Abstract
This study aimed to investigate the efficacy of the combination of neuron-specific enolase (NSE) measurement and initial neurological examination in predicting the neurological outcomes of patients with cardiac arrest (CA) by retrospectively analyzing data from the Korean Hypothermia Network prospective registry. NSE levels were recorded at 48 and 72 h after CA. The initial Full Outline of UnResponsiveness (FOUR) and Glasgow Coma Scale (GCS) scores were recorded. These variables were categorized using the scorecard method. The primary endpoint was poor neurological outcomes at 6 months. Of the 475 patients, 171 (36%) had good neurological outcomes at 6 months. The areas under the curve (AUCs) of the categorized NSE levels at 72 h, GCS score, and FOUR score were 0.889, 0.722, and 0.779, respectively. The AUCs of the combinations of categorized NSE levels at 72 h with categorized GCS scores and FOUR score were 0.910 and 0.912, respectively. Each combination was significantly higher than the AUC value of the categorized NSE level at 72 h alone (with GCS: p = 0.015; with FOUR: p = 0.026). Combining NSE measurement and initial neurological examination improved the prediction of neurological outcomes.
Similar content being viewed by others
Introduction
Many prognostication tools have been developed to predict the neurological state of patients with comatose mental status after out-of-hospital cardiac arrest (OHCA). However, no single test has an accuracy of 100%. Serological testing is a cheaper, easier, and more rapid prognosticator than imaging or electroencephalography (EEG) and benefits from not being influenced by the required administration of sedatives to patients1. The neuron-specific enolase (NSE) assay is the most promising and extensively studied serological test2. The increased level of NSE in comatose post-cardiac arrest patients with or without targeted temperature management (TTM) is associated with poor prognosis3,4. International guidelines suggest that NSE level alone should not be used to predict poor neurological outcomes because of the possibility of high false-positive rates5. If the NSE level alone is unreliable, it appears reasonable to use it in conjunction with another neurological test. Although several studies have reported attempts to combine NSE with an additional prognosticator for the improvement of diagnostic accuracy, it is unclear as to which prognosticator is best combined with NSE.
The Glasgow Coma Scale (GCS) and Full Outline of UnResponsiveness (FOUR) scale are well-known neurological grading scales that are essential for the examination of unconscious patients6. In previous studies, the combination of initial neurological examination and other prognostic tools showed better performance than either test alone in predicting neurological outcomes after OHCA6,7,8,9. However, the potential benefits accruing from a combination of initial neurological examination and the NSE assay have not been fully addressed. As the cutoff NSE level that is predictive of poor outcomes varies across studies, it might be difficult to employ specific cutoff NSE levels to evaluate the dichotomized prognosis of post-cardiac arrest patients and to combine them with other prognosticators2,4,5,10,11,12,13,14,15. Considering these limitations, it might be a good alternative to convert the continuous variables into ordered categories and then assign them differentiated scores. Several scoring systems based on this concept have been developed to predict neurological outcomes after cardiac arrest6,16. This study aimed to investigate whether the combination of initial neurological examination and the NSE assay using a scorecard method could improve the prediction of neurological outcomes in patients with OHCA.
Results
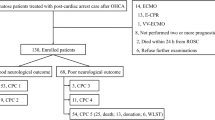
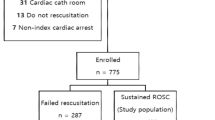
Of the 10,258 patients with OHCA, 1373 had their data recorded in the Korea Hypothermia Network prospective (KORHN-pro) registry. Of the 1373 patients, 898 patients were excluded for the following reasons: incomplete NSE-level data at 48 and 72 h after return of spontaneous circulation (ROSC; n = 839); incomplete FOUR scores after ROSC (n = 32); incomplete data concerning neurological outcomes at 6 months (n = 10); withdrawal of life-sustaining therapy (WLST) decision (n = 11); and initial GCS score > 8 (n = 6). The remaining 475 patients were eligible for participation in this study (Fig. 1).
Flow chart depicting the patient selection process. OHCA: out-of-hospital cardiac arrest; ROSC: return of spontaneous circulation; TTM: targeted temperature management; DNR: do not resuscitate; CPC: cerebral performance category; NSE: neuron-specific enolase; FOUR: Full Outline of UnResponsiveness; WLST: withdrawal of life-sustaining therapy; GCS: Glasgow Coma Scale.
Of the 475 patients, 171 (36%) had good neurological outcomes 6 months after ROSC. The median age of patients was 59 (interquartile range [IQR], 48–69) years, and 72.8% (346/475) of the patients were men. Analgesics, sedatives, and neuromuscular blocking agents were administered to 77.3% (367/475), 90.5% (430/475), and 85.9% (408/475) of patients, respectively. In the study population, the following tests were used for prognostication: brain computed tomography (CT), 453/475 (95.4%); measurement of somatosensory evoked potentials, 186/475 (39.2%); EEG, 297/475 (62.5%); and brain magnetic resonance imaging, 272/475 (57.3%).
Table 1 shows the demographic and clinical features of the patients stratified according to their neurological outcomes. Following ROSC, the total GCS score and FOUR score were higher in the good outcome group than in the poor outcome group (p < 0.001 for both scores). NSE levels at 48 and 72 h were higher in patients with poor outcomes than in those with good outcomes (p < 0.001 for both time points).
The results of the multivariate analyses are shown in Table 2. Each model confirmed that the NSE levels at 48 and 72 h after ROSC, GCS score, and FOUR score were independently associated with poor neurological outcomes at 6 months.
Supplementary Table S1 shows the associations between the 6-month neurological outcomes and strata of NSE level at 48 and 72 h, GCS score, and FOUR score. The proportion of patients with poor 6-month neurological outcomes increased as the NSE level increased at 48 and 72 h. The proportion of patients with poor 6-month neurological outcomes decreased as the GCS score and FOUR score increased. The weighted scores applied to each category of NSE levels at 48 and 72 h, GCS score, and FOUR score are shown in Supplementary Table S2.
Table 3 and Fig. 2 show the performance data of each predictor and their combinations in predicting 6-month poor neurological outcomes. For each categorized predictor and their combinations, the area under the curve (AUC) of the categorized NSE level at 48 and 72 h, GCS score, and FOUR score were 0.879 (95% confidence interval [CI], 0.846–0.907), 0.889 (95% CI 0.858–0.916), 0.722 (95% CI 0.680–0.762), and 0.779 (95% CI 0.739–0.816), respectively (Table 3). The AUCs of the combinations of the categorized NSE level at 72 h with the categorized GCS score and FOUR score were 0.910 (95% CI 0.885–0.936) and 0.912 (95% CI 0.886–0.938), respectively. Each combination was significantly higher than the AUC value of the NSE level at 72 h alone (with GCS, ΔAUC = 0.021 [95% CI 0.004–0.038], p = 0.0153; with FOUR, ΔAUC = 0.023 [95% CI 0.003–0.043], p = 0.0257). The predictive performances of the combinations of the categorized NSE level at 48 h with the categorized GCS score and FOUR score were also higher than the performance of any variable alone (Table 3).
Comparison of the receiver operating characteristic curves in combinations of categorized predictors. AUC for the combination of categorized FOUR score and categorized NSE 72 h: 0.912 (criteria > 11, sensitivity = 76.3%, specificity = 94.7%); for the combination of categorized FOUR score and categorized NSE 48 h: 0.906 (criteria > 11, sensitivity = 76.3%, specificity = 94.7%); and for the combination of categorized GCS score and categorized NSE 72 h: 0.910 (criteria > 10, sensitivity = 77.6%, specificity = 93.6%). Comparison of ROC curves with Bonferroni correction: FOUR + NSE 72 h vs. FOUR + NSE 48 h, p = 0.453; FOUR + NSE 72 h vs. GCS + NSE 72 h, p = 0.770; FOUR + NSE 48 h vs. GCS + NSE 72 h, p = 0.660. AUC, area under the curve; GCS, Glasgow Coma Scale; FOUR, Full Outline of UnResponsiveness; NSE, neuron-specific enolase.
The receiver operating characteristic (ROC) curves were also analyzed to determine the predictive performance of the original variables (continuous or nominal variables): serum NSE level, neurological examination scores, and their combinations. The results are presented in Table 3.
Discussion
This study showed that the combination of initial neurological examination and serum NSE assay is superior to either test alone for predicting poor neurological outcomes 6 months after cardiac arrest. We also demonstrated the feasibility of NSE values using weighted categorical values when combined with another prognostication tool.
Current international guidelines do not recommend the use of NSE level as the sole predictor in the prognostic assessment of patients with cardiac arrest5. Efforts have been made to combine the NSE level with another prognosticator for the prediction of neurological outcomes in patients with OHCA. Lee et al. reported that a combination of NSE level and quantitative parameters in brain CT improved prognostic performance when compared with either component alone in predicting poor neurological outcomes in patients with OHCA17. Ryoo et al. recently combined the NSE level at 48 h and lactate level measured after ROSC in the prognostic assessment of patients18; the combination yielded no synergic effect. Luescher et al. observed that NSE level measured on the third day following cardiac arrest significantly improved the clinical risk scores for outcome predictions19. Pfeifer et al. reported that the combination of NSE level at 72 h after cardiopulmonary resuscitation (CPR) and GCS score allows for a more reliable prediction of outcomes20. Our sample size of 475 patients was relatively larger than those of the aforementioned studies (97–336 patients), and the cutoff NSE levels used in the aforementioned studies also varied (41.8–82.5 ng/mL). In addition, in contrast to our study, the NSE level used in the combinations was treated as a continuous variable. In our study, weighted categorical values were used in combination with other predictors instead of absolute serum NSE cutoff values. Although the areas under the ROC (AUROCs) of the original continuous NSE values at 72 h and categorized NSE values at 72 h differed slightly (0.895 versus 0.889, p = 0.254), synergism through combination was maintained. In this study, the NSE assays performed at the participating hospitals were not uniform. Given the variability of the cutoff NSE value across previous studies and differences in NSE assay methods across institutions, it might be helpful to categorize the NSE value when applying it to the prognostication of post-resuscitation patients in clinical practice. Clinicians might already be familiar with ways to select and weigh distinct variables and to convert them to scores, as this concept has been incorporated into a risk prediction model in intensive care units (e.g., the Simplified Acute Physiology Score, Acute Physiology, and Chronic Health Evaluation score). In addition, several scoring models have been suggested for predicting the neurological outcomes of patients with cardiac arrest6,14.
Based on previous studies, no clinical neurological signs can reliably predict poor outcomes at < 24 h after cardiac arrest2. International guidelines do not recommend the use of neurological examinations in the early phases following ROSC8. TTM is usually administered to patients who are non-responsive to verbal commands or patients with coma after ROSC. Comatose mentality is defined as the state in which an individual has a GCS score of ≤ 8. Therefore, grades in each element of the GCS or FOUR scale might vary among patients with cardiac arrest who underwent TTM. Several studies have reported that the motor grade of GCS measured early after ROSC is associated with neurological prognosis7,21,22. The higher the patient's motor grade after ROSC, the better the patient’s neurological prognosis. Some studies have combined the initial FOUR score with other prognostic tools6,8,9. Youn et al. reported that combining initial brain stem reflex FOUR score with continuous EEG patterns is superior to any individual test in predicting survival after cardiac arrest8. Their subsequent study also revealed improved prognostic performance when the initial FOUR score was combined with the parameters of brain CT and continuous EEG patterns 9. In contrast with previous studies that used scores of only one element of the GCS and FOUR scale, we used the sum of the scores in each element of the GCS and FOUR scale. The total score might better reflect neurological prognosis than the individual elements of the neurological grading scales. In our study, the discriminative power of the total score of the two neurological grading scales was higher than that of their individual elements.
The FOUR scale includes additional information that is not assessed in the GCS, such as brainstem reflexes, visual tracking, breathing patterns, and respiratory drive6,8. Due to these differences between the FOUR scale and GCS, the predictive power of the FOUR score for poor outcomes might be superior to that of the GCS score. However, whether the advantages of the FOUR score remain when each neurological grading scale is combined with the NSE level has not been shown. This result might be attributed to the use of a small number of categories derived for both the neurological grading scales in the combination process, the similar AUC values of the element with the highest AUC in both coma scales, as well as the moderate association between brainstem reflex and motor response in the FOUR scale (r = 0.545). Few studies have compared the use of the GCS score and FOUR score to predict the prognosis of patients with cardiac arrest. Fugate et al. found that the FOUR score is an accurate predictor of outcomes in survivors of cardiac arrest, similar to the GCS score23. According to Weiss et al., the FOUR score provides a more accurate prognosis of poor neurological outcomes in patients with OHCA than does the GCS score24. However, Topcuoglu et al. reported results that conflict with those of Weiss et al.25.
Our study has some limitations. First, the possibility of selection bias cannot be ruled out because approximately 30% of the participating hospitals did not measure NSE levels during neurological prognostication. This could also limit the generalizability of the research results. However, baseline characteristics and neurological outcomes were similar when comparing included and excluded patients in the final analysis (Supplementary Table S3). Second, as the NSE level measured with the Roche method is reportedly 1.3 times higher than the NSE level measured using the Diasorin method26, our study is also limited by differences in serum NSE testing methods. Furthermore, information on hemolysis was not included in the registry. As NSE levels are influenced by hemolysis (a potential disadvantage of NSE), the lack of these data may also have diminished the validity of our findings. Third, although we excluded patients who elected WLST from the analysis, the results of the neurological examination and NSE level might be prone to the risk of self-fulfilling prophecy because treating physicians could not be blinded. This could have influenced treatment aggressiveness in patients who did not have WLST but were determined to have a poor prognosis. However, information on treatment aggressiveness of individual patients was not included in our registry. Fourth, the use of sedative drugs and neuromuscular blocking agents could have influenced the neurological examination. However, our registry did not include information regarding the timing, dosage, and duration of such pharmacotherapies administered after arrival at the hospital. Finally, the scorecard used in this study may need to be further refined using a more robust sample size, and external validation may be required.
In conclusion, the combination of categorized serum NSE levels and initial neurological examination improved the prediction of neurological outcomes 6 months after cardiac arrest compared with either test alone. Further studies are warranted to validate these findings.
Methods
Data resources and study setting
This was a retrospective analysis of data collected prospectively by the KORHN-pro registry from November 2015 to December 2018. Data were collected from patients who were admitted to 22 hospitals across South Korea. This study was approved by all participating hospitals, including the Institutional Review Board of Samsung Changwon Hospital (IRB No. SCMC 2015-10-055-099) and registered at a clinical trial registry platform (ClinicalTrials.gov Identifier: NCT02827422). Informed written consent was obtained from all patients enrolled in this study. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines and checklist27 and complied with the tenets of Declaration of Helsinki.
The Korean emergency medical service (EMS) system is operated exclusively by the National Fire Agency. EMS providers must continue resuscitation efforts until ROSC is achieved at the scene or until arrival at the hospital. The EMS level is basic-to-intermediate, and the use of sedatives by EMS providers is not permitted. All emergency departments generally provide advanced cardiac life support, acute cardiac care, and post-resuscitation care, including the administration of sedatives and neuromuscular blockers. The enrolled patients underwent TTM according to the protocol of each hospital.
The process for data management of the KORHN-pro registry has been described in several previous studies28,29,30. Prehospital, resuscitation, and outcome data were collected according to the Utstein style. The principal investigator from each participating hospital reviewed the hospital records of OHCA survivors who underwent TTM. Neurological outcomes at discharge and at 1 and 6 months after ROSC were investigated by researchers who were blinded to patient data. Neurological outcomes were evaluated using a telephone survey or face-to-face interview with the surviving discharged patients or their relatives. Three clinical research associates monitored the data and assessed their quality by sending queries to the investigators. Finally, a data manager examined the data and decided whether the records were acceptable or required revision.
Study population
The study included all patients with OHCA aged > 18 years, who were treated with TTM. The exclusion criteria were as follows: (1) confirmation of hemorrhagic or ischemic stroke as the cause of cardiac arrest, (2) cerebral performance category (CPC) of 3 or 4 before cardiac arrest, (3) body temperature < 30 °C upon arrival, (4) non-provision of post-resuscitation care, including TTM, (5) meaningful response to verbal commands following ROSC, (6) non-measurement of serum NSE level at 48 or 72 h after ROSC, (7) non-assessment of FOUR score or GCS score after ROSC, (8) initial GCS score > 8, (9) WLST, and (10) unknown neurological outcome at 6 months.
Data collection and endpoint
All data were extracted from the web-based registry. The variables investigated in this study were as follows: age, sex, medical history, place of cardiac arrest (public, non-public), witnessed cardiac arrest, bystander CPR, time from collapse to ROSC, initial monitored rhythm (shockable, non-shockable), prehospital defibrillation, causes of arrest (cardiac, non-cardiac), initial serum lactate level measured after ROSC, GCS score and FOUR score obtained within 1 h of ROSC, target temperature of TTM (< 35 °C, ≥ 35 °C), serum NSE levels measured at 48 and 72 h after ROSC, and CPC scores at 1 and 6 months after cardiac arrest. The total GCS score of intubated patients was calculated by assigning one point for verbal response. The neurological outcomes were dichotomized as good (CPC 1 or 2) or poor (CPC 3 through 5). The primary outcome was poor neurological outcome at 6 months after cardiac arrest.
NSE levels at 24, 48, and 72 h after ROSC were entered into the registry. Of the 22 sites, 16 sites provided data for this biomarker. Two different NSE measurement instruments were used at the 16 sites. Hemolysis index was not included separately in the registry data. The NSE levels at 24, 48, and 72 h were entered into the registry for 575 (41.9%), 620 (45.2%), and 565 patients (41.2%), respectively; NSE measurements were available at all three time points for 349 (25.4%) patients. Only patients whose NSE levels were measured at 48 and 72 h were included in the analysis because these time points are known to be associated with the highest sensitivities and specificities10.
Statistical analyses
Continuous variables are expressed as means with standard deviations or as medians and IQRs. Categorical variables are expressed as numbers and percentages. Demographic and clinical characteristics were compared between groups with good and poor neurological outcomes using the Student’s t-test, Mann–Whitney U test, Chi-squared test, or Fisher’s exact test, as appropriate.
Multivariate analyses were performed to identify independent predictors of poor neurological outcomes after adjusting for potential confounders. All variables shown in Table 1 were included in the multivariate model. NSE levels at 48 and 72 h after ROSC and GCS score and FOUR score obtained within 1 h of ROSC were confirmed for independent variables in multivariate analyses. Continuous variables were then converted to categorical variables by rounding up or down, whichever was appropriate after reaching the cutoff, using the R software optimal binning method (“smbinning” package), based on the reference variable of neurological outcomes at 6 months. The odds ratio (OR) and beta-coefficient of these variables in the unadjusted analyses were used to derive the scorecard. Weighted scores were assigned an integer value based on the relative magnitude of the OR and beta-coefficient with fixed point to double odds of 1.5. Scores were then adjusted for each category of the NSE level, GCS score, and FOUR score to ensure that total scores increased correspondingly with categories of predicted probabilities. After applying a weighted score for each category of the NSE level, GCS score, and FOUR score, we determined the AUC of each categorized variable using the ROC curve analysis. The AUC of the combination of the categorized NSE level and categorized FOUR score or GCS score was then determined from ROC curves of sums of each weighted score. ROC curve analysis was also used to determine the predictive performance of the original variables (continuous variables or nominal variables): serum NSE level, neurological examination scores, and their combinations. Comparisons of the AUROC curves were performed as recommended by DeLong et al. Statistical analyses were conducted using SPSS 24.0 (SPSS Inc., Chicago, IL, USA), R software version 3.5.2, and MedCalc 15.2.2 (MedCalc Software, Mariakerke, Belgium). A two-sided p-value of < 0.05 was considered statistically significant.
Data availability
Datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Sandroni, C. et al. Prognostication after cardiac arrest. Crit. Care 22, 150 (2018).
Peberdy, M. A. et al. Part 9: Post-cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 122, S768–S786 (2010).
Rundgren, M. et al. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation 80, 784–789 (2009).
Reisinger, J. et al. Prediction of neurological outcome after cardiopulmonary resuscitation by serial determination of serum neuron-specific enolase. Eur. Heart J. 28, 52–58 (2007).
Callaway, C. W. et al. Part 8: Post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 132, S465–S482 (2015).
Kim, H. S. et al. Prognostic value of OHCA, C-GRApH and CAHP scores with initial neurologic examinations to predict neurologic outcomes in cardiac arrest patients treated with targeted temperature management. PLoS ONE 15, e0232227 (2020).
Roger, C. et al. Neuron specific enolase and Glasgow motor score remain useful tools for assessing neurological prognosis after out-of-hospital cardiac arrest treated with therapeutic hypothermia. Anaesth. Crit. Care Pain Med. 34, 231–237 (2015).
Youn, C. S. et al. Post Cardiac Arrest Service. Combination of initial neurologic examination and continuous EEG to predict survival after cardiac arrest. Resuscitation 94, 73–79 (2015).
Youn, C. S. et al. Post Cardiac Arrest Service. Combination of initial neurologic examination, quantitative brain imaging and electroencephalography to predict outcome after cardiac arrest. Resuscitation 110, 120–125 (2017).
Stammet, P. et al. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33 °C and 36 °C. J. Am. Coll. Cardiol. 65, 2104–2114 (2015).
Huntgeburth, M. et al. Changes in neuron-specific enolase are more suitable than its absolute serum levels for the prediction of neurologic outcome in hypothermia-treated patients with out-of-hospital cardiac arrest. Neurocrit. Care 20, 358–366 (2014).
Pfeifer, R. et al. Hypothermia after cardiac arrest does not affect serum levels of neuron-specific enolase and protein S-100b. Acta Anaesthesiol. Scand. 58, 1093–1100 (2014).
Storm, C. et al. Serial measurement of neuron specific enolase improves prognostication in cardiac arrest patients treated with hypothermia: A prospective study. Scand. J. Trauma Resusc. Emerg. Med. 20, 6 (2012).
Oksanen, T. et al. Predictive power of serum NSE and OHCA score regarding 6-month neurologic outcome after out-of-hospital ventricular fibrillation and therapeutic hypothermia. Resuscitation 80, 165–170 (2009).
Zandbergen, E. G. et al. PROPAC Study Group. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology 66, 62–68 (2006).
Martinell, L. et al. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit. Care 21, 96 (2017).
Lee, B. K. et al. Combining brain computed tomography and serum neuron specific enolase improves the prognostic performance compared to either alone in comatose cardiac arrest survivors treated with therapeutic hypothermia. Resuscitation 84, 1387–1392 (2013).
Ryoo, S. M. et al. Prognostic abilities of serial neuron-specific enolase and lactate and their combination in cardiac arrest survivors during targeted temperature management. J. Clin. Med. 9, 159 (2020).
Luescher, T. et al. Neuron-specific enolase (NSE) improves clinical risk scores for prediction of neurological outcome and death in cardiac arrest patients: Results from a prospective trial. Resuscitation 142, 50–60 (2019).
Pfeifer, R. et al. Outcome after cardiac arrest: Predictive values and limitations of the neuroproteins neuron-specific enolase and protein S-100 and the Glasgow Coma Scale. Resuscitation 65, 49–55 (2005).
Hifumi, T. et al. Effect of admission Glasgow coma scale motor score on neurological outcome in out-of-hospital cardiac arrest patients receiving therapeutic hypothermia. Circ. J. 79, 2201–2208 (2015).
Natsukawa, T. et al. At what level of unconsciousness is mild therapeutic hypothermia indicated for out-of-hospital cardiac arrest: A retrospective, historical cohort study. J. Intensive Care 3, 38 (2015).
Fugate, J. E. et al. The FOUR score predicts outcome in patients after cardiac arrest. Neurocrit. Care 13, 205–210 (2010).
Weiss, N. et al. Daily FOUR score assessment provides accurate prognosis of long-term outcome in out-of-hospital cardiac arrest. Rev. Neurol. 171, 437–444 (2015).
Topcuoglu, M. A. et al. Prognostic value of magnetic resonance imaging in post-resuscitation encephalopathy. Intern. Med. 48, 1635–1645 (2009).
Rundgren, M. et al. Serum neuron specific enolase - impact of storage and measuring method. BMC. Res. Notes 7, 726 (2014).
PLOS Medicine Editors. Observational studies: Getting clear about transparency. PLoS Med. 11, e1001711 (2014).
Choi, Y. H. et al. Korean Hypothermia Network Investigators. Renal replacement therapy is independently associated with a lower risk of death in patients with severe acute kidney injury treated with targeted temperature management after out-of-hospital cardiac arrest. Crit. Care 24, 115 (2020).
Oh, J. H. et al. Korean Hypothermia Network Investigators. Association between acute kidney injury and neurological outcome or death at 6 months in out-of-hospital cardiac arrest: A prospective, multicenter, observational cohort study. J. Crit. Care 54, 197–204 (2019).
Hong, J. Y. et al. Korean Hypothermia Network Investigators. Grey-white matter ratio measured using early unenhanced brain computed tomography shows no correlation with neurological outcomes in patients undergoing targeted temperature management after cardiac arrest. Resuscitation 140, 161–169 (2019).
Acknowledgements
We would like to thank the investigators of the Korean Hypothermia Network.
Author information
Authors and Affiliations
Contributions
J.H.L., C.S.Y., and Y.H.K. conceptualized the research idea and study design; J.H.L., D.W.L., and J.-H.K. were involved in data acquisition; M.S.S. and K.W.J. performed data analysis/interpretation; S.Y.H. and Y.H.K. performed statistical analyses; S.Y.H. provided supervision/mentorship. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J.H., Kim, Y.H., Lee, J.H. et al. Combination of neuron-specific enolase measurement and initial neurological examination for the prediction of neurological outcomes after cardiac arrest. Sci Rep 11, 15067 (2021). https://doi.org/10.1038/s41598-021-94555-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94555-0
This article is cited by
-
Quantitative analysis of apparent diffusion coefficients to predict neurological prognosis in cardiac arrest survivors: an observational derivation and internal–external validation study
Critical Care (2024)
-
Different neuroprognostication thresholds of neuron-specific enolase in shockable and non-shockable out-of-hospital cardiac arrest: a prospective multicenter observational study in Korea (the KORHN-PRO registry)
Critical Care (2023)
-
External validation of the 2020 ERC/ESICM prognostication strategy algorithm after cardiac arrest
Critical Care (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.