Abstract
Objective
Halting and reversing glaucoma therapy-related ocular surface disease (GTR-OSD) will improve the success of long-term medical therapy, impacting millions of patients worldwide.
Methods
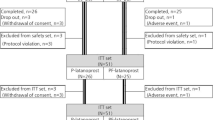
A single-centre, masked, prospective, placebo-controlled, crossover trial of 41 well-controlled open-angle glaucoma subjects with moderate to severe GTR-OSD on preserved latanoprost and dorzolamide/timolol fixed combination (DTFC) therapy was conducted. Subjects were randomized to preservative-free (PF) tafluprost and DTFC with either placebo or cyclosporine 0.1% drops for 6 months and were then crossed over to the opposite therapy. Oxford score of ocular staining was the primary outcome; osmolarity, matrix-metalloproteinase-9 (MMP-9) testing, tear film break-up time (TFBUT), meibomian gland dysfunction (MGD), punctum evaluation, adverse events and diurnal intraocular pressure (IOP) comprised secondary outcomes.
Results
GTR-OSD findings improved with PF therapy. At 6 months the triple PF with placebo group showed improvement compared to baseline in mean Oxford score (mean difference [MD]:−3.76; 95% confidence interval [CI]:−4.74 to −2.77; p < 0.001), osmolarity (MD:−21.93; 95%CI:−27.61 to −16.24 mOsm/l; p < 0.001), punctum stenosis (p = 0.008) and conjunctival hyperaemia (p < 0.001). Similar improvements occurred in the cyclosporine enhanced period, which also provided greater improvement in MMP-9 positivity (24 vs 66%; p < 0.001) and TFBUT (p = 0.022). The cyclosporine group was superior vs placebo in mean Oxford score (MD:−0.78; 95%CI:−1.40 to −0.15); p < 0.001), itchiness and objective adverse events (p = 0.034). Cyclosporine elicited more stinging vs placebo (63 vs 24%; p < 0.001). Both PF regimens reduced mean diurnal IOP more than preserved therapy (14.7 vs 15.9 mmHg; p < 0.001).
Conclusions
Changing from preserved to PF glaucoma medications improves ocular surface health and IOP control. Topical cyclosporine 0.1% further reverses GTR-OSD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–34.
Banitt M, Jung H. Ocular surface disease in the glaucoma patient. Int Ophthalmol Clin. 2018;58:23–33.
Wong ABC, Wang MTM, Liu K, Prime ZJ, Danesh-Meyer HV, Craig JP. Exploring topical anti-glaucoma medication effects on the ocular surface in the context of the current understanding of dry eye. Ocul Surf. 2018;16:289–93.
Clayton JA. Dry eye. N. Engl J Med. 2018;378:2212–23.
Pflugfelder SC, Baudouin C. Challenges in the clinical measurement of ocular surface disease in glaucoma patients. Clin Ophthalmol. 2011;5:1575–83.
Holló G, Katsanos A, Boboridis KG, Irkec M, Konstas AGP. Preservative-free prostaglandin analogs and prostaglandin/timolol fixed combinations in the treatment of glaucoma: efficacy, safety and potential advantages. Drugs. 2018;78:39–64.
Zhang X, Vadoothker S, Munir WM, Saeedi O. Ocular surface disease and glaucoma medications: a clinical approach. Eye Contact Lens. 2019;45:11–8.
Figus M, Agnifili L, Lanzini M, Brescia L, Sartini F, Mastropasqua L, et al. Topical preservative-free ophthalmic treatments: an unmet clinical need. Expert Opin Drug Deliv. 2021;18:655–72.
Stalmans I, Lemij H, Clarke J, Baudouin C on behalf of the GOAL study group. Signs and symptoms of ocular surface disease: the reasons for patient dissatisfaction with glaucoma treatments. Clin Ophthalmol. 2020;14:3675–80.
Wolfram C, Stahlberg E, Pfeiffer N. Patient-reported nonadherence with glaucoma therapy. J Ocul Pharm Ther. 2019;35:223–8.
Konstas AGP, Labbe A, Katsanos A, Meier-Gibbons F, Irkec M, Boboridis KG, et al. The treatment of glaucoma using topical preservative-free agents: an evaluation of safety and tolerability. Expert Opin Drug Saf. 2021;20:453–66.
Goldstein MH, Silva FQ, Blender N, Tran T, Vantipalli S. Ocular benzalkonium chloride exposure: problems and solutions. Eye. 2022;36:361–8.
Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112:1446–54.
Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma. 2013;22:730–5.
Chamard C, Larrieu S, Baudouin C, Bron A, Villain M, Daien V. Preservative-free versus preserved glaucoma eye drops and occurrence of glaucoma surgery. A retrospective study based on the French national health insurance information system, 2008-2016. Acta Ophthalmol. 2020;98:e876–881.
European Glaucoma Society. Terminology and guidelines for glaucoma. 5th ed. Savona, Italy: PubliComm; 2020.
Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R, CIGTS Study Group. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118:1766–73.
Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–5.
Portela RC, Fares NT, Machado LF, São Leão AF, de Freitas D, Paranhos A Jr, et al. Evaluation of ocular surface disease in patients with glaucoma: clinical parameters, self-report assessment, and keratograph analysis. J Glaucoma. 2018;27:794–801.
Boboridis KG, Konstas AGP. Evaluating the novel application of cyclosporine 0.1% in ocular surface disease. Expert Opin Pharmacother. 2018;19:1027–39.
Labbe A, Terry O, Brasnu E, Van Went C, Baudouin C. Tear film osmolarity in patients treated for glaucoma or ocular hypertension. Cornea. 2012;31:994e9.
Mohammed I, Kulkarni B, Faraj LA, Abbas A, Dua HS, King AJ. Profiling ocular surface responses to preserved and non-preserved topical glaucoma medications: A 2-year randomized evaluation study. Clin Exp Ophthalmol. 2020;48:973–82.
Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17:341–9.
Steven DW, Alaghband P, Lim KS. Preservatives in glaucoma medication. Br J Ophthalmol. 2018;102:1497–503.
Saini M, Dhiman R, Dada T, Tandon R, Vanathi M. Topical cyclosporine to control ocular surface disease in patients with chronic glaucoma after long-term usage of topical ocular hypotensive medications. Eye. 2015;29:808–14.
Zhou XQ, Wei RL. Topical cyclosporine A in the treatment of dry eye: a systematic review and meta-analysis. Cornea. 2014;33:760–7.
Kymionis GD, Bouzoukis DI, Diakonis VF, Siganos C. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol. 2008;2:829–36.
Lee SY, Wong TT, Chua J, Boo C, Soh YF, Tong L. Effect of chronic anti-glaucoma medications and trabeculectomy on tear osmolarity. Eye. 2013;27:1142–50.
Leonardi A, Messmer EM, Labetoulle M, Amrane M, Garrigue JS, Ismail D, et al. Efficacy and safety of 0.1% ciclosporin A cationic emulsion in dry eye disease: a pooled analysis of two double-masked, randomised, vehicle-controlled phase III clinical studies. Br J Ophthalmol. 2019;103:125–31.
Konstas AG, Kahook MY, Araie M, Katsanos A, Quaranta L, Rossetti L, et al. Diurnal and 24-h intraocular pressures in glaucoma: monitoring strategies and impact on prognosis and treatment. Adv Ther. 2018;35:1775–804.
Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–7.
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276–83.
Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Investig Ophthalmol Vis Sci. 2011;52:2006–49.
Kim DW, Seo JH, Lim SH. Evaluation of ocular surface disease in elderly patients with glaucoma: expression of matrix metalloproteinase-9 in tears. Eye. 2021;35:892–900.
Batra R, Tailor R, Mohamed S. Ocular surface disease exacerbated glaucoma: optimizing the ocular surface improves intraocular pressure control. J Glaucoma. 2014;23:56–60.
Dubrulle P, Labbé A, Brasnu E, Liang H, Hamard P, Meziani L, et al. Influence of treating ocular surface disease on intraocular pressure in glaucoma patients intolerant to their topical treatments: a report of 10 cases. J Glaucoma. 2018;27:1105–11.
Baudouin C, Denoyer A, Desbenoit N, Hamm G, Grise A. In vitro and in vivo experimental studies on trabecular meshwork degeneration induced by benzalkonium chloride (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2012;110:40–63.
Baudouin C, Messmer EM, Aragona P, Geerling G, Akova YA, Benítez-del-Castillo J, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100:300–6.
Periman LM, Mah FS, Karpecki PM. A review of the mechanism of action of cyclosporine A: the role of cyclosporine A in dry eye disease and recent formulation developments. Clin Ophthalmol. 2020;14:4187–200.
Schargus M, Ivanova S, Kakkassery V, Dick HB, Joachim S. Correlation of tear film osmolarity and 2 different MMP-9 tests with common dry eye tests in a cohort of non-dry eye patients. Cornea. 2015;34:739–44.
Acknowledgements
The authors would like to thank all medical staff of the 1st University Department of Ophthalmology and the patients who participated in this study.
Funding
This study was supported in part by Santen. The sponsor had no involvement in the design of the trial, the analysis or interpretation of results, or the publication of the paper.
Author information
Authors and Affiliations
Contributions
AGK and KGB contributed to the conception of the study, the examination of patients, and the preparation of the manuscript. GPA, ICV and EP contributed to the examination of patients and drafting parts of the manuscript. ABH contributed to the conception of the study, the statistical analysis and the preparation of the manuscript. AK contributed to the interpretation of results and the preparation of the manuscript. LJK contributed to the conception of the study, the interpretation of results and the preparation of the manuscript. All authors approved the submitted version of the manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
AGK: Research funding from Allergan, Bayer, Omni Vision, Pharmaten and Santen; travel support and congress expenses from Vianex and Zwitter; honoraria from Allergan, Esteve Pharmaceuticals, Santen and Vianex. KGB: Honoraria from Laboratoires Théa, Bausch & Lomb, Santen and Novartis. GPA, ABH, ICV and EP: No conflicts of interest. AK: Honoraria and congress expenses from Cooper SA, Santen, Vianex, Zwitter; research funding from Laboratoires Théa. LJK: Grants and research support from Allergan, Diopsys, Heidelberg Engineering, Alcon, Zeiss, Olleyes; consultant/advisory board: Olleyes (stock options); honoraria from Allergan, Glaukos, Bausch & Lomb; Stock shareholder: Glaukos, Mati Therapeutics, Aerie, Olleyes; employment (salary): Glaukos (Chief Medical Officer).
Ethics approval
The research protocol was approved by the Institutional Review Board of the Medical School of Aristotle University and was registered with ClinicalTrials.gov (NCT04673604). The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Konstas, AG., Boboridis, K.G., Athanasopoulos, G.P. et al. Changing from preserved, to preservative-free cyclosporine 0.1% enhanced triple glaucoma therapy: impact on ocular surface disease—a randomized controlled trial. Eye 37, 3666–3674 (2023). https://doi.org/10.1038/s41433-023-02578-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02578-w