of cells is approximately two to three minutes49. Stylet withdrawal by aphids after a short probe in the outer leaf tissues suggests the presence of probing deterrents in these tissues. This is often observed in incompatible plant-aphid associations23, on resistant plant cultivars48, or when the non-acceptable xenobiotics are applied to aphid host-plants50. The soybean genotypes studied evoked a spectrum of behavioral responses from A. pisum. We demonstrated that the pea aphids withdrew their stylets four to seven minutes after the beginning of a probe, which means that the probing aphids must have encountered the deterrent factors either in the first (epidermis) or second/third (mesophyll) tissue layer in soybean leaves. This was especially noticeable in the cultivar ‘Aldana’, on which the proportion of no-probing time in relation to other aphid activities was highest as compared to other soybean cultivars studied. The mean duration of a probe was approximately four minutes and almost all aphids on this cultivar failed to reach phloem vessels and commence feeding during eight hours of access to the plants, which means that the probes included chiefly stylet activities in non-phloem tissues and points at the strong activity of antixenosis factors in the outer leaf tissues. The described characteristic behavior was also typical of A. pisum probing on resistant lupine cultivars and unpalatable species of forage and grain legumes, on which non-probing activities prevailed over stylet penetration, the probes were terminated usually 3–5 min after stylet insertion in plant tissues, and the phloem phase was short or did not occur24,25,26,48. Low number of probes before the first phloem phase, short duration of no-probing, relatively short time to reach phloem phase, and sap ingestion sustained over many hours with no interruption indicate that little or no antixenosis factors are present in tissues encountered before reaching sieve elements and in the phloem vessels by A. pisum on susceptible legumes24,25,26,48. The predominance of no-probing activities occurred also on soybean cultivars ‘Annushka’, ‘Augusta’, ‘Madlen’, ‘Mavka’, ‘Simona’, ‘Violetta’, and ‘Viorica’. However, aphids on these cultivars were able to reach phloem and commence feeding. Among these cultivars, the frequency of phloem sap ingestion phase on ‘Annushka’ and ‘Augusta’ was higher than in ‘Madlen’, ‘Mavka’, ‘Simona’, ‘Violetta’, and ‘Viorica’ while the total duration of phloem phase was highest in ‘Madlen’. The reduced duration of phloem sap uptake results in the impediment of aphid survival and development, which happened to the cabbage aphid Brevicoryne brassicae L. on resistant rapeseed cultivars51. Among soybean cultivars studied, the lowest survival of newborn nymphs was on ‘Aldana’ and highest on ‘Madlen’.
Aphids on all soybean cultivars showed stylet activities associated with the ingestion of sap from xylem vessels. Generally, the mean duration of xylem phase was comparable to the duration of the phloem phase. However, on ‘Aldana’ the xylem phase was the key activity associated with ingestion from vascular tissues, as the phloem phase was practically absent on this cultivar. Water uptake by aphids is generally considered as an osmoregulatory strategy in response to phloem sap dietary osmotic pressure and dehydration caused by drought52. On the other hand, it has been proposed that xylem sap ingestion is initiated to reduce the negative impact of plant toxins53, which may also have been the case in the present study.
Plant allelochemical antixenosis against aphids is based mainly on bioactive compounds, such as hydroxamic acids, alkaloids, polyphenols, flavonoids, terpenoids or saponins54. In our study, we concentrated on flavonoids which are well known for their detrimental effect on insect herbivores including the pea aphid55,56,57,58. The correlation analysis revealed that the total amount of the group of flavonoids analyzed did not affect the pea aphid probing behavior significantly. It was especially noticeable in cultivar ‘Augusta’, on which the aphid feeding success was relatively high despite the highest observed content of the analyzed flavonoids of all soybean cultivars studied. Apigenin and genistein were detected in all cultivars. Both apigenin and genistein are known for their anti-herbivore properties. Apigenin is highly toxic to larvae of southern house mosquito Culex quinquefasciatus Say (Diptera: Culicidae), affects the fecundity, mortality, and food consumption of Formosan termite Coptotermes formosanus Shiraki (Blattodea: Isoptera: Rhinotermitidae), shows antifeedant activity against striped flea beetles Phyllotreta striolata (Coleoptera: Chrysomelidae)59,60,61. Apigenin reduced the pea aphid abundance and phloem sap ingestion on alfalfa, caused a reduction in the number and duration of probes when added to saponin mixtures in artificial diets, and was accumulated in vegetative parts of aphid-infested pea plants62. In soybean, genistein had negative effects on the behavior and biology of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae), Piezodorus guildinii (Hemiptera: Pentatomidae), and Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae)63,64,65. The application of genistein in artificial diet decreased the feeding efficiency and reduced the survival rate of A. pisum and the increases in genistein conferred resistance against the pea aphid in M. sativa57,66. Considering the presence of both genistein and apigenin in all soybean cultivars studied it is reasonable to infer that these flavonoids are responsible for the general negative response of the pea aphid to G. max. The subtle differences observed in the acceptability of soybean cultivars may be due to the content of daidzein, glycitein, isorhamnetin, kaempferol and rutin, which were identified in individual cultivars. However, these flavonoids differ in their role in constitutive and induced plant resistance against biotic stressors. Daidzein and genistein are associated with the observed antibiosis resistance of soybeans to the soybean aphid, and are induced in soybean leaves by the feeding of Spodoptera litura (L.) (Lepidoptera: Noctuidae) and A. gemmatalis67,68,69. In the present study, daidzein was found in the only one relatively acceptable to the pea aphid cultivar ‘Madlen’ and relatively non-accepted ‘Violetta’. Glycitein is induced by the feeding of A. gemmatalis but not by S. litura67. In our research, we detected glycitein in the relatively non-accepted cultivar ‘Mavka’. Isorhamnetin is associated with the resistance of cowpea Vigna unguiculata L. Walp. against Aphis fabae (Scop.) and has promising potential as an anthelmintic against Haemonchus contortus (Rudolphi, 1803) Cobb (Nematoda: Trichostrongylidae)69,70. In our study, isorhamnetin was found in the relatively acceptable cultivar ‘Augusta’. Kaempferol occurs in higher quantity in cowpea cultivars resistant to cowpea aphid as compared to susceptible cultivars69. An increase in the content of kaempferol was observed due to the feeding of A. gemmatalis on soybeans71. The level of kaempferol in broccoli did not change due to herbivory of B. brassicae and M. persicae72 and did not correlate with the number of A. pisum colonizing P. sativum seedlings38. In our study, kaempferol was detected only in the cultivar ‘Aldana’ that was the least acceptable soybean cultivar to A. pisum in the present study. Rutin is generally considered as associated with plant resistance against herbivores73. High concentration of rutin was found in soybean cultivars resistant to A. gemmatalis and P. guildinii63,64. Rutin is toxic to the woolly apple aphid Eriosoma lanigerum (Hausmann)73,74. In our study, rutin occurred in cultivars ‘Aldana’, ‘Augusta’, and ‘Viorica’, which differed in their susceptibility to A. pisum, ‘Aldana’ being relatively least acceptable and ‘Augusta’ relatively most acceptable cultivar to the pea aphid.
Aphid stylets penetrate plant tissues mostly within the apoplast, between the cellulose and hemicellulose fibres of the secondary cell walls and their work is mostly mechanical with still not well known support of salivary enzymes27,75,76,77. Mechanical problems with the stylets, visualized in EPG recordings as waveform ‘F’ during mesophyll phase of probing have not been well defined, yet77,78. Various studies reported that the derailed mechanics may occur in aphids on resistant plants79 and in aposymbiotic aphids80, may depend on the age of plants79 or may be a function of insect age and plant resistance level53. The incidence of ‘F’ in our study was negligible, which allows us to conclude that it is likely that no mechanical obstacles occur in leaf tissues of soybean cultivars studied. Thus, biophysical antixenosis was not the reason of the rejection of these cultivars by A. pisum biotype ‘G’.
The statistical analysis (PCA) performed independently for aphid probing and plant chemistry showed no similarities in the groupings of soybean cultivars studied. Therefore, no unequivocal classification of cultivars that would have included all analyzed traits was possible. Nevertheless, taking into account the pea aphid probing behavior and relative feeding success as well as the survival, the soybean cultivars studied can be categorized according to A. pisum preferences and the assumed backgrounds of these preferences into four groups. Group I—relatively susceptible—cultivar ‘Madlen’, on which the pea aphid feeding success and survival were highest. Group II—medium susceptible—‘Annushka’ and ‘Augusta’, on which the feeding success and survival were lower than in ‘Madlen’ but higher than in ‘Mavka’, ‘Simona’, ‘Violetta’, and ‘Viorica’. Group III—medium resistant—‘Mavka’, ‘Simona’, ‘Violetta’, and ‘Viorica’, on which the feeding success and survival were lower than in ‘Annushka’ and ‘Augusta’ but higher than in ‘Aldana’. Group IV—highly resistant—‘Aldana’ on which the pea aphid feeding success and the survival were lowest.
In conclusion, we have confirmed that soybean is a relatively unsuitable host for the pea aphid, which we have cautiously determined in our previous studies26. On all soybean cultivars, A. pisum readily probed into leaf tissues but the probes were usually terminated before reaching vascular tissues. In consequence, the phloem phase was significantly delayed or did not occur, the ingestion of phloem sap was limited or prevented, and aphid survival was dramatically impeded. Thus, we can infer the existence of antixenosis factors in peripheral leaf tissues of all soybean cultivars studied. Nevertheless, as stylet penetration of A. pisum in peripheral and vascular tissues was not entirely impeded on any cultivar of soybeans, G. max may be considered a possible source of semi-persistent and persistent viruses, respectively, that may be acquired by the pea aphid and transferred to other legumes and vice versa. Antixenosis in soybean cultivars studied is primarily of biochemical nature. The potency of antixenosis factors differs among soybean cultivars, which was reflected in differences in the acceptance of these cultivars by A. pisum. In our opinion, the spectrum and not the amount of flavonoids in soybean leaves was responsible for the varying pea aphid response to individual cultivars. The content of apigenin and genistein in all soybean cultivars studied probably made all of them relatively unacceptable to A. pisum. We hypothesize that kaempferol in ‘Aldana’ might be responsible for the observed strong antixenosis resistance of this cultivar to A. pisum. However, the impact of individual soybean flavonoids needs a further study. There was no knowledge on the background of susceptibility or resistance of soybean cultivars to A. pisum infestation prior to our study. The results of our survey provide the first detailed data that can be used for reference studies in the future.
Material and methods
Plants and aphids
Eight cultivars of genetically unmodified soybeans were studied: ‘Aldana’, ‘Annushka’, ‘Augusta’, ‘Madlen’, ‘Mavka’, ‘Simona’, ‘Violetta’, and ‘Viorica’. These cultivars were selected because they represent various regions of origin in Eastern and Central Europe: Bulgaria (‘Simona’), Lithuania (‘Violetta’), Poland (‘Aldana’, ‘Augusta’, ‘Madlen’, ‘Mavka’), Romania (‘Viorica’), and Ukraine (‘Annushka’)81, and belong to different maturity groups in these regions: ‘Annushka’ and ‘Augusta’ are very early maturing cultivars, ‘Aldana’, ‘Simona’, ‘Violetta’, ‘Viorica’—early, ‘Mavka’—semi-early, and ‘Madlen’—late maturing cultivars82. The seeds were provided by Hodowla Soi Agroyoumis Sp. z o. o. (Kordeckiego 20, 37-420 Rudnik nad Sanem, Poland). Plants were grown in commercial soil in 9 cm diam. plastic pots, in the chamber Sanyo MLR-351H (Sanyo Electronics Co. Ltd.) at 20 °C, 65% r.h., and L16:8D photoperiod. The plants were watered regularly and no fertilizers were applied.
The laboratory culture of P. sativum-derived Acyrthosiphon pisum (biotype ‘G’ according to19) was maintained on Pisum sativum cv. ‘Milwa’ in the laboratory at 20 °C, 65% r.h., and L16:8D photoperiod. The seeds of P. sativum were purchased from HR Smolice Sp. z o. o. Grupa IHAR (Oddział Przebędowo, 62–095 Murowana Goślina, Poland).
The study did not involve the collection of plants and insects in nature. Plants used in the present study were grown from commercially available seeds. Insects used in the present study were collected from the laboratory culture kept at the Department of Botany and Ecology, University of Zielona Gora, Poland since 2000. The research complies with relevant institutional, national, and international guidelines and legislation.
Free-choice test
To evaluate the antixenosis resistance in soybean cultivars, we performed free-choice tests. Pisum sativum cv. ‘Milwa’ was used as susceptible check for all soybean cultivars. Plants for the tests were cultured as described. Shoots of plants at 14 BBCH growth stage (trifoliate leaf on the 4th node unfolded)83 were excised. The cut end of each shoot was covered with moist cotton wool and placed in an eppendorf vial. The prepared shoots were placed randomly at equidistance from each other in a circular manner on the bottom of a glass vial (240 mm diam, 10 mm high) and 50 apterous females of A. pisum were introduced in the centre of the arena. The vial was covered with a gauze, transferred to the growing chamber Sanyo MLR-350 H (Sanyo Electronics Co. Ltd.) and kept there at 21 ± 1 °C, 65% r.h., and L16:8D photoperiod. The number of aphids on each plant shoot was counted 24 h later. The experiment was replicated 15 times.
Survival tests
One adult apterous female of A. pisum was placed on a plant at 14 BBCH growth stage (trifoliate leaf on the 4th node unfolded)83 for 24 h. After 24 h, the female and all progeny except one nymph were removed. Each plant was isolated within a plastic cylinder with a fine mesh on top. The development of the nymph was monitored daily. The experiment was replicated 15 times for each soybean cultivar. The tests were conducted in an environmental chamber Sanyo MLR-351H (Sanyo Electronics Co. Ltd.) at L16:D8 photoperiod, 21 ± 1 °C, and 70% r.h.
Aphid probing behavior
The probing behavior of A. pisum was monitored using the technique of electronic registration of aphid probing in plant tissues, known as Electrical Penetration Graph or electropenetrography (EPG)27. Aphid and plant were made parts of an electric circuit, which was completed when the aphid inserted its stylets into the plant. Weak voltage was supplied in the circuit, and all changing electric properties were recorded as EPG waveforms that could be correlated with aphid activities and stylet position in plant tissues27. In the present study, adult apterous aphids were attached to a golden wire electrode with conductive silver paint and starved for 1 h prior to the experiment. Probing behavior of A. pisum on soybean cultivars was monitored for 8 h continuously with 4- and 8-channel DC EPG recording equipment. Signals were saved on the computer and analyzed using the PROBE 3.1 software provided by W. F. Tjallingii (www.epgsystems.eu). The following aphid behaviors were distinguished: no penetration (waveform ‘np’—aphid stylets outside the plant), pathway phase—penetration of non-phloem tissues (waveforms ‘ABC’), derailed stylet movements (waveform ‘F’), salivation into sieve elements (waveform ‘E1’), ingestion of phloem sap (waveform ‘E2’), and ingestion of xylem sap (waveform ‘G’). The E1/E2 transition patterns were included in E2. Waveforms ‘F’ occurred sporadically, therefore these events were combined with pathway activities in all calculations and defined as non-phloem activities. The waveform patterns that were not terminated before the end of the experimental period (8 h) were included in the calculations. In sequential parameters, when time to waveforms related to phloem phase (E1 or E2) was calculated, the time from the 1st probe until the end of the recording was used if no phloem phase occurred. In non-sequential parameters, when a given waveform had not been recorded for an individual, the duration of that waveform was given the value of 0.
Aphids for EPG experiments were 2–3 days old (2–3 days after the final molt) viviparous apterous A. pisum. The plants of G. max used in the bioassays were at 14 BBCH growth stage (trifoliate leaf on the 4th node unfolded)83. Each aphid was given access to a freshly prepared plant. Each plant-aphid set was considered as a replication and was tested only once. The number of replications for each plant cultivar/aphid combination was 24. However, only the replications that included complete 8-h recording were kept for analysis, which were: ‘Aldana’, n = 16; ‘Annushka’, n = 16; ‘Augusta’, n = 22; ‘Madlen’, n = 16; ‘Mavka’, n = 13; ‘Simona’, n = 16; ‘Violetta’, n = 15, ‘Viorica’, n = 18. Recordings that terminated due to aphid falling from the plant or where EPG signal was unclear were discarded from analysis. All bioassays started at 10:00–11:00 h MEST (Middle European Summer Time). Aphids show distinct diurnal feeding activity, with peak activity during day time, independently of host plants27,84,85.
High-performance liquid chromatography of flavonoids
The dried soybean leaves, of different botanical varieties, i.e., ‘Annushka’, ‘Aldana’, ‘Augusta’, ‘Madlen’, ‘Mavka’, ‘Simona’, ‘Violetta’ and ‘Viorica’ (1.2 g of each) were homogenized in an aqueous ethanol solution (80%) using a Diax 900 homogenizer. The resulting suspension was centrifuged (12,000 rpm, 10 min) and the supernatant solution was collected in a graduated flask and the pellet was reconditioned. This operation was repeated three times, and the obtained extracts were combined. The homogenization procedure in combination with the extraction was carried out in such a way that the final volume of the extract was 100 ml. From the prepared ethanol extracts, 10 ml was taken and evaporated to dryness in a rotary evaporator under reduced pressure at 40 °C. The dry extracts were dissolved in 100% methanol to a volume of 1 ml. Resulting methanolic extracts containing flavonoids compounds were analyzed by HPLC–ESI–MS/MS.
The content of: ampelopsin, apigenin, daidzein, genistein, glycitein, hesperetin, hesperidin, isorhamnetin, kaempferol, luteolin, naryngin, quercetin, rutin, taxifolin was determined. The selection of the flavonoid spectrum for analysis was based on literature data86,87,88,89.
Individual pure flavonoids were purchased from Sigma–Aldrich (Poland). Ethanol, HPLC gradient grade methanol and acetonitrile were supplied by Merck (Germany). Formic acid was purchased from Sigma-Aldrich (Poland). Stock standard solutions of individual flavonoids (50 mg/1) were prepared by dissolving appropriate amounts of solid reagents in methanol. Mixed working standard solutions of flavonoid compounds at 20, 10, 5, 2.5 and 1 mg/1 or lower concentrations were prepared by appropriate dilutions of stock standard solutions.
The chromatographic analysis was carried out with a Shimadzu LC system, comprising a LC20-AD binary pump, a DGU-20A5 degasser, a CTO-20AC column oven and a SIL-20AC autosampler, connected to a 3200 QTRAP hybrid triple quadrupole (Applied Biosystem, MDS SCIEX, USA) with electrospray ionization source (ESI) operated in negative-ion mode. Phenolic compounds were separated on a Phenomenex Luna C-18 column (100 × 2.0 mm × 3.0 µm) with a pre-column, both maintained at 30 °C. A 7.4 mmol/l solution of formic acid (pH 2.8, eluent A) and acetonitrile (eluent B) were used. The mobile phase was delivered at 0.2 ml/min in a linear gradient mode as follows: 0–2 min 10% B, 30 min 60% B, 40 min 100% B, 55 min 10% B. Flavonoids were identified by comparing their retention times and m/z values of precursor and resulting fragmentation product ions in their MS and MS/MS spectra, respectively, to those obtained for respective standard solutions analyzed under the same conditions. The quantification of flavonoids was done using calibration curves obtained in the SRM (single reaction mode) mode90,91.
Statistical analysis
All statistical calculations were performed using StatSoft, Inc. (2014) STATISTICA (data analysis software system), version 12. Parameters of the free-choice test and aphid performance (nymph survival) were analyzed using Kruskal–Wallis test and post-hoc multiple comparisons of mean ranks for all groups (Dunn’s test). EPG parameters describing aphid probing behavior were calculated manually and individually for every aphid and the mean and standard errors were subsequently calculated using the EPG analysis Excel worksheet created by the authors especially for this study. All aphids were included in analysis. If the specific trait did not occur in the individual EPG recording, the value for this trait was given zero. Data thus obtained were analyzed by Kruskal–Wallis test and post-hoc multiple comparisons of mean ranks for all groups (Dunn’s test). Additionally, the relationships among all the traits were estimated on the basis of correlation coefficients. The graphic distribution of cultivars, described by means of the observed traits, was obtained by means of the principal components analysis (PCA). Correlation and PCA analyses were done in GenStat 18.
References
Pagano, M. C. & Miransari, M. The importance of soybean production worldwide. In Abiotic and Biotic Stresses in Soybean Production Vol. 1 (ed. Miransari, M.) 1–26 (Academic Press, 2016).
FAOSTAT. Food and Agriculture Organisation Statistical Database http://www.apps.fao.org/faostat. Accessed 23 May 2021.
MacDonald, R. S. et al. Environmental influences on isoflavones and saponins in soybeans and their role in colon cancer. J. Nutr. 135, 1239–1242 (2005).
Tidke, S. A. et al. Assessment of anticancer, anti-inflammatory and antioxidant properties of isoflavones present in soybean. Res. J. Phytochem. 12, 35–42 (2018).
Hill, J. H. & Whitham, S. A. Control of virus diseases in soybeans. Adv. Virus Res. 90, 355–390 (2014).
Tian, B. et al. Host adaptation of soybean dwarf virus following serial passages on pea (Pisum sativum) and soybean (Glycine max). Viruses 9, 155 (2017).
Wang, R. Y., Kritzman, A., Hershman, D. E. & Ghabrial, S. A. Aphis glycines as a vector of persistently and nonpersistently transmitted viruses and potential risks for soybean and other crops. Plant Dis. 90, 920–926 (2006).
Hesler, L. S., Dashiell, E., Jonathan, A. E. & Lundgren, G. Characterization of resistance to Aphis glycines in soybean accessions. Euphytica 154, 91–99 (2007).
Baldin, E. L. L. et al. Feeding behavior of Aphis glycines (Hemiptera: Aphididae) on soybeans exhibiting antibiosis, antixenosis, and tolerance resistance. Fla. Entomol. 101, 223–228 (2018).
Chang, H.-X. & Hartman, G. L. Characterization of insect resistance loci in the USDA soybean germplasm collection using genome-wide association studies. Front. Plant Sci. 8, 670. https://doi.org/10.3389/fpls.2017.00670 (2017).
Bansal, R., Mian, M. A. R. & Michel, A. Characterizing resistance to soybean aphid (Hemiptera: Aphididae): Antibiosis and antixenosis assessment. J. Econ. Entomol. https://doi.org/10.1093/jee/toab038 (2021).
Klein, A. T. et al. Investigation of the chemical interface in the soybean−aphid and rice−bacteria interactions using MALDI-Mass Spectrometry Imaging. Anal. Chem. 87, 5294–5301 (2015).
Hohenstein, J. D. et al. Transcriptional and chemical changes in soybean leaves in response to long-term aphid colonization. Front. Plant Sci. 10, 310 (2019).
Blackman, R. L. & Eastop, V. F. Taxonomic issues. In Aphids as Crop Pests (eds van Emden, H. F. & Harrington, R.) 1–36 (CABI, 2017).
Wale, M., Jembere, B. & Seyoum, E. Occurrence of the pea aphid, Acyrthosiphon pisum (Harris) (Homoptera: Aphididae) on wild leguminous plants in West Gojam, Ethiopia, Sinet. Ethiopian J. Sci. 26, 83–87 (2003).
Chan, C. K., Forbes, A. R. & Raworth, D. A. Aphid-transmitted viruses and their vectors of the world. Agric. Can. Tech. Bull. 3E, 1–216 (1991).
Rashed, A. et al. Vector-borne viruses of pulse crops, with a particular emphasis on North American cropping system. Ann. Entomol. Soc. Am. 111, 205–227 (2018).
Stavrinides, J., McCloskey, J. K. & Ochman, H. Pea Aphid as both host and vector for the phytopathogenic bacterium Pseudomonas syringae. Appl. Environ. Microbiol. 75, 2230–2235 (2009).
Peccoud, J., Ollivier, A., Plantegenest, M. & Simon, J.-C. A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. PNAS 106, 7495–7500 (2009).
Caillaud, M. C. & Via, S. Specialized feeding behavior influences both ecological specialization and assortative mating in sympatric host races of pea aphids. Am. Nat. 156, 606–621 (2000).
Ferrari, J., Godfray, H. C., Faulconbridge, A. S., Prior, K. & Via, S. Population differentiation and genetic variation in host choice among pea aphids from eight host plant genera. Evolution 60, 1574–1584 (2006).
Mitku, G. & Damte, T. Development, reproduction, and host preference of Acyrthosiphon pisum (Harris) (Homoptera: Aphididae) on selected lentil genotypes and resistance index of these selected lentil genotypes to pea aphid. Int. J. Entomol. Res. 4, 16–22 (2019).
Powell, G., Tosh, C. R. & Hardie, J. Host plant selection by aphids: behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 51, 309–330 (2006).
Kordan, B. et al. European yellow lupine Lupinus luteus and narrow-leaf lupine Lupinus angustifolius as hosts for the pea aphid Acyrthosiphon pisum. Entomol. Exp. Appl. 128, 139–146 (2008).
Kordan, B. et al. Susceptibility of forage legumes to infestation by the pea aphid Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae). Crop Pasture Sci. 69, 775–784 (2018).
Kordan, B. et al. Antixenosis potential in pulses against the pea aphid (Hemiptera: Aphididae). J. Econ. Entomol. 112, 465–474 (2019).
Pettersson, J., Tjallingii, W. F. & Hardie, J. Host-plant selection and feeding. In Aphids as Crop Pests (eds van Emden, H. F. & Harrington, R.) 173–195 (CABI, 2017).
Martin, B., Collar, J. L., Tjallingi, W. F. & Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 78, 2701–2705 (1997).
Garzo, E., Moreno, A., Plaza, M. & Fereres, A. Feeding behavior and virus-transmission ability of insect vectors exposed to systemic insecticides. Plants 9, 895. https://doi.org/10.3390/plants9070895 (2020).
Onstad, D. W. & Knolhoff, L. Arthropod resistance to crops. In Insect Resistance Management (ed. Onstad, D. W.) 293–326 (Academic Press, 2014).
Smith, C. M. & Clement, S. L. Molecular bases of plant resistance to arthropods. Annu. Rev. Entomol. 57, 309–328 (2012).
Stout, M. J. Reevaluating the conceptual framework for applied research on host-plant resistance. Insect Sci. 20, 263–272 (2013).
Smith, C. M. & Chuang, W. P. Plant resistance to aphid feeding: behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag. Sci. 70, 528–540 (2014).
Dogimont, C., Bendahmane, A., Chovelon, V. & Boissot, N. Host plant resistance to aphids in cultivated crops: Genetic and molecular bases, and interactions with aphid populations. C. R. Biol 333, 566–573 (2010).
Chandran, P. et al. Feeding behavior comparison of soybean aphid (Hemiptera: Aphididae) biotypes on different soybean genotypes. J. Econ. Entomol. 106, 2234–2240 (2013).
Simmonds, M. S. J. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 64, 21–30 (2003).
Mai, V. C. et al. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 221–222, 1–12 (2014).
Morkunas, I. et al. Pea aphid infestation induces changes in flavonoids, antioxidative defence, soluble sugars and sugar transporter expression in leaves of pea seedlings. Protoplasma 253, 1063–1079 (2016).
Woźniak, A. et al. The dynamics of the defense strategy of pea induced by exogenous nitric oxide in response to aphid infestation. Int. J. Mol. Sci. 18, 329. https://doi.org/10.3390/ijms18020329 (2017).
Buer, C. S., Muday, G. K. & Djordjevic, M. A. Implications of long-distance flavonoid movement in Arabidopsis thaliana. Plant Signal. Behav. 3, 415–417 (2008).
Petrussa, E. et al. Plant flavonoids: Biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 14, 14950–14973 (2013).
Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 20, 576–585 (2015).
Alseekh, S., de Souza, L. P., Benina, M. & Fernie, A. L. The style and substance of plant flavonoid decoration; Towards defining both structure and function. Phytochemistry 174, 112347. https://doi.org/10.1016/j.phytochem.2020.112347 (2020).
Klingauf, F. A. Host plant finding and acceptance. In Aphids, Their Biology, Natural Enemies and Control Vol. 2 (eds Minks, A. K. & Harrewijn, P.) 209–223 (Elsevier, 1987).
Tjallingii, W. F. & Mayoral, A. M. Criteria for host plant acceptance by aphids. In Proceeding 8th International Symposium Insect–Plant Relationships (eds Menken, S. B. J. et al.) 280–282 (Kluwer Academic Publishers, 1992).
Wensler, R. J. & Filshie, B. K. Gustatory sense organs in the food canal of aphids. J. Morph. 129, 473–492 (1969).
Gabryś, B. & Tjallingii, W. F. The role of sinigrin in host plant recognition by aphids during initial plant penetration. Entomol. Exp. Appl. 104, 89–93 (2002).
Philippi, J. et al. Correlation of the alkaloid content and composition of narrow-leafed lupins (Lupinus angustifolius L.) to aphid susceptibility. J. Pest Sci. 89, 359–373 (2016).
Van Hoof, H. A. An investigation of the biological transmission of a non-persistent virus. Doctoral thesis (Van Putten and Oortmijer, 1958).
Dancewicz, K., Szumny, A., Wawrzeńczyk, C. & Gabryś, B. Repellent and antifeedant activities of citral-derived lactones against the peach potato aphid. Int. J. Mol. Sci. 21, 8029. https://doi.org/10.3390/ijms21218029 (2020).
Kordan, B. et al. Variation in susceptibility of rapeseed cultivars to the peach potato aphid. J. Pest. Sci. 94, 435–449 (2021).
Pritchard, J. & Vickers, L. H. Aphids and stress. In Aphids as Crop Pests (eds Van Emden, H. F. & Harrington, R.) 132–147 (CABI, 2017).
Pompon, J. & Pelletier, Y. Changes in aphid probing behaviour as a function of insect age and plant resistance level. Bull. Entomol. Res. 102, 550–557 (2012).
van Emden, H. F. Host-plant resistance. In Aphids as Crop Pests (eds van Emden, H. F. & Harrington, R.) 515–532 (CABI, 2017).
Gould, K. S. & Lister, C. Flavonoid functions in plants. In Flavonoids, Chemistry, Biochemistry and Applications (eds Andersen, Ø. M. & Markham, K. R.) 397–442 (CRC Press, 2006).
Goławska, S., Kapusta, I., Łukasik, I. & Wójcicka, A. Effect of phenolics on the pea aphid, Acyrthosiphon pisum (Harris) population on Pisum sativum L. (Fabaceae). Pestycydy. 3–4, 71–77 (2008).
Goławska, S. & Łukasik, I. Antifeedant activity of luteolin and genistein against the pea aphid, Acyrthosiphon pisum. J. Pest Sci. 85, 443–450 (2012).
Goławska, S. et al. Alfalfa (Medicago sativa L.) apigenin glycosides and their effect on the pea aphid (Acyrthosiphon pisum). Polish J. Environ. Stud. 19, 913–919 (2010).
Johnson, A. D. & Singh, A. Larvicidal activity and biochemical effects of apigenin against filarial vector Culex quinquefasciatus. Int. J. Life. Sci. Sci. Res. 3, 1315–1321 (2017).
Boué, S. M. & Raina, A. K. Effects of plant flavonoids on fecundity, survival, and feeding of the formosan subterranean termite. J. Chem. Ecol. 29, 2575–2584 (2003).
Xu, D. et al. Antifeedant activities of secondary metabolites from Ajuga nipponensis against adult of striped flea beetles, Phyllotreta striolata. J. Pest Sci. 82, 195–202 (2009).
Goławska, S., Sprawka, I. & Łukasik, I. Effect of saponins and apigenin mixtures on feeding behavior of the pea aphid, Acyrthosiphon pisum Harris. Biochem. Syst. Ecol. 55, 137–144 (2014).
Zavala, J. A., Scopel, A. L. & Ballaré, C. L. Effects of ambient UV-B radiation on soybean crops: Impact on leaf herbivory by Anticarsia gemmatalis. Plant Ecol. 156, 121–130 (2001).
Bentivenha, J. P. F. et al. Role of the rutin and genistein flavonoids in soybean resistance to Piezodorus guildinii (Hemiptera: Pentatomidae). Arthropod Plant Interact. 12, 311–320 (2018).
Hoffmann-Campo, C. B., Harborne, J. B. & McCaffery, A. R. Pre-ingestive and post-ingestive effects of soya bean extracts and rutin on Trichoplusia ni growth. Entomol. Exp. Appl. 98, 181–194 (2001).
Yuan, E. et al. Increases in genistein in Medicago sativa confer resistance against the Pisum host race of Acyrthosiphon pisum. Insects. 10, 97. https://doi.org/10.3390/insects10040097 (2019).
Meng, F. et al. QTL underlying the resistance to soybean aphid (Aphis glycines Matsumura) through isoflavone-mediated antibiosis in soybean cultivar ‘Zhongdou 27’. Theor Appl. Genet. 123, 1459–1465 (2011).
Murakami, S. et al. Insect-induced daidzein, formononetin and their conjugates in soybean leaves. Metabolites 4, 532–546 (2014).
Lattanzio, V. et al. Role of endogenous flavonoids in resistance mechanism of Vigna to aphids. J. Agric. Food Chem. 48, 5316–5320 (2000).
Delgado-Núñez, E. J. et al. Isorhamnetin: A nematocidal flavonoid from Prosopis laevigata leaves against Haemonchus contortus eggs and larvae. Biomolecules 10, 773 (2020).
Gómez, J. D., Vital, C. E., Oliveira, M. G. A. & Ramos, H. J. O. Broad range flavonoid profiling by LC/MS of soybean genotypes contrasting for resistance to Anticarsia gemmatalis (Lepidoptera: Noctuidae). PLoS ONE https://doi.org/10.1371/journal.pone.0205010 (2018).
Khan, M. A. M., Ulrichs, C. & Mewis, I. Effect of water stress and aphid herbivory on flavonoids in broccoli (Brassica oleracea var. italica Plenck). J. Appl. Bot. Food Qual. 84, 178–182 (2011).
Bale, J. S., Ponder, K. L. & Pritchard, J. Coping with stress. In Aphids as Crop Pests (eds van Emden, H. F. & Harrington, R.) 287–309 (CABI, 2007).
Atteyat, M., Abu-Romman, S., Abu-Darwish, M. & Ghabeish, I. Impact of flavonoids against woolly apple aphid, Eriosoma lanigerum (Hausmann) and its sole parasitoid, Aphelinus mali (Hald). J. Agric. Sci. 4, 227–236 (2012).
Tjallingii, W. F. & Hogen Esch, T. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 18, 317–328 (1993).
Cherqui, A. & Tjallingii, W. F. Salivary proteins of aphids, a pilot study on identification, separation and immunolocalisation. J. Insect Physiol. 46, 1177–1186 (2000).
Silva-Sanzana, C., Estevez, J. M. & Blanco-Herrera, F. Influence of cell wall polymers and their modifying enzymes during plant–aphid interactions. J. Exp. Bot. 71, 3854–3864 (2020).
Alvarez, A. E. et al. Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to aphid Myzus persicae. Entomol. Exp. Appl. 121, 145–157 (2006).
Alvarez, A. E. et al. Infection of potato plants with potato leafroll virus changes attraction and feeding behaviour of Myzus persicae. Entomol. Exp. Appl. 125, 135–144 (2007).
Machado-Assefh, C. R. & Alvarez, A. E. Probing behavior of aposymbiotic green peach aphid (Myzus persicae) on susceptible Solanum tuberosum and resistant Solanum stoloniferum plants. Insect Sci. 25, 127–136 (2018).
Common Catalogue of Varieties of Agricultural Plant Species [CCA]. 37th complete edition. Official Journal of the European Union C 13/1 (2019). Accessed 23 May 2021.
Porejestrowe doświadczalnictwo odmianowe. Charakterystyka odmian. http://www.coboru.gov.pl/Polska/Rejestr/odm_w_rej.aspx?kodgatunku=SOS. Accessed 23 May 2021.
Meier, U. Growth stages of mono- and dicotyledonous plants: BBCH. Monograph (Julius Kühn-Institut, 2018).
Beer, K., Joschinski, J., Sastre, A. A., Kraus, J. & Helfrich-Forster, C. A damping circadian clock drives weak oscillations in metabolism and locomotor activity of aphids (Acyrthosiphon pisum). Sci. Rep. 7, 1–5. https://doi.org/10.1038/s41598-017-15014-3 (2017).
Joschinski, J., Beer, K., Helfrich-Forster, C. & Krauss, J. Pea aphids (Hemiptera: Aphididae) have diurnal rhythms when raised independently of a host plant. J. Insect. Sci. 16, 1–5 (2016).
Graham, T. L. Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol. 95, 594–603 (1991).
Lee, J. H. et al. Characterization of isoflavones accumulation in developing leaves of soybean (Glycine max) cultivars. J. Korean Soc. Appl. Biol. Chem. 52(2), 139–143 (2009).
Magarelli, G. et al. Rutin and total isoflavone determination in soybean at different growth stages by using voltammetric methods. Microchem. J. 117, 149–155 (2014).
Perlatti, B. et al. Application of a quantitative HPLC-ESI-MS/MS method for flavonoids in different vegetables matrices. J. Braz. Chem. Soc. 27(3), 475–483 (2016).
Biesaga, M. & Pyrzyńska, K. Stability of bioactive polyphenols from honey during different extraction methods. Food Chem. 136, 46–54 (2013).
Sergiel, I., Pohl, P. & Biesaga, M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 145, 404–408 (2014).
Author information
Authors and Affiliations
Contributions
K.S., B.K., I.S., M.B., J.M., B.G. conceived, designed, and conducted research; J.B. performed statistical analysis, K.S. and B.G. wrote the manuscript, all Authors contributed to analysis of data, read and approved manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stec, K., Kordan, B., Sergiel, I. et al. Antixenosis in Glycine max (L.) Merr against Acyrthosiphon pisum (Harris). Sci Rep 11, 15289 (2021). https://doi.org/10.1038/s41598-021-94703-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94703-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.