Abstract
Given that nonadherence is related to subject characteristics and drug tolerance and preserved eye drops tend to be more intolerable than preservative-free ones, we conducted a phase 4, parallel-grouped, investigator-blind, active-control, randomized, multicenter study. A total of 51 patients with intraocular pressure (IOP) ≥ 15 mmHg diagnosed with open-angle glaucoma or ocular hypertension were randomly assigned to the preserved latanoprost group (n = 26) and the preservative-free latanoprost group (n = 25). The efficacy variables were corneal/conjunctival staining grade, Ocular Surface Disease Index (OSDI), adherence at 12 weeks after the first administration; corneal/conjunctival staining grade at 4 weeks; and IOP, tear break-up time (TBUT), and hyperemia score at 4 and 12 weeks. The safety variables included visual acuity and drug tolerance questionnaire results. There was no statistically significant difference in corneal/conjunctival staining grade, OSDI, or TBUT between the groups at 4 and 12 weeks. However, the adherence rate was higher and the hyperemia score was lower in the preservative-free group than in the preserved group. The severity and duration of stinging/burning sensation were lower in the preservative-free group than in the preserved group. Overall, preservative-free latanoprost showed better ocular tolerance assessed by hyperemia scores and stinging/burning symptoms following higher adherence than preserved latanoprost.
Similar content being viewed by others
Introduction
Glaucoma is the leading cause of global irreversible blindness, and the number of glaucoma patients aged over 40 years is estimated to increase to 111.8 million in 2040 worldwide1. Since the main strategy for treating glaucoma is adequate control of intraocular pressure (IOP) using antiglaucoma eye drops, physicians have been focused on how patients become more adherent to eye drop usage.
A large number of glaucoma patients experience ocular symptoms and signs upon and between instillation of antiglaucoma eye drops, which can affect the quality of life and adherence to therapy2,3,4. The ocular adverse effect appears to be the second most common reason for switching medication following low efficacy, which can lead to treatment failure and progression of visual function loss in glaucoma patients2.
Laboratory and clinical studies with benzalkonium chloride (BAK)-containing eye drops, an antimicrobial preservative, have shown a higher incidence of ocular signs and symptoms than BAK-free formulations5,6. The development of a preservative-free latanoprost eye drop is inevitable based on the known deleterious effects of BAK and the increasing interest in patients’ quality of life. A parallel-group noninferiority study comparing preserved and preservative-free latanoprost described that they have the same efficacy in terms of IOP control7. A study by Misiuk-Hojlo et al. showed that the rate of moderate-to-severe conjunctival hyperemia and subjective ocular symptoms were less likely to be seen during the 3-month follow-up after switching from preserved latanoprost to preservative-free latanoprost8. To the best of our knowledge, this is the first prospective parallel-grouped study that directly compared the ocular signs, symptoms, and adherence between the preserved and preservative-free latanoprost groups.
We assessed the corneal/conjunctival staining score, Ocular Surface Disease Index (OSDI, Allergan, Inc., Irvine, CA, USA) score, hyperemia score, tear break-up time (TBUT), adherence, and drug tolerance to compare the differences between the two groups.
Results
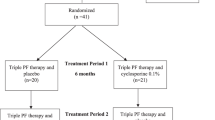
A total of 55 (29 in the preserved group and 26 in the preservative-free group) out of 57 patients who were screened (two failed on screening due to ‘withdrawal of consent’) were finally enrolled in the study (Fig. 1). Fifty-one patients (four were excluded due to ‘withdrawal of consent’ before safety assessment at visit 3) were included in the safety set and the intention-to-treat (ITT) set (26 in the preserved group and 25 in the preservative-free group).. Four out of 51 were excluded from the per-protocol (PP) set due to protocol violation (all four of them instilled eye drops in the morning at visit 4) and a low adherence rate (two of them showed an adherence rate of < 80%). There were no differences in the demographic features between the groups (Table 1).
Primary efficacy endpoint
There were no significant differences in corneal/conjunctival staining scores, or OSDI scores between the two groups in the ITT and PP sets at 12 weeks. The change in the adherence rate in the preservative-free group of the ITT set was increased at 12 weeks without statistical significance (3.72 ± 21.88%) compared with that of the preserved group (− 2.81 ± 6.66%), whereas the change showed statistical significance in the PP set (p = 0.019, 3.41 ± 10.82% vs. − 2.92 ± 6.77% in the preservative-free group vs. the preserved group, respectively, Fig. 2, Table 2). Analysis of covariance (ANCOVA) also supported the superiority (p = 0.021).
Secondary efficacy endpoint
No statistically significant differences in corneal/conjunctival staining score at 4 weeks, changes in IOP at 4 and 12 weeks, or TBUT at 4 and 12 weeks were noted. However, the sum of bulbar and limbal hyperemia scores of the preservative-free group at 12 weeks was significantly lower than that of the preserved group in the ITT set (ANCOVA p = 0.049, 1.88 ± 1.01 vs. 2.46 ± 1.24, respectively). In the PP set, only the bulbar hyperemia score of the preservative-free group at 12 weeks was significantly lower than that of the preserved group (ANCOVA p = 0.037, 0.86 ± 0.64 vs. 1.24 ± 0.66, respectively, Table 2).
Safety measure
The best-corrected visual acuity (BCVA) was significantly increased in the preservative-free group at 4 weeks and in both groups at 12 weeks. The severity and duration of stinging and burning sensation were significantly lower and shorter in the preservative-free group at 4 and 12 weeks than in the preserved group (ANCOVA, p < 0.001). Other symptoms, such as sticky sensation, itching, blurring, sandiness and grittiness, dryness, light sensitivity, and pain and soreness showed no difference between the preservative-free and preserved groups (Table 3).
The total incidence of adverse events (AEs) was 19.61% (10/51 patients, 13 cases). AEs were reported in 15.38% (4/26 patients, 4 cases) of the preserved group and 24% (6/25 patients, 9 cases) of the preservative-free group. The incidence of ocular surface disorders was 3.85% in the preserved group (1/26 patients, 1 case of dry eye) and 8% in the preservative-free group (2/25 patients, 1 case of dry eye, 1 case of epiphora).
Discussions
IOP control, which can be mainly achieved by effective and regular self-administration of glaucoma eye drops, is associated with reduced progression of visual field defects according to major large-scale clinical trials9,10,11. Nonadherence to glaucoma eye drops is a significant barrier to the successful treatment of glaucoma. If we put the limited value of self-reporting data aside, PF prostaglandin analogs are relatively novel options and employing them can be one step in the delivery of successful medical therapy balancing good efficacy with tolerability and adherence12. A recent survey-based study reported that preservative-free eye drops may provide benefits for adherence in relation to side effects. Individuals who experienced side effects with glaucoma eye drops reported higher rates of nonadherence than those who did not (37.6% vs. 18.4%; p = 0.004)11. The self-reported nonadherence rates were 32.0%, 25.0%, and 12.5% for the preserved eye drops, combined preserved and preservative-free eye drops, and preservative-free eye drops only groups, respectively. Interestingly, even the combination of preservative-free eye drops with preserved eye drops can also decrease the rate of nonadherence. The most common side effects of glaucoma eye drops were burning sensation (49.6%), and redness (39.2%). Although the vast majority of glaucoma patients reported a high degree of satisfaction with their current eye drops with or without experiencing side effects, the nonadherence rate was significantly lower in the no side effects group, indicating that the avoidance of even minor side effects can be beneficial to patients.
Given the differences between the unit-dose pipettes and the multiple-use bottles, one can assume that the type of eye drop container might affect patient adherence. Na et al. reported that the proportions of proper consumers of glaucoma eye drops were higher in the unit groups than in the bottle groups. In their study, three quarters or more of subjects in the unit groups were in the narrower range of proper consumption, suggesting that prescribing eye drops with unit-dose pipettes could lead to more consistent consumption of medication13. Proper consumption is also important for the study design itself since the change of adherence, side effects, and drug tolerance can all be affected by it. We assume that the lack of statistical significance in the ITT set of our study despite the positive change in adherence rate (Fig. 2) was due to outliers influenced by improper consumers (protocol violations) whereas statistical significance was seen in the PP set. Due to the limitation of our study design, we were not able to determine the extent to which the container affected adherence. Further studies are needed to verify this hypothesis.
A number of studies have reported that chronic application of preserved eye drops induces significant detrimental effects on the ocular surface. BAK, the most commonly used preservative in eye drops to date, was originally introduced by Gustav Raupenstrauch as an antiseptic disinfectant in Germany for the control of the Cholera pandemic in 1889. BAK is highly toxic to fish (LC50 = 280 μg a.i./L), moderately toxic to birds (LD50 = 136 mg/kg bw), and slightly toxic to mammals (LD50 = 430 mg/kg bw) on an acute exposure basis14. BAK disrupts the lipid layer of the cell membrane of pathogens, such as Vibrio cholerae whereas it also exerts harmful effects on the ocular surface when added to eye drops by destabilizing the tear film, causing inflammation, squamous metaplasia, and fibrotic changes in the conjunctiva5,15,16,17,18.
Despite the studies supporting the toxic effect of BAK on the ocular surface, the beneficial aspect of using BAK in eye drops is still debated. The assumption that BAK can improve the pharmacologic effects of antihypertensive agents as a penetration enhancer through the cornea has been contradicted by a considerable number of clinical studies19,20. BAK-free travoprost showed a similar IOP-lowering effect as BAK-containing travoprost21. A comparative study reported that there was no significant difference in the tafluprost concentration in rabbit aqueous humor between BAK-free and BAK-containing eye drops22. Among the preserved glaucoma eye drops, latanoprost and its generics contain higher concentrations of BAK (0.02%) than all the other monotherapy eye drops, which draws the conceptualization of this study.
In our study, there were no significant differences in corneal/conjunctival staining scores, or OSDI scores between the preserved and non-preserved groups at all measurements. However, this finding does not necessarily mean that BAK is not toxic to the ocular surface; preferably, it could mean that the acute detrimental effect of BAK does not last long if the exposure time is a fairly short term. A recent study described that preserved latanoprost caused a significantly acute decrease in transepithelial electric resistance (TER) measurement of the corneal epithelium at 1 min after the first instillation23. Surprisingly, the decrease disappeared at 24 h as well as at 1 week after once-daily application of the preserved latanoprost, and these findings were confirmed by scanning electron microscopy analyses. TER reflects the barrier function of the corneal epithelium; therefore, corneal TER is considered suitable for the quantitative assessment of corneal permeability and irritancy. This regenerative power seems to be a repetitive process during the daily exposures to BAK and it might be the reason why the ocular surface findings and OSDI scores failed to show significant differences since the follow-up period in this study was 1 month and 3 months from the first visit.
Alternatively, the detrimental effect on the corneal surface might have been more significant if the follow-up period was sufficiently long to result in chronic changes, given that BAK toxicity is cumulative. Moreover, the majority of the subjects in our study (except four subjects in the PP set) were naive to glaucoma treatment, which might explain why their ocular surface was less vulnerable than expected. The benefit will be far greater when multiple medications are used24. This needs to be addressed in the future as latanoprost eye drops are most commonly prescribed eye drops in many countries, including South Korea25. In the same vein, a systematic review is not enough to explain a global switch from preserved eye drops to PF eye drops, although a meta-analysis has shown that there are no clinically significant differences in ocular hyperemia or tear break-up time26. The lack of difference may be due to variations in design, study quality, and outcome definition.
This study used a parallel-group design instead of a crossover design. The crossover design requires a much smaller number of patients for similar statistical power because subjects act as their own controls, resulting in a lower financial cost and exposure of fewer patients to each agent. In contrast, there is a theoretical risk that the effects of the first intervention might carry over into the second intervention, possibly confounding the detection of effects27. The parallel-group design is more versatile in a study with relatively stable disease if a multicenter approach is possible. Therefore, we conducted a parallel study that was more beneficial for investigating the effect of eye drops on ocular surface status. In addition, a preemptive validation of ocular assessment was performed to minimize potential bias between the examiners.
In conclusion, preservative-free latanoprost shows better ocular tolerance assessed by hyperemia scores and stinging and burning symptoms following higher adherence than preserved latanoprost in open-angle glaucoma or ocular hypertensive eyes with unfailingly comparable IOP-lowering effects. Close examination of the detrimental effect of BAK on the ocular surface should be verified by a long-term study design, since glaucoma medications are normally considered a chronic option.
Methods
Subjects enrollment and study design
This study was a parallel-grouped, investigator-blind, active-control, randomized, multicenter (four institutions), and phase 4 clinical trial approved by the Institutional Review Board of CHA Bundang Medical Center on 20/02/2019, which fully adhered to the Declaration of Helsinki. The study was registered on clinicaltrials.gov on 06/02/2021 and posted on 08/02/2021 as NCT04743622. All subjects provided informed consent before the screening. Patients were randomized into two groups; a preservative-free latanoprost (Monoprost, Samil Pharmaceutical Company Ltd., Seoul, South Korea) group and preserved latanoprost (Xalatan, Pfizer Inc., Puurs, Belgium) group according to the interactive web-based randomization system (IWRS, TnW software Ltd., Seoul, South Korea) running 24 h a day during the whole study period. All the variables were uploaded to web-based e-CRF (case report form, http://www.ecrf.kr, ver 1.0, TnW software Ltd., Seoul, South Korea) software. All investigators were blinded throughout the whole study period due to the same external package for the investigation product, but the participants could know after receiving and unpacking the contents. Patients were instructed to instill eye drops once daily at 9 PM (± 1 h) from day 0 and were instructed to visit the clinic at 4 and 12 weeks (Fig. 3).
Patients aged 19 years or older with open-angle glaucoma or ocular hypertension were enrolled in four different institutions from April 2019 to June 2020. Glaucomatous changes in the eye were confirmed by reproducible glaucomatous visual field defects corresponding to typical optic disc/retinal nerve fiber layer changes by glaucoma specialists. All subjects underwent a full ophthalmologic examination including BCVA, Goldmann applanation tonometry by masked examiner(s) in each institution, central corneal thickness (CCT), gonioscopy, visual field test using a Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA), fundus photography, red-free photography, and spectral-domain optical coherence tomography.
The Inclusion criterion was an IOP of 15 ~ 40 mmHg in at least one eye at the screening visit after the proper period for washout (e.g., cholinergic eye drops and carbonic anhydrase inhibitors for 5 days and all other glaucoma eye drops for 4 weeks). Patients were excluded if their BCVA was worse than 20/80; if their CCT was not within 470–591 μm; if they had any eye disease (e.g., ischemic optic neuropathy, proliferative diabetic retinopathy, age-related macular degeneration, etc.) that could affect the visual field results significantly, if they had active ocular inflammatory conditions; if they had lacrimal punctal occlusion procedures in the past 3 months; if they needed to use eye drops for severe dry eye disease with hyaluronic acid, cyclosporine, or diquafosol; or if they were pregnant or currently nursing.
Outcome measures
According to the aim of this study, the primary endpoints were the difference in the corneal staining grade (assessed by the Oxford grading system; 0–5), conjunctival staining grade (assessed by the NEI scale; the conjunctiva was divided into 6 areas; 0–3 for each area), OSDI score, and adherence change at 12 weeks between the preserved and preservative-free groups. Adherence was assessed using a self-report sheet, which was collected at 4 and 12 weeks (0–100%)28,29,30. The secondary endpoints were the difference in corneal staining grade, conjunctival staining grade, OSDI score, TBUT, and hyperemia score (assessed using the Efron grading scale; 0–4) at 4 weeks and the difference in IOP, TBUT, and hyperemia score at 12 weeks between the two groups31,32.
The safety outcome measures included BCVA, AEs, and drug tolerance. Drug tolerance data were acquired using a drug tolerance questionnaire sheet to collect the frequency and severity of the symptoms that occurred after the instillation of eye drops, such as stinging/burning, sticky sensation, itching, blurring, sandiness/grittiness, dryness, light sensitivity, and pain/soreness. The level of each symptom was instructed to range from 0 (none) to 3 (severe, which immensely interferes with the subject’s daily life). The duration of each symptom was graded as 0 (prompt: < 5 min) or 1 (continuous: ≥ 5 min).
Statistical analysis
This study aimed to evaluate the superiority of preservative-free latanoprost over preserved latanoprost in terms of ocular surface conditions such as corneal/conjunctival staining, and hyperemia score. The superiority test was based on the 95% confidence interval of the difference between the two groups using an independent t test. Although there was no equally designed study similar to ours, superiority was concluded if the difference in the hyperemia score was 0.82 or more according to a study that evaluated the difference in eye redness before and after switching the subjects’ eye drop from preserved latanoprost to preservative-free latanoprost8. Given that a standard deviation of 1.0 for the hyperemia score was proposed assuming a dropout rate of 20%, a total of 62 patients (31 in each group) should be enrolled to provide 80% power for the superiority calculation. To maximize the accuracy of the assessment among all investigators, a blinded person created a validation image set of conjunctival hyperemia, which was used to check the agreement between each investigator. The Kendall’s taus of bulbar and limbal hyperemia scores between each assessor were 0.563–0.939 (p < 0.019) and 0.662–0.977 (p < 0.026), generally representing a moderate-to-high correlation.
Comparisons of the primary/secondary efficacy and safety endpoints were performed using ANCOVA to explore any possible effect of the dropout pattern on the analysis after searching for potentially significant variables using an independent t-test. Discrete or categorical variables were compared using chi-square analysis. A paired t-test was used to compare the V4–V3 change in the adherence rate between the two groups. All analyses were performed using PASW software (version 18.0; SPSS, Inc., Chicago, IL, USA). Statistical significance was set at p < 0.05.
Efficacy and safety assessments were performed using ITT and PP. Only patients who did not violate the protocol with an adherence rate of more than 80% were included in the PP set.
Data availability
All relevant data are within the paper.
References
Tham, Y. C. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121(11), 2081–2090 (2014).
Zimmerman, T. J. H. S., Gelb, L., Tan, H. & Kim, E. E. The impact of ocular adverse effects in patients treated with topical prostaglandin analogs: Changes in prescription patterns and patient persistence. J. Ocul. Pharmacol. Ther. 25, 145–152 (2009).
Rossi, G. C. M. P. G., Scudeller, L. & Bianchi, P. E. Ocular surface disease and glaucoma: How to evaluate impact on quality of life. J. Ocul. Pharmacol. Ther. 29, 390–394 (2013).
Lemij, H. G. H. J. & van der Windt, C. Patient satisfaction with glaucoma therapy: Reality or myth?. Clin. Ophthalmol. Auckl. N.Z. 9, 785–793 (2015).
Jaenen, N. B. C., Pouliquen, P., Manni, G., Figueiredo, A. & Zeyen, T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur. J. Ophthalmol. 86(4), 418–423 (2007).
LA Baudouin, C., Liang, H., Pauly, A. & Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 29, 312–314 (2010).
Rouland, J. F. et al. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br. J. Ophthalmol. 97(2), 196–200 (2013).
Misiuk-Hojlo, M. et al. The RELIEF study: Tolerability and efficacy of preservative-free latanoprost in the treatment of glaucoma or ocular hypertension. Eur. J. Ophthalmol. 29(2), 210–215 (2019).
Van Veldhuisen P. E. F. Gaasterland, D. E. et al. The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 130, 429–440 (2000).
Society EG. Terminology and guidelines for glaucoma. Savona: Publicomm srl (2014).
Wolfram, C., Stahlberg, E. & Pfeiffer, N. Patient-reported nonadherence with glaucoma therapy. J. Ocul. Pharmacol. Ther. 35(4), 223–228 (2019).
Hollo, G., Katsanos, A., Boboridis, K. G., Irkec, M. & Konstas, A. G. P. Preservative-free prostaglandin analogs and prostaglandin/timolol fixed combinations in the treatment of glaucoma: Efficacy. Saf. Potential Adv. Drugs 78(1), 39–64 (2018).
Na, K. Y. C., Park, J. & Kim, Y. Y. Eye drop dispenser type and medication possession ratio in patients with glaucoma: Single-use containers versus multiple-use bottles. Am. J. Ophthalmol. 188, 9–18 (2018).
U.S. Environmental Protection Agency Office of Prevention P, and Toxic Substances. Reregistration Eligibility Decision For Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC). Frank T Sanders. (2009).
Baudouin, C. Detrimental effect of preservatives in eyedrops: Implications for the treatment of glaucoma. Acta Ophthalmol. 86(7), 716–726 (2008).
Rossi, G. C. P. G., Scudeller, L., Raimondi, M., Lanteri, S. & Bianchi, P. E. Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur. J. Ophthalmol. 23, 296–302 (2013).
Pisella, P. J. P. P. & Baudouin, C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br. J. Ophthalmol. 86, 418–423 (2002).
PB P. BENZALKONIUM CHLORIDE (ZEPHIRAN CHLORIDE®) AS A SKIN DISINFECTANT. Arch Surg. 61, 23–33 (1950).
Okabe, K. K. H. & Okabe, J. Effect of benzalkonium chloride on transscleral drug delivery. Invest. Ophthalmol. Vis. Sci. 46, 703–708 (2005).
Majumdar, S. H. K. & Repka, M. A. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int. J. Pharm. 348, 175–178 (2008).
Gross, R. L. P. J. et al. Duration of IOP reduction with travoprost BAK-free solution. J. Glaucoma 17, 217–222 (2008).
Pellinen, P. L. J. Corneal penetration into rabbit aqueous humor is comparable between preserved and preservative-free tafluprost. Ophthalmic Res. 41, 118–122 (2009).
Inoue, D. M., Mohamed, Y. H., Uematsu, M. & Kitaoka, T. Corneal damage and its recovery after instillation of preservative-free versus preserved latanoprost eye drops. Cutan. Ocul. Toxicol. 39, 158–64 (2020).
Konstas, A. G. et al. The treatment of glaucoma using topical preservative-free agents: An evaluation of safety and tolerability. Expert Opin. Drug Saf. 20(4), 453–466 (2021).
Kim, C. Y. et al. Treatment patterns and medication adherence of patients with glaucoma in South Korea. Br. J. Ophthalmol. 101(6), 801–807 (2017).
Hedengran, A., Steensberg, A. T., Virgili, G., Azuara-Blanco, A. & Kolko, M. Efficacy and safety evaluation of benzalkonium chloride preserved eye-drops compared with alternatively preserved and preservative-free eye-drops in the treatment of glaucoma: A systematic review and meta-analysis. Br. J. Ophthalmol. 104(11), 1512–1518 (2020).
Richens, A. Proof of efficacy trials: Cross-over versus parallel-group. Epilepsy Res. 45(1–3), 43–47 (2001).
Amparo FWH, Yin J, Marmalidou A, & Dana R. Evaluating corneal fluorescein staining using a novel automated method. Invest. Ophthalmol. Vis. Sci. 58, BIO168–BIO173 (2017).
Bron Aj, E. V. & Smith, J. A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 22, 640–650 (2003).
Lemp, M. A. Report of the National Eye Institute/Industry workshop on clnical trials in dry eyes. CLAO J. 21, 221–232 (1995).
Efron N. Clinical application of grading scales for contact lens complications. Optician 213, 26–34 (1997).
Begley, C. et al. Review and analysis of grading scales for ocular surface staining. Ocul. Surf. 17(2), 208–220 (2019).
Acknowledgements
This study was funded by Samil Pharmaceutical Company Ltd.; however, the design, data collection, statistical analyses, and drafting of this manuscript were solely conducted by the authors.
Author information
Authors and Affiliations
Contributions
Conception and design of the study (S.R.); collection and management of data (D.W.K., J.S., C.L., M.K., S.L.); analysis and interpretation of data (C.L., M.K., S.R.); writing of the article (D.W.K., J.S., S.R.); and approval of manuscript (D.W.K., J.S., C.L., J.S., S.R.).
Corresponding author
Ethics declarations
Competing interests
This study was funded by Samil Pharmaceutical Company Ltd., Seoul, South Korea. All the authors declare no other competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, D.W., Shin, J., Lee, C.K. et al. Comparison of ocular surface assessment and adherence between preserved and preservative-free latanoprost in glaucoma: a parallel-grouped randomized trial. Sci Rep 11, 14971 (2021). https://doi.org/10.1038/s41598-021-94574-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94574-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.