Abstract
Species community structures respond strongly to habitat changes. These are either driven by nature or human activities. The biota of East African drylands responds highly sensitively to natural and anthropogenic impacts. Thus, seasonality strongly influences resource availability in a cyclic manner during the year, with cyclic appearance of the different developmental stages of invertebrates, while man-made landscape transformations profoundly and permanently modify habitat structures and, as a consequence, species communities. Butterflies are an excellent model group for the study of the effects of seasonality, and to test for biodiversity responses to anthropogenic activities such as habitat modification, degradation and destruction. We performed transect counts of adult butterflies in riparian forests and their adjoining areas, either dry savannahs with occasional pasturing (i.e. near-natural status) or farmland areas with fields, gardens and settlements (i.e. highly degraded status with lack of original vegetation). Transects were set along the river beds as well as at 250 m and 500 m distances parallel to these rivers, with eight transects per distance class and site (i.e. 48 transects in total). We recorded habitat structures for each transect. Counts were conducted during the dry and the rainy season, with 16 repetitions for each single transect, i.e. eight per season and transect. We compiled trait data on morphology, geographic distribution, ecology, behaviour, and life-history for all butterfly species encountered. Our results show higher species richness and numbers of individuals in farmland transects compared with the savannah region. Seasonal fluctuations of the detectable species abundances between the rainy and dry season were severe. These fluctuations were much more pronounced for the savannah than the farmland area, i.e. was buffered by human activities. Farmland and savannah support two distinct butterfly communities, with generalist species being more common in the farmland communities. Strict habitat associations were comparatively weak and typical dry savannah and riparian forest species were not clearly restricted to the near natural landscape.
Similar content being viewed by others
Introduction
Various natural and anthropogenic factors drive ecosystems, and thus have important impacts on their species communities. Some of these factors are cyclic and temporary, others directed and permanent. A cyclic natural factor is seasonality, which strongly influences resource availability in ecosystems, with the consequence that it severely impacts the activity and occurrence of species, influences their developmental cycles, affects abundances, and thus community composition and structure over time1. Such seasonal community modifications are particularly pronounced for organisms with short generation cycles, such as most arthropods. For example, studies on butterfly imagoes in south-eastern Kenya’s coastal forests showed that their community structures and abundances differ markedly between the dry and rainy season2. The majority of anthropogenic activities impact ecosystems in a more permanent way, and subsequently also their species community structures, as revealed by various studies. Such anthropogenic activities often result in complete habitat destruction or at least strong modification of the habitat configuration, e.g. from interconnected into fragmented systems3. This frequently leads to a significant and permanent reduction of general habitat quality4, which severely impacts the occurrence of species and community assemblages5.
Species respond differently to the loss, fragmentation and degradation of habitats. Species with specialised habitat demands respond more sensitively to anthropogenic habitat transformations than species with a wide ecological amplitude5, while species requiring specific resources tolerate habitat modifications only to a rather limited extent6. In consequence, sedentary specialists suffer particularly under habitat destruction and subsequent habitat fragmentation7. Thus, changes in habitat conditions particularly impact those species with narrow adaptations to these particular environmental conditions8.
The drylands of East Africa belong to the tropical regions most strongly affected by extreme annual changes in the climatic conditions9, leading to remarkable fluctuations in species richness, community composition and abundance10. Until recently, this part of Africa was mainly covered by dry savannah, transgressed by temporarily water-carrying rivers. These rivers are naturally bordered by dense riparian vegetation, home of a unique flora and fauna with many specialised and endemic species11. The substantial increase of settlements and hence human activities (in particular subsistence agriculture) along these rivers and in general across the dryland areas has caused severe degradation of both the riparian forests12,13 and the dry savannahs14. The resilience of both ecosystems is rather limited, and many questions about the effects of human and natural impacts on these ecosystems and the species living therein remain unresolved.
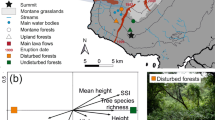
Therefore, we analysed butterfly species communities along two rivers and in their adjoining dryland areas in southern Kenya. We established line-transects and performed standardized butterfly counts (modified after Pollard15). Transects were established along the banks of rivers and parallel to them at average distances of 250 m and 500 m, respectively. We established identical study designs in two landscape types in the vicinity of the city of Kitui in southern Kenya (Fig. 1): 1. In a densely populated and thus degraded landscape dominated by subsistence agriculture; and 2. In a still widely intact dry savannah with riparian forests along the river, only moderately affected by pasturing of live-stock. We recorded habitat structures for each transect, and counted butterflies along all transects during the dry and the rainy season. During each transect count, we recorded all individuals after their determination to species level. Morphological, distributional, ecological, behavioural, and life-history traits were assigned to each species encountered. Based on these data, we address the following research questions:
-
1.
How do species richness, abundance and community structures differ in near natural habitats and in anthropogenic landscapes?
-
2.
Are community structures in near natural habitats and in anthropogenic landscapes affected differentially by seasonal shifts?
-
3.
How strongly do the riparian habitats influence the community structures in the adjoining areas?
Results
In total, we recorded 13,748 butterfly imagoes, representing 71 butterfly species along the transects of both study sites along two different river systems. Answering our first research question, we recorded more species in the agricultural site at Nzeeu River than in the transects established in the near native savannah environment at Kainaini River (Table 1, Fig. 2a). Irrespective of the differences in land-use in the two study sites, the dry season was significantly less rich in butterfly species on the wing compared to the rainy season (Tables 1, 2). Thus, totals of 35 and 53 species were found at Nzeeu during the dry and rainy seasons, respectively, i.e. an increase of 51%. The difference was more pronounced in the near-natural site at Kainaini with 11 and 51 species, respectively, i.e. an increase of 363% (Table 1, Fig. 2a). These differences in species numbers were also reflected by strong fluctuations in abundance of imagoes. At Nzeeu, we recorded 11.2 times more butterfly individuals during the rainy (5131) than during the dry season (459); this factor increased to 98.5 at Kainaini (8081 vs. 82; Table 1). Thus, comparing sites, the agricultural site at Nzeeu River had higher abundances during the dry season, the near-natural savannah site near Kainaini River during the rainy season. However, the latter was triggered by the very high abundance of one single butterfly species, i.e. Belenois aurota with 5805 individuals at Kainaini and 1028 at Nzeeu. Despite the more similar total abundances in the rainy season, we found significant differences in species richness between the Kainaini and Nzeeu river systems in direct comparisons of respective transects in both seasons, with the Kainaini transects always being less species-rich but only less rich in butterfly individuals during the dry season (Fig. 2a, b, Tables 1, 2).
Species richness (a), and number of individuals (b), along the Nzeeu (orange) and Kainaini (yellow) rivers; given is the mean per transect. (c) Average scores of the dominant (explained variance: 91.5%; red: dry season, violet: rainy season) and subdominant (explained variance: 3.5%; green: dry season, brown: rainy season) PCoA eigenvectors across transects. (d) Bray–Curtis similarities of the intermediate (I) and far (F) transects to the near river transects (N), as well as average Bray–Curtis similarity within the eight near river transects NW (green bars; only calculated for the rainy season; data insufficient for dry season) and the average similarities across the eight near river transects NB (blue bars; data insufficient for Kainaini during the dry season). Error bars in (a), (b), and (c) denote standard errors from eight sample points in each transect. Error bars in (d) are based on 1000 bootstrap samples. Abbreviations: N—near river transects; I—transects at intermediate distance from river (i.e. 250 m); F—transects far from river (i.e. 500 m); NW—average Bray–Curtis similarity within the eight walks in the near river transects; NB—average similarities across the eight near river transects.
Habitat conditions quantified by tree and shrub cover did not significantly influence abundances, species richness and community composition of the transects, except for a marginally significant positive correlation of tree cover and species richness. Similarly, butterfly ecological traits did not significantly co-vary with habitat conditions (see Appendix S1).
With respect to our second research question, we found a higher species turnover (β-diversity) of the transects located in the Kainaini river system than at Nzeeu during the rainy season; this difference was not apparent during the dry season (Table 1, Fig. 2c). Permanova confirmed structural differences between both sites and seasons, while pointing only to marginal differences with respect to distance from the rivers (Table 3).
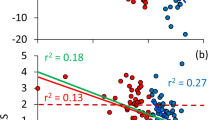
These differences in community compositions were not reflected by clear morphological differences between transect groups (Appendix S1), except for the forewing length/thorax width index that was consistently higher during the rainy season (Appendix S1), but we found significant differences in trait expression (Table 4, Fig. 3). Thus, based on mean values considering the number of individuals per species, habitat specialisation and savannah index differed significantly between the dry and the rainy season (Table 4, Fig. 3), indicating respective shifts in community composition of the adult individuals. The transects located in the Kainaini river system were comparatively richer in species occurring in open landscapes than at Nzeeu. Thus, the savannah index (Fig. 3d) was comparatively higher, the forest (Fig. 3b), tree (Fig. 3e) and wetness indices (Fig. 3f) lower at Kainaini River, indicating higher proportions of savannah species than in the Nzeeu site (P(F1,94) < 0.01). These differences were more pronounced for the butterflies on the wing during the rainy (P(F1,94) < 0.01) than during the dry season (P(F1,94) > 0.05). Seasons did not significantly differ with respect to the degree of hemeroby (Table 4, pairwise P(F1,94) > 0.05). Mostly similar values were obtained when these comparisons were based on means calculated from only presence-absence data (Appendix S1). However, contrary to the means obtained for numbers of individuals, the presence-absence data show a tendency toward higher values for habitat and larval food plant specialisation, underlining the presence of such specialist species, but in relatively low numbers.
Average abundance-based butterfly ecological traits in transects along the Nzeeu (orange) and Kainaini (yellow) rivers near Kitui city in southern Kenia. Error bars denote standard errors. Traits used are (a) geographic distribution (4 categories), (b) forest index (5 categories), (c) habitat specialisation (3 categories), (d) savannah index (5 categories), (e) tree index (3 categories), (f) wetness index (3 categories), (g) larval foodplant specialisation (3 categories), (h) larval food plant type (dicotyledonous, monocotyledonous), and (i) hemeroby index (4 categories). Categories (apart from larval food plant type) are always in increasing order. Abbreviations: N—near river transects; I—transects at intermediate distance from river (i.e. 250 m); F—transects far from river (i.e. 500 m).
Answering our third research question, we found species composition to differ only marginally between the transect groups with different distances from the rivers, indicating considerable compositional similarity between transects near and far from the river (Tables 2, 3, Fig. 2d). At Nzeeu, but not at Kainaini, the respective compositional similarity of butterfly imagoes was higher during the rainy than during the dry season (Fig. 2d). Furthermore, average similarity across the three distance classes was even higher than between the transects near the river (Fig. 2d). We found the lowest compositional similarity (i.e. highest species turnover) within each transect group (Fig. 2d). There were no clear linear gradients correlated with distance from the river. The only case of a linear decrease was observed for the wetness index at the Nzeeu site during the dry season decreasing with increasing distance from the river (Fig. 3f).
Discussion
Species richness and abundance
We found a generally higher butterfly species richness and higher abundances (the latter only during the dry season) across the anthropogenic landscape disturbed along Nzeeu River if compared with the mostly undisturbed natural dry savannahs along Kainaini River. In contrast, abundances were higher in the mostly undisturbed study site during the rainy season. This picture is mostly congruent with results from other studies, showing higher species richness and abundances in diverse anthropogenic landscapes compared to natural habitats7,16,17,18,19. This also holds true for tropical ecosystems of East Africa where, for example in western Kenya, a higher bird species richness was found across heterogeneous agricultural land than in the adjoining natural forest patches20. Similarly, diversely structured urban areas with gardens may provide an even higher species richness and higher abundances than adjoining natural habitats do21,22. This result might be due to the accumulation of ecological niches and resources frequently found in anthropogenic landscapes. Particularly in dryland areas, such as in East Africa, artificial water irrigation produces more constant resource availability throughout the year, which boosts species richness and abundance23,24. In consequence, only specialist species adapted to survive rather dry climatic conditions were found along the near-natural Kainaini River site during the dry season and were much less frequent than in the anthropogenic landscape with higher artificial water availability.
Our transects along Nzeeu River represent a disturbed and heterogeneous environment consisting of a mosaic of gardens around habitations, fallow land, small fields for subsistence agriculture, trees and shrubs (the latter dominated by the exotic and persistently flowering shrub Lantana camara), as well as disturbed riparian forest remnants. This complex habitat diversity combined with year-round water irrigation of some parts of it provides numerous niches and a plenitude of resources for many butterfly species. However, the total number of individuals during the rainy season was higher in our savannah transects than in the agricultural land. This underlines the high potential of such near-natural sites to produce rather high numbers of individuals and the capacity for massive abundance of single species (e.g. Larsen25), as for Belenois aurota in our study.
Community structure and specialist species
Apart from species richness and abundances, a closer look at the species composition is important in evaluating the ecological performance and conservation value of the two types of study sites. While, at a first glance, there is a positive effect on numbers of species and individuals in anthropogenically disturbed habitats, there is a lack of species with specific habitat requirements (e.g. several blues of the genera Euchrysops and Lepidochrysops, or the nymphalid Pardopsis punctatissima). However, these species were generally found at low abundances, also in the near-natural savannah habitats. Furthermore, several typical savannah species occurred at reduced levels of abundance in the study site dominated by human impacts (e.g. Pinacopteryx eriphia, Charaxes xoolina, Junonia hierta). This agrees with previous studies underlining that anthropogenic landscapes, although frequently richer in species numbers and abundances, do not hold specialist species26. Thus, rare bee species vanished after human disturbances of habitats in eastern North America27, and, in a study in the Taita Hills of southern Kenya, butterflies of cloud forests are mainly observable within the natural forest28. Thus, considering only the total number of species and individuals might lead to wrong interpretations of data. Consequently, a detailed look at the community structures and trait assemblages is necessary.
Our data show that community structures differed significantly between our two study sites. Differences in community assembly were particularly pronounced during the dry season, but less during the rainy season. The butterfly community in the anthropogenic landscape along Nzeeu River is comparatively diverse and consequently was expected to also span a broad trait space. In contrast, the butterflies found in the near-natural habitats along Kainaini River represent a partly distinct community consisting more of savannah elements, but reached almost the species richness found along Nzeeu River during the rainy season. However, the butterfly community in the anthropogenic landscape is more dominated by ubiquists, exemplified by considerably higher numbers of species such as Catopsilia florella and Hypolimnas misippus. Here we also found common species like Neptis saclava and Bicyclus safitza which require more dense vegetation (as provided by the garden and farmland structures) but have few additional habitat requirements. In contrast, typical species of dry savannah habitats (such as Pinacopteryx eriphia, Charaxes xoolina and Junonia hierta) are considerably more frequent in the largely undisturbed savannah environment along Kainaini River. Thus, our data reflect the typical pattern that community structures follow environmental conditions and thus mirror human disturbances, as also demonstrated for other tropical ecosystems28,29, and that generalist species frequently replace specialist species after human disturbances28,30. In summary, anthropogenic landscapes may host a high number of species and individuals, but lack the specialist species that respond highly sensitively to habitat disturbances.
Seasonal shifts
Seasonal shifts in tropical ecosystems significantly impact the prevailing environmental conditions, because the availability of resources strongly depends on the amount of precipitation31. Insect populations are known to react very strongly to these seasonal effects, particularly in the dryland ecosystems of East Africa2. However, the detectable effect of seasonality in our study was less pronounced in the anthropogenic landscape (5131 counted butterfly imagoes during the rainy season, 459 individuals during the dry season) than in the near-natural savannah site (8081 vs. 82). Obviously, the seasonal fluctuation in the number of adult individuals is buffered in anthropogenic habitats by multiple factors: 1. Permanent water irrigation ensures resource availability throughout the year, and, 2. Species at home in anthropogenic landscapes often have broader ecological amplitudes, and their response might thus be more plastic than that of specialist species. Previous studies also showed similar seasonal patterns in the number of adult individuals and differences in community structures, depending on landscape configuration20. Thus, seasonal shifts of adult butterfly community composition and abundance in the East African coastal forest ecosystem is also buffered in anthropogenic landscapes (e.g. agriculture, forest edges and tree plantations), but are very pronounced in the natural dryland forest habitat2. Consequently, the here observed seasonality pattern of communities represents a general feature in butterflies (and insects more generally) of the drylands of tropical Africa.
Spill-over effects
Answering our third research question, we found no significant differences in species richness, abundance and community structure between transects in the riparian forest on the one hand compared to the transects in the dry savannah and the agricultural land on the other. Furthermore, our data do not show any significant gradient of butterfly diversity spill-over from the riparian forests into the adjoining landscapes. Our findings contrast with other studies indicating positive spill-over effects from (near) natural habitats into anthropogenic landscapes32. Such spill-over effects might have a positive influence on ecosystem functions (i.e. services) such as an increase in pollination due to visiting pollinators from (near) natural habitats33, which might enhance agricultural productivity32,34,35,36. In our study, however, the size of the gallery forests seems to be too small, and they are too closely intertwined with the adjacent ecosystems, so that potential spill-over effects are not detectable.
Methods
Study sites
Our study sites are located on the Yatta Plateau in south-eastern Kenya. This region is characterized by dry savannahs. Annual rainfall (average: 810 mm) occurs during two periods, from March to May (average: 330 mm) and from October to January (average 480 mm) (c.f. Jaetzold et al.37). The commonest soil types are ferralsols and luvisols, which are of low fertility37. 97.1% of the human population in our study region depend on subsistence crop farming38, and the population has almost doubled in number from 1999 to 200938. Consequently, fallow periods for fields are omitted, which further decreases soil fertility, and increases pressure on pristine habitats.
The dry savannah landscape is traversed by temporary (seasonal) rivers. These rivers are bordered by riparian vegetation, consisting of a diverse and unique plant community. However, this vegetation is frequently exploited for timber, charcoal and brick production39,40. The region is further affected by climate change, with an increase in rainfall variability and mean temperature37. These factors lower the reliability of agricultural production and food security, hence leading to severe destruction of pristine habitats.
We selected two study sites, affected by different anthropogenic pressures, but which are subject to identical biotic and abiotic preconditions (including seasonality): Firstly, a highly degraded anthropogenic landscape along Nzeeu River, south of Kitui city. Secondly, a largely intact dryland environment along Kainaini River located near the university campus of the South Eastern Kenya University, north of Kitui city (Fig. 1). The landscape along Nzeeu River is densely populated by subsistence farmers. Thus, the original riparian and savannah vegetation has been mostly transformed into arable fields for the cultivation of maize, sorghum, peas, and mangos. Furthermore, the riparian vegetation, where it still exists, has largely been replaced by invasive exotic plant species (e.g. Lantana camara)12. The landscape of our second study site along Kainaini River represents a still largely intact riparian forest with adjoining dry savannahs. It remains mostly undisturbed, except for some moderate live-stock pasturing by nearby subsistence settlers.
Butterfly assessments
We counted butterflies in both habitat types along line-transects, each 150 m long. We set 24 transects along each of the two rivers, with eight transects along the river bank, eight 250 m distant to the river, and another eight 500 m distant to the river (in total: 2 × 24 transects = 48 transects). The minimum distance between transects was at least 200 m, to minimize spatial autocorrelation. Exact GPS coordinates of each transect are given in Appendix S2.
We recorded all butterflies encountered during transect counts (species, number of individuals of each species). Each transect was visited eight times during the dry season (August/September 2019) and eight times during the rainy season (January/February 2020). Data collection was performed between 9 a.m. and 4 p.m. Each butterfly individual within 5 m of the transect line (horizontally to vertically) was recorded by visual observation and, if needed, a butterfly net (see Pollard15, with modifications). While recording butterflies, the observers walked very slowly and spent about 15 min per transect. Species were identified either immediately while the butterfly was on the wing, or individuals were netted and then determined in the field. Individuals of species for which ad hoc identification was critical (e.g. many blues and skippers) were caught with the net, photographed (upper and under wing side) and released again. The photograph-based identification of these individuals was performed later using literature25. Apart from species and number of individuals per species, we recorded cloud cover during each transect walk (classified as: clear, slightly cloudy, mostly cloudy, overcast), exact time, and date. Field teams comprised two observers and one person making notes of all observations. Transects are displayed in Fig. 1. All butterfly data collected are compiled in Appendix S3.
Traits
The occurrence of a species in a specific environment strongly depends on its ecology, behaviour, and life-history41. Therefore, we considered these characteristics for each butterfly species recorded in the field. These trait data were compiled from Larsen25 and web-sites (e.g. www.gbif.org, www.lepiforum.de/non-eu.pl). We considered the following characteristics: wing span (mm), ratio length/width of the forewing (relative), ratio forewing length/thorax width (relative), geographic distribution (4 categories), savannah index (5 categories), forest index (5 categories), tree index (3 categories), wetness index (3 categories), habitat specialisation (3 categories), larval foodplant specialisation (3 categories), larval food plant type (dicotyledonous, monocotyledonous), and hemeroby index (4 categories). Detailed classifications are provided in Appendix S4.
Habitat parameters
Habitat structures impact species´ occurrence, abundances and community structures42. In our study, we considered habitat structures for each transect. Habitat parameters were recorded (counted and estimated) every 20 m along each transect. We estimated the following habitat parameters: Canopy cover (percentage of leaf cover vs. sky measured with the CanopeoApp); herb, shrub and tree cover (percentage coverage of each layer within a radius of 3 m); flowers on herbs, shrubs and trees (estimated within a radius of 3 m, and subsequently allocated to the classes 0, 1–10, 11–50, 51–100 and > 100 flowers); occurrence of Lantana camara shrub, and exotic trees (estimated coverage within a radius of 3 m, and subsequently allocated to the classes 0 (no), 1 (rare), 2 (present) and 3 (dominant), respectively); and water availability (presence/absence) within a radius of 3 m. All raw data of habitat parameters are provided in Appendix S5.
Statistics
We first arranged the raw data in three matrices: a 71 × 14 species × trait matrix T, a 71 × 96 species × transect matrix M, and a 6 × 96 habitat characteristics × transect matrix H. Matrix multiplication of E = T−1MA−1, where A is the vector of total abundances in the transects, returned a matrix E of average trait expression in each transect.
To answer the first research question, we compared species richness, abundances, and trait expression between the transects and used general linear modelling (glm) to detect differences in richness and trait expression with respect to the study sites (i.e. the two river systems with their different land-use patterns), season, distance from the rivers, as well as to environmental variables. Some of the habitat variables and trait expressions were highly positively correlated (Appendix S1). Consequently, the glm included only variables correlated by less than r = 0.7 (i.e. shrub cover, tree cover, habitat specialisation, savannah index, larval foodplant specialisation, and hemeroby).
To infer differences in community structure between transects (second research question), we first calculated the two most dominant eigenvectors, which explained 91.5% and 3.5% of variance, of a principal components analysis of the M matrix. These eigenvectors cover differences in species composition between and within transects. We used glm and two-way Permanova to relate these differences to season, distance to river, and study sites (i.e. different land-use types in the two river systems). Additionally, we assessed the degree of β-diversity among sets of transects with the proportional turnover metric of Tuomisto43: \(\beta =1-\frac{\alpha }{\gamma }\); where α denotes the average species richness per transect and γ the corresponding total richness.
To infer species spill-over effects from the riparian forests into the adjoining savannah (third research question), we calculated the Bray–Curtis similarities for three groups of transects within each season and study site. First, we compared average pairwise Bray–Curtis values between transects of intermediate and greater distance with the near-river transects within each study site. Second, we calculated the average Bray–Curtis similarities between all transects within each study site (2)—season (2)—distance class to river (3) combination. Third, we calculated the average within-transect Bray–Curtis similarity for the rainy season, to infer small scale compositional variability. The latter calculations were impossible for the dry season, due to the overall low number of recorded species. Calculations were done with Statistica 12.
References
Maicher, V. et al. Seasonal shifts of biodiversity patterns and species’ elevation ranges of butterflies and moths along a complete rainforest elevational gradient on Mount Cameroon. J. Biogeogr. 47, 342–354 (2020).
Habel, J. C., Seibold, S., Ulrich, W. & Schmitt, T. Seasonality overrides differences in butterfly species composition between natural and anthropogenic forest habitats. Anim. Conserv. 21, 405–413 (2018).
Miller-Rushing, A. J. et al. How does habitat fragmentation affect biodiversity? A controversial question at the core of conservation biology. Biol. Conserv. 232, 271–273 (2019).
van Halder, I., Barnagaud, J.-Y., Jactel, H. & Barbaro, L. Woodland habitat quality prevails over fragmentation for shaping butterfly diversity in deciduous forest remnants. For. Ecol. Manag. 357, 171–180 (2015).
Thomas, J. A. Butterfly communities under threat. Science 353, 216–218 (2016).
Clavel, J., Julliard, R. & Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization?. Front. Ecol. Environ. 9, 222–228 (2011).
Birkhofer, K., Smith, H. G., Weisser, W. W., Wolters, V. & Gossner, M. M. Land-use effects on the functional distinctness of arthropod communities. Ecography 38, 889–900 (2015).
Habel, J. C. & Schmitt, T. Vanishing of the common species: Empty habitats and the role of genetic diversity. Biol. Conserv. 218, 211–216 (2018).
Jaetzold, R., Schmidt, H., Hornet, Z. B. & Shisanya, C. A. Farm Management Handbook of Kenya. Volume II: Natural Conditions and Farm Information. (Ministry of Agriculture/GTZ, 2012).
Wagner, T. Seasonal changes in the canopy arthropod fauna in Rinorea beniensis in Budongo Forest, Uganda. Plant Ecol. 153, 169–178 (2001).
Bennett, A. F., Nimmo, D. G. & Radford, J. Q. Riparian vegetation has disproportionate benefits for landscape-scale conservation of woodland birds in highly modified environments. J. Appl. Ecol. 51, 514–523 (2014).
Habel, J. C., Teucher, M., Ulrich, W. & Schmitt, T. Documenting the chronology of ecosystem health erosion along East African rivers. Remote Sens. Ecol. Conserv. 4, 34–43 (2018).
Schmitt, C. B., Kisangau, D. & Matheka, K. W. Tree diversity in a human modified riparian forest landscape in semi-arid Kenya. For. Ecol. Manage 433, 645–655 (2019).
Mganga, K. Z., Nyariki, D. M., Musimba, N. K. R. & Amwata, D. A. Determinants and rates of land degradation: Application of stationary time-series model to data from a semi-arid environment in Kenya. J. Arid Landsc. 10, 1–11 (2018).
Pollard, E. A method for assessing changes in the abundance of butterflies. Biol. Conserv. 12, 115–134 (1977).
Winfree, R., Griswold, T. & Kremen, C. Effect of human disturbance on bee communities in a forested ecosystem. Conserv. Biol. 21, 213–223 (2007).
McCracken, M. E. et al. Social and ecological drivers of success in agri-environment schemes: The roles of farmers and environmental context. J. Appl. Ecol. 52, 696–705 (2015).
Mallinger, R. E., Gibbs, J. & Gratton, C. Diverse landscapes have a higher abundance and species richness of spring wild bees by providing complementary floral resources over bees’ foraging periods. Landsc. Ecol. 31, 1523–1535 (2016).
Kunz, W. Species Conservation in Managed Habitats: The Myth of a Pristine Nature. (Wiley, 2016).
Mulwa, R. K., Böhning-Gaese, K. & Schleuning, M. High bird species diversity in structurally heterogeneous farmland in Western Kenya. Biotropica 44, 801–809 (2012).
Ives, C. D. et al. Cities are hotspots for threatened species: The importance of cities for threatened species. Glob. Ecol. Biogeogr. 25, 117–126 (2016).
Soanes, K. & Lentini, P. E. When cities are the last chance for saving species. Front. Ecol. Environ. 17, 225–231 (2019).
Amprako, L., Stenchly, K., Wiehle, M., Nyarko, G. & Buerkert, A. Arthropod communities in urban agricultural production systems under different irrigation sources in the northern region of Ghana. Insects 11, 488 (2020).
Bouallala, M., Neffar, S. & Chenchouni, H. Vegetation traits are accurate indicators of how do plants beat the heat in drylands: Diversity and functional traits of vegetation associated with water towers in the Sahara Desert. Ecol. Ind. 114, 106364 (2020).
Larsen, T. B. The Butterflies of Kenya and Their Natural History. (Oxford University Press, 1996).
Miller, J. E. D., Damschen, E. I., Harrison, S. P. & Grace, J. B. Landscape structure affects specialists but not generalists in naturally fragmented grasslands. Ecology 96, 3323–3331 (2015).
Harrison, T., Gibbs, J. & Winfree, R. Anthropogenic landscapes support fewer rare bee species. Landsc. Ecol. 34, 967–978 (2019).
Schmitt, T. et al. The relevance of cloud forest fragments and their transition zones for butterfly conservation in Taita Hills, Kenya. Biodivers. Conserv. 29, 3191–3207 (2020).
Sambhu, H., Northfield, T., Nankishore, A., Ansari, A. & Turton, S. Tropical rainforest and human-modified landscapes support unique butterfly communities that differ in abundance and diversity. Environ. Entomol. 46, 1225–1234 (2017).
Mangels, J., Fiedler, K., Schneider, F. D. & Blüthgen, N. Diversity and trait composition of moths respond to land-use intensification in grasslands: Generalists replace specialists. Biodivers. Conserv. 26, 3385–3405 (2017).
Guan, K. et al. Simulated sensitivity of African terrestrial ecosystem photosynthesis to rainfall frequency, intensity, and rainy season length. Environ. Res. Lett. 13, 025013 (2018).
Tscharntke, T. et al. Landscape constraints on functional diversity of birds and insects in tropical agroecosystems. Ecology 89, 944–951 (2008).
Habel, J. C. & Ulrich, W. Ecosystem functions in natural and anthropogenic ecosystems across the East African coastal forest landscape. Biotropica 52, 598–607 (2020).
Klein, A.-M. et al. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313 (2007).
Klein, A.-M., Steffan-Dewenter, I. & Tscharntke, T. Pollination of Coffea canephora in relation to local and regional agroforestry management: Pollination of lowland coffee. J. Appl. Ecol. 40, 837–845 (2003).
Ricketts, T. H. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv. Biol. 18, 1262–1271 (2004).
Jaetzold, R., Hornetz, B., Shisanya, C. A. & Schmidt, H. Farm management handbook of Kenya. Volume II: Natural conditions and farm management information. vol. 2 (Ministry of Agriculture, Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ), 2007).
Government of Kenya. County poverty rates 2005/06. Kenya open data. https://www.opendata.go.ke/ (2014).
Teucher, M. et al. A Kenyan endemic bird species Turdoides hindei at home in invasive thickets. Basic Appl. Ecol. 16, 180–188 (2015).
Nzau, J. M., Rogers, R., Shauri, H. S., Rieckmann, M. & Habel, J. C. Smallholder perceptions and communication gaps shape East African riparian ecosystems. Biodivers. Conserv. 27, 3745–3757 (2018).
Birkhofer, K. et al. Land-use type and intensity differentially filter traits in above- and below-ground arthropod communities. J. Anim. Ecol. 86, 511–520 (2017).
Altermatt, F. & Holyoak, M. Spatial clustering of habitat structure effects patterns of community composition and diversity. Ecology 93, 1125–1133 (2012).
Tuomisto, H. A consistent terminology for quantifying species diversity? Yes, it does exist. Oecologia 164, 853–860 (2010).
Acknowledgements
We thank Lara Bauer, Kerstin Link, Michaela Maislinger, Anna Sommer, Marielle Schleifer, Julia Schuster, Daniel Stieringer, and Eva-Teresa Raphaela Szekeres for help in the field. This activity was conducted in close collaboration with the South Eastern Kenya University. We acknowledge the German Academic Exchange Service for funding the DAAD Quality Network Biodiversity Kenya (2016–2019). We dedicate this publication to Prof. Ralph Jätzold (University of Trier), one of the great old men in Africa research and a great friend of Kenya and its people; he passed away peacefully at high age by the end of last year.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
T.S. and J.C.H. designed the study, T.S., J.C.H., A.D. and M.T. led data collection in the field, W.U. did the statistics, all contributed while writing and reviewing the mauscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmitt, T., Ulrich, W., Delic, A. et al. Seasonality and landscape characteristics impact species community structure and temporal dynamics of East African butterflies. Sci Rep 11, 15103 (2021). https://doi.org/10.1038/s41598-021-94274-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94274-6
This article is cited by
-
Diverging responses to natural and anthropogenic habitats in aftrotropical birds and butterflies
Biodiversity and Conservation (2024)
-
Population ecology and behaviour of two Afrotropical forest butterflies
Journal of Insect Conservation (2023)
-
Fluctuating insect diversity, abundance and biomass across agricultural landscapes
Scientific Reports (2022)
-
Seasonal dynamics and polyphenism of butterfly communities in the coastal plains of central Western Ghats, India
Journal of Biosciences (2022)
-
Species community structures of Afrotropical butterflies differ depending on the monitoring method
Biodiversity and Conservation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.