Abstract
We hypothesized that the scarcity of N-nucleotides might contribute to the inability of the neonate to mount a robust allergic immune response. To test this, we used terminal deoxyribunucleotidyl Transferase deficient (TdT−/−) mice, which express “fetal-like” T cell receptor and immunoglobulin repertoires with largely germline-encoded CDR3 regions. Intraperitoneal sensitization was followed by aerosol provocation with either PBS or the allergen OVA in both TdT−/− mice and wild-type mice to develop allergic respiratory inflammation. The effects of this procedure were investigated by lung function test, immunological analysis of serum and brochoalveolar lavage. The local TH2 cytokine milieu was significantly attenuated in TdT−/− mice. Within this group, the induction of total IgE levels was also significantly reduced after sensitization. TdT−/− mice showed a tendency toward reduced eosinophilic inflow into the bronchial tubes, which was associated with the elimination of respiratory hyperreactivity. In conclusion, in a murine model of allergic airway inflammation, the expression of fetal-like antigen receptors was associated with potent indications of a reduced ability to mount an asthma phenotype. This underlines the importance of somatically-generated antigen-receptor repertoire diversity in type one allergic immune responses and suggests that the fetus may be protected from allergic responses, at least in part, by controlling N addition.
Similar content being viewed by others
Introduction
Allergic asthma is a chronic inflammatory disease of the airways characterized by bronchial hyperreactivity and variable airway obstruction1,2.
Allergic sensitization is described as a misled classical affinity-driven immune response associated with an imbalance towards a TH2 milieu3,4. TH2-cell derived cytokines such as interleukin 4 (IL-4) promote B cell isotype switching to immunoglobulin E (IgE) which plays a key role in allergic asthma by acting as a link between an allergen and the mast cell to which the IgE is attached by its constant domain to membrane-bound Fcε receptors5,6,7.
We and others have previously shown that in type 1 allergic responses the third complementarity determining region of the H chain (CDR-H3), which encodes for the center of classic immunoglobulin antigen binding sites, often plays a key role in forming the interface between the allergen and IgE8,9,10,11. In contrast, in atopic dermatitis the circulating IgE repertoire reflects superantigen like activation12.
During fetal life, human and murine T cell receptor (TCR) and immunoglobulin (Ig) repertoires are restricted in diversity due to a reduction or absence of terminal deoxynucleotidyl transferase (TdT) activity in the fetal liver, fetal bone marrow, and fetal thymus; and thus a reduction or absence of N nucleotides within their CDR3 regions13,14. After birth, the diversity of both the TCR and Ig repertoires expands as a consequence of the contribution of N nucleotide addition to CDR313,14,15,16,17,18,19. The diversification of the adaptive antigen receptor repertoires in utero and after birth parallels the developing ability to respond to a broad range of antigens and to generate robust secondary immune responses, including allergic responses.
Although the classic immunoglobulin antigen binding site forms the Ig core of the allergen–antibody interaction, the importance of qualitative properties of the CDR-H3 region for the development of an allergic phenotype has not yet been conclusively clarified. Previous studies show that gene targeted mouse lines with altered diversity gene loci region exhibit differing allergic phenotypes depending on the hydrophobicity of their CDR-H3 regions8,20. A mouse strain expressing predominantly neutral amino acids in CDR-H3 developed a strong allergic phenotype to ovalbumin17, whereas a repertoire enriched for positively charged CDR-H3 led to a weakened allergic phenotype. However, to the best of our knowledge the role of N regions on the development of allergic respiratory inflammation has not yet been characterized.
The aim of the present study was to investigate the influence of CDR3 repertoires lacking N nucleotides on the development of the allergic phenotype. We found that in a murine model of allergic airway inflammation terminal deoxynucleotidyl transferase (TdT−/−) deficient mice, which express “fetal-like” antigen receptor repertoires without N-nucleotides21, give substantial indications to develop an impaired immune response.
Results
Induction of total IgE levels after sensitization with OVA is significantly reduced in TdT−/− mice
To assess allergic sensitization, serum concentrations of IgG1 and IgE in the TdT−/− and wt mice were determined. Before sensitization, the serum levels of each of the corresponding antibodies were just above the limits of detection (Supplementary Fig. S1). The same was shown for the levels of antibody classes of the animals exposed to PBS (control) nebulization (Fig. 1). In contrast, a significant increase was observed for both IgG1 and IgE levels when sensitized with aerosolic OVA. This was true for both the overall levels and the OVA-specific levels. When compared to controls, the TdT−/− mice demonstrated a significant increase of all analyzed antibody classes (p < 0.0001). The same tendency was observed for the increase in antibody concentrations due to sensitization to the wild type, (total IgG1 p < 0.001, OVA-specific IgG1, total IgE and OVA-specific IgE p < 0.0001). However, in contrast to OVA-specific antibody levels, total IgE levels in the TdT−/− mice were significantly lower than in wt (p < 0.0001).
Serum immunoglobulin levels. In serum of wt and TdT−/− mice, both sensitized and non-sensitized, immunoglobulin levels of (a) total IgG1, (b) total IgE, (c) OVA-specific IgG1 and (d) OVA-specific IgE were measured using ELISA. Sensitization induced a significant rise in OVA-specific IgG1 and IgE levels. No significant differences between wt and TdT−/− mice were observed in OVA-specific IgE levels. Total IgE and total IgG1 levels were significantly increased after sensitization for both strains. The increase in IgG1 levels was significantly higher and the increase in IgE levels was significantly lower in TdT−/− mice when compared to wt (mean shown as blue lines, SEM shown as black bars).
TH2 cytokines in the bronchoalveolar lavage are reduced in TdT−/− mice
The local TH cytokine pattern in BAL fluids of the animals was characterized by using a cytometric bead assay to determine IL-4, IL-5, IL-6, IL-10, IL-13 and interferon-γ (INF-γ) levels (Fig. 2). When compared to non-sensitized mice, sensitization with OVA followed by local allergen challenge resulted in a significant rise in IL-4 levels in wt mice (p < 0.001, 263.9 ± 25%; mean ± SEM) and a marked increase in TdT−/− mice (p = 0.06, 178.3 ± 16.7%; mean ± SEM) in BAL fluids. The same tendency was observed for IL-13 levels (wt p < 0.0001, 972.4 ± 162.5%; TdT−/− p < 0.01, 473.3 ± 85.7; mean ± SEM) and IL-5 (wt p < 0.0001, 546.5 ± 58.7%; TdT−/− p < 0.001, 433.2 ± 62.0; mean ± SEM). However, when compared to wt mice, this increase was attenuated in sensitized TdT−/− mice for IL-13 and for IL-4 (p < 0.05). The induction of IL-5 levels following OVA sensitization was comparable between the two genotypes. Sensitization with OVA did not lead to a significant increase in either IL-10, INF-γ or IL-6 levels in TdT−/− mice, while IL-6 levels were significantly elevated after sensitization in wt (p < 0.01). The measured levels of the cytokines IL-6, IL-10 and INF-γ showed no differences between the two genotypes.
Cytokine level in BAL fluids. In BAL fluids of wt and TdT−/− mice, both sensitized and non-sensitized, cytokine levels of (a) IL-4, (b) IL-5, (c) IL-6 (d) IL-10 (e) IL-13 and (f) INF-γ were measured using Cytokine Multiplex Assay. (a–c) Sensitization with OVA resulted in a marked increase in cytokine levels of IL-4 in TdT−/− (p = 0.06) compared to non-sensitized mice. A significant rise was observed in IL-4 levels in wt (p < 0.001), in IL-13 (Wt p < 0.0001; TdT−/− p < 0.01) and IL-5 (Wt p < 0.0001; TdT−/− p < 0.001) compared to non-sensitized mice. However, this increase was attenuated in sensitized TdT−/− mice compared to wt mice (p < 0.05 (IL13 and IL-4)). (d–f) The levels of the cytokines IL-6, IL-10 and INF-γ showed no differences between the two genotypes. All levels were normalized to wt control (mean shown as blue lines, SEM shown as black bars).
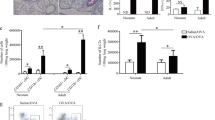
Eosinophilic influx into the airways is reduced in TdT−/− mice
Eosinophilic infiltration is associated with the development of local allergic airway inflammation. Thus, to assess the local inflammatory response in the airways, the influx of eosinophilic granulocytes into the BAL fluids was determined (Fig. 3). The number of eosinophils in BAL fluids in non-sensitized mice of either genotype proved negligible. As expected, sensitization followed by aerosolic challenge with OVA resulted in a significant increase in the levels of eosinophils in the BAL fluids of both wt (p < 0.05, 3.66 × 105 ± 0.37 × 105 cells/mL; mean ± SEM) and TdT−/− mice (p < 0.0001, 2.04 × 105 ± 0.36 × 105 cells/mL; mean ± SEM). However, when compared to wt, the eosinophilic influx was markly reduced in the TdT−/− animals (p = 0.06).
Content of eosinophils in bronchoalveolar lavage (BAL) fluids. In BAL fluids of wt and TdT−/− mice, both sensitized and non-sensitized, content of eosinophils were measured using a pulse area counter. No eosinophils were detectable in the BAL fluids of non-sensitized animals. This applies to both genotypes, wt and TdT−/−. Sensitization and aerosolic challenge with OVA caused a significant influx of eosinophils (wt p < 0.001 and TdT−/− p < 0.0001) in both genotypes. However, this influx was markly reduced in TdT−/− mice compared to wt mice (p = 0.06; mean shown as blue lines, SEM shown as black bars).
Airway hyperresponsiveness is unset in TdT−/− mice
To assess airway hyperresponsiveness, the response to inhaled methacholine was investigated (Fig. 4). The provocation concentration 50 (PC50) value was determined for statistical comparison of the individual groups. This value describes the concentration of methacholine (in mg/ml) that leads to a 50% decrease in the MEF50 value. For the wt animals, PC50 was significantly reduced in the group of OVA sensitized and challenged mice compared to the PBS control (p < 0.01). There is considerable indication that the degree of airway reactivity differed between the two genotypes, as in TdT−/− mice, PC50 values were indistinguishable between sensitized and non-sensitized animals. Thus, OVA-induced airway hyperreactivity was abolished in the TdT−/− group.
Lung function analysis—methacholine response. Head-out body plethysmography was used to assess lung function on day 29. Methacholine provocation was performed in the headout body plethysmograph to evaluate airway responsiveness. The PC50 value represents the provocation concentration of methacholine required to induce significant airway obstruction. In wt animals, PC50 was significantly reduced in the group of OVA-sensitized and challenged mice compared to the PBS control (p < 0.01). In TdT−/− mice, no significant difference was found between OVA-sensitized animals and the control, thus airway hyperreactivity was attenuated (mean shown as blue lines, SEM shown as black bars).
Discussion
In a mouse model of experimental allergic inflammation, we found that CDR3 repertoires somatically diversified by N nucleotide addition are required for the development of a fully established allergic airway inflammation.
During ontogeny, as new cell types appear and tissues and organs are created, the developing humoral immune system encounters a progressively increasingly complex array of self-antigens. Sequential exposure to these normal ‘neo’antigens poses a risk for the development and survival of potentially pathogenic autoreactive immunoglobulins. The evolutionary adaptation in mammals that allows implantation of their embryos in the mother's womb creates an additional immunological problem22. Intimate contact with the mother's uterine tissue and leakage between the fetal and maternal circulations make maternal cells, tissues and organs yet another potential target for the developing immune system of the embryo and fetus. The infant inherits only half of maternal gene polymorphisms, thus the developing embryo and fetus can be considered a 'semi-allograft', with the potential for an attack on the mother by antibodies generated by her child.
Unsurprisingly, there is strong evidence that humoral immune responses in the mammalian embryo and fetus are selectively suppressed. This was made evident more than 40 years ago when the existence of a homologous, controlled, programmed hierarchy of antigen responsiveness was identified in lambs, mice, and humans23,24. This finding appeared paradoxical given that lymphocyte antigen receptor repertoires, immunoglobulin (Ig)25 and T cell receptor (TCR)26, were presumed to be generated in a stochastic fashion through the process of random VDJ rearrangement and N addition. However, it was subsequently shown that both the process of VDJ rearrangement and the presence and extent of N addition are regulated during embryonic, fetal and neonatal development, restricting the diversity of both repertoires in the womb.
The focus of antigen receptor diversity is the third complementary determining region of the V domain (CDR3), which is somatically created by V(D)J rearrangement and N nucleotide addition. CDR3 regions are located at the center of the antigen binding site, as classically defined, and thus typically play a commanding role in antigen recognition and binding27. Although restriction of antigen receptor CDR3 repertoires is common during mammalian ontogeny, the precise array and combination of mechanisms used vary by species. In mouse, the primary mechanism of repertoire control is the absence of terminal deoxynucleotidyl transferase (TdT) activity, the source of N nucleotide addition, until after birth, which restricts the diversity of both the Ig heavy (H) chain28,29 and TCRβ13 repertoires to germline content. Use of individual VH and, to a lesser extent, DH are also regulated30.
We and others have tested for benefits and detriments to lymphocyte development and immune function in TDT deficiency. The primary benefit to the absence of N nucleotides, and thus a focus on a germline-encoded TCR and Ig repertoire, is enhanced efficiency of positive selection and a more rapid population of lymphoid organs31,32.
With two exceptions, the effect on antibody production, T cell function, specificity, and pathogen neutralization is either neutral (e.g. ovalbumin KLH)33 or negative34. In T cells, the peripheral repertoire is more polyreactive and less peptide-oriented than is the N+ repertoire31. Total antibody production to a large array of antigens is neutral or reduced32. N nucleotide deficient CD8+ memory T cells mediate poor recall responses compared to adults and are comprised of a repertoire of lower avidity35,36. Responses towards some epitopes are skewed36, and heterosubtypic immunity to influenza virus is abrogated34. This manuscript was focused on testing one additional gap in our knowledge, i.e. the contribution of N nucleotide diversity to CD4 T cell mediated immune responses in general, and to allergens in specific.
Here we demonstrate a role of N addition in the function of a TH2 CD4 T cell-dependent IgE immune response to an allergen. We observe that the absence of N nucleotides leads to a complex disturbance of an allergen-induced inflammatory network, indicating a differentially altered sensitization phase and effector phase of this CD4 T cell dependent allergic response. The initial sensitization with ovalbumin using Alum as an adjuvant specifically led to an altered TdT deficient TH2 type antibody response since total IgG1 levels were increased whereas total IgE levels were decreased when compared to wt mice.
Allergen-specific IgE levels proved similar in TdT−/− and wildtype mice indicating that the absence of N nucleotides did not entirely impair the absolute quantity of specific antibody production. This finding is in accordance with previous reports on other antigens21,31,37,38,39. However, the TH2 cytokines IL-4 and IL-13 were reduced in the BAL fluids of TdT−/− mice, indicating that at least part of the problem reflected an effect of the altered repertoire on the intrinsic function of CD4 T cells, themselves, potentially due to the skewing of epitope recognition that occurs in the absence of N addition36. Unexpectedly, the allergic inflammation appeared to be reduced in the TdT−/− mice in comparison to wt, as shown by reduced influx of eosinophils and an attenuated bronchial hyperresponsiveness to methacholine. However, head-out body plethysmography must be interpreted cautiously because of the difference in sample numbers, and it remains unclear whether the apparent difference between the wt and TdT−/− control groups reflects an intrinsic difference in airway response between the two genotypes or experimental variance.
Increased total IgG1 levels and reduced total IgE levels indicate that in TdT−/− mice the cytokine milieu contains more TH1 components than in wt controls. The weakened TH2 cytokine bias might result from a disruption of the positive feedback loop that usually links the sensitization phase that yields specific IgE under the influence of TH2 cytokines and the effector phase that follows re-exposure to the allergen and TH2 cytokine release by mast cells and basophils40,41,42,43,44,45, potentially due to changes in epitope recognition patterns. Based on the results of this study, future investigations should be conducted to provide better insight into the underlying mechanisms.
The polyreactivity of the B-cell repertoire of TdT−/− mice is reduced compared to wt animals38. Thus, it remains unclear if the allergen specific IgE antibodies expressed by TdT−/− mice have the same affinity as those of wildtype mice. Just like T cell patterns of epitope recognition, it can be speculated that specific IgE produced by TdT−/− mice might not recognize the complete range of epitopes of ovalbumin bound by wild type IgE, thereby narrowing its reactivity to the allergen46. CDR-H3 length has significant influence on the tertiary structure of the antigen binding site47 and the length of CDR-H3 regions correlates with the nature of recognized antigens48. Thus TdT−/− mice might be able to produce a similar quantity of allergen specific IgE as wildtype mice, but a broader, wildtype spectrum of allergen specific antibodies might be required to yield a clinically relevant allergic airway response. This would support the hypothesis that allergies may represent a misled oligoclonal allergen-specific immune response.
The OVA-induced airway hyperresponsiveness to methacholine ws reduced in TdT−/− mice when compared to wild-type mice. The expression of IL-5 is normally triggered by elevated levels of IL-4, IL-13 and IgE. Intriguingly, the concentration of IL-5 was not reduced in BAL fluids of TdT−/− mice. These observations support the hypothesis that the initial IgE-mediated inflammation was triggered without T-cell stimulation. Allergic inflammation does not exclusively depend on a TH2-dependent, IgE-mediated allergic type 1 immune response. Other factors, such as innate lymphoid cells (ILC2s), can also contribute by secreting IL-5 and IL-13, which in turn contribute to the adaptive type 2 immune response49,50,51,52. Since the concentration of IL-5 in BAL fluids in TdT−/− mice was similar to wildtype, but the number of eosinophils tend to be reduced, mediators other than IL-5 might be involved in regulating the influx of eosinophils into the BAL fluids53,54. We favor the view that the reduced number of eosinophils in the BAL fluids can be attributed to the disturbed overall cytokine profile in TdT−/− mice.
Although the diversity of both the Ig and TCR repertoire is restricted during ontogeny in both human and mouse, the precise timing and details differ55. The range of CDR-H3 lengths in humans is much larger compared to mice, although the murine CDR-H3 repertoire does not represent a subset of that of humans56. Moreover, in mouse the Ig and TCR repertoires remain deficient in N addition until days after birth; whereas in human N addition is limited in the embryo, begins to increase in the fetus in the second trimester of pregnancy, and achieves an adult phenotype by 6 months after birth57. Thus, it is unclear if these results can be transferred to human on a one-for-one basis. It also needs to be taken into account that these results might not necessarily reflect all type one allergies other than OVA. However, recent studies have shown that protection against allergy may begin in the womb, with maternal IgG being transferred to the embryo from the second trimester on, offering protection against allergen sensitization58. Thus, it is possible that exposure to allergens in the human womb in mothers who lack protective IgG may promote expression of a ‘locked-in’ allergen-sensitive Ig or TCR repertoire that will result in an increased risk of allergy after birth. Consequently, the differences in repertoire pre and post birth could lead to markedly different outcomes.
Studies of T cell function in the absence of TdT have either been general (e.g.33, or focused on CD8 T cell function35,36,59,60. Our findings suggest that equal attention should be placed on the study of the effects of the role of N addition in CD4 T cell epitope recognition and function61.
The mucosa associated lymphatic tissue is a regulator of the adaptive immune response and closely interacts with intestinal microbiota which are considered key regulators of health and disease62,63,64. To date, to the best of our knowledge, the microbiome of TdT−/− mice has not been characterized in detail. However, the microbiome of RAG deficient mice that are unable to perform somatic recombination of the immunoglobulin heavy an light chains, is severely biased65,66,67,68,69. Thus, it is likely that TdT−/− mice also exhibit an altered microbiome. It can be speculated that an altered microbiome may have an influence on the establishment of the allergic airway inflammation in TdT−/− mice.
This study was able to shed light on just the section of the complex immune network that depends on the activity of TdT. The results that we obtained indicate that the absence of TdT leads to an alteration in the pattern of cytokine production after allergic sensitization and challenge; a feature that had not been described previously. Further studies are therefore necessary in order to present a detailed mechanism in this context. To identify further TdT-affected key components of the immune system, -omics experiments should be considered. In addition, it is necessary to investigate which specific genetic changes concerning the TCR might lead to a rescue of the TdT phenotype.
In conclusion, we found indications that the allergic phenotype is attenuated in a murine model of allergic airway inflammation in mice expressing “fetal-like” Ig and TCR repertoires, as reflected by the absence of N nucleotides. We hypothesize that a type 1 allergy is not only mediated by the antibodies specifically reacting in the ELISA, but properties of the N deficient TCR repertoire may also be involved in establishing and maintaining a normal TH2 cytokine profile that leads to the influx of eosinophils and airway hyperreactivity.
Material and methods
Study approval
The study was ethically approved by the governmental authority (Regierungspräsidium Giessen, Dezernat V54-Veterinärwesen; reference V54-19c20-15 (1) MR36-Nr. 05/2007). All procedures were performed in accordance with relevant guidelines and regulations.
Mice
We have used a previously described TdT−/− mouse strain on a BALB/c background21,31 using wildtype mice as controls (Harlan Winkelmann (Borchen, Germany)). All animals were kept under pathogen-free conditions in single ventilated cage systems. At a constant temperature of 20 °C, the mice were exposed to an artificial light–dark rhythm of 12/12 h. The animals were offered an ovalbumin-free diet and water ad libitum for food and drinking water intake. The study was carried out in compliance with ARRIVE guidelines.
Test protocol
The chronological sequence of operations and analyses was designed as follows (Fig. 5). Before starting of the test series, the concentration of individual antibody classes was measured in serum by ELISA. On days 1, 14, and 21, the mice were intraperitoneally sensitized with either the allergen ovalbumin (OVA) or with PBS as a control. On days 26, 27, and 28, the aerosol provocation was again performed with either OVA or PBS. The resulting effects on serum antibody concentration were subsequently determined by ELISA. In addition, lung function was examined. Bronchoalveolar lavage (BAL) was performed to assess both the number of eosinophils and the amount of characteristic cytokines in the BAL fluids.
Test protocol. Mice were sensitized to ovalbumin (OVA) by intraperitoneal injections on days 1, 14, and 21. To induce an allergic airway inflammation, mice received allergen challenges via the airways delivered by nebulization with OVA on days 26, 27 and 28. The control group received PBS instead of OVA. To quantify the immunoglobulin classes in serum using ELISA, blood samples were taken on the day before the first sensitization and on day 30. Head-out body plethysmography including methacholine provocation was used to assess the lung function on day 29. Bronchoalveolar lavage was performed on day 30. BAL fluids were evaluated for cytokine levels as well as eosinophil content.
Protocol of allergic sensitization
The study included four groups of mice: (A) nonsensitized wt mice (Wt PBS), (B) sensitized wt mice (Wt OVA), (C) nonsensitized TdT−/− mice, and (D) sensitized TdT−/− mice. Mice were sensitized to ovalbumin (OVA) as previously described8,20. 10 µg of OVA grade IV (Sigma, Germany) were adsorbed to 1.5 mg Al(OH)3 (Imject® Alum; Pierce, Rockford, Ill., USA) and administered by intraperitoneal injections on days 1, 14, and 21. To induce an allergic airway inflammation, mice received allergen challenges via the airways delivered by nebulization of 1% (w/v) OVA grade V (Sigma), diluted in PBS, for 20 min on days 26, 27, and 28. Control mice received PBS intraperitoneally by intraperitoneal injections on days 1, 14, and 21 and were challenged on days 26, 27, and 28 with aerosolic PBS (Table 1).
Determination of antibody titers
To quantify the immunoglobulin levels in serum, blood samples were taken on the day before the first sensitization and on day 30. Serum concentrations of total and allergen-specific IgE and IgG1 were measured by ELISA as previously described20. Antibodies and standards were purchased from BD (Heidelberg, Germany). Serum samples were diluted 1:500 (IgG1) and 1:100,000 (IgE) for OVA sensitized mice. For non-sensitized controls, samples were diluted 1:10 (IgG1) and 1:2 (IgE), respectively. Determination of OVA-specific IgE was performed as described in8.
Assessment of lung function
As previously described, head-out body plethysmography was used to assess lung function on day 2940. Methacholine provocation was performed in the headout body plethysmograph to evaluate airway responsiveness (AR). The animals were not anaesthesized during the determination of lung function. After a period of acclimatization and recording of the baseline, the mice inhaled aerosol PBS. Methacholine was then applied in ascending concentration (6.25–100 mg/mL). The mean expiratory flow at which 50% of the tidal volume in ml/s has been exhaled (MEF50) was used to assess airflow limitation. The PC50 value represents the provocation concentration of methacholine required to induce significant airway obstruction.
Bronchoalveaolar lavage
On day 30, bronchoalveaolar lavage was performed as described previously8: the trachea was cannulated and the airways were lavaged with 1.6 mL ice-cold PBS supplemented with proteinase inhibitors (Complete®; Boehringer, Mannheim Germany). Using a pulse area counter (Casy®, Schärfe, Reutlingen, Deutschland), the cell numbers were ascertained. Cells were centrifugated onto slides, differentially stained with DiffQuik® (Behring, Marburg, Germany), and classified by light microscopy.
Determination of cytokine levels in bronchoalveolar lavage fluids
In the BAL fluids, the concentration of interleukin 4 (IL-4), IL-5, IL-6, IL-10, IL-13, and interferon-γ (INF-γ) was determined. For the analysis, the Basic Kit FlowCytomix for mouse/rat (Bender Medsystems; Vienna, Austria) was utilized according to manufacturer's specifications using a FACSCalibur FlowCytometer (BD, Franklin Lakes, USA).
Statistical analysis
Data are presented as mean ± SEM. The two‐tailed Mann–Whitney–U-test was used to determine statistical significance. p < 0.05 was considered statistically significant. For labelling in illustrations, the following symbols are used: *p < 0.05; **p < 0.01; ***p < 0.001; ****0.0001. Statistics were performed using GraphPad Prism software V8.02 (GraphPad Software, La Jolla, CA, USA).
Abbreviations
- BAL:
-
Bronchoalveolar lavage
- CD:
-
Cluster of differentiation
- CDR3:
-
Complementarity determining region 3
- CDR-H3:
-
Immunoglobulin heavy-chain complementarity-determining region 3
- DH :
-
Diversity
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- ILC2:
-
Innate lymphoid cell
- MEF:
-
Mean expiratory flow
- OVA:
-
Ovalbumin
- PBS:
-
Phosphate buffered saline
- PC:
-
Provocation concentration
- SEM:
-
Standard error of the mean
- TCR:
-
T cell receptor
- TdT:
-
Terminal deoxyribonucleotidyl transferase
- Wt:
-
Wild type
References
Hoogsteden, H. C., Verhoeven, G. T., Lambrecht, B. N. & Prins, J. B. Airway inflammation in asthma and chronic obstructive pulmonary disease with special emphasis on the antigen-presenting dendritic cell: Influence of treatment with fluticasone propionate. Clin. Exp. Allergy 29(Suppl 2), 116–124 (1999).
Kubo, M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol. Rev. 278, 162–172 (2017).
Scadding, G. Cytokine profiles in allergic rhinitis. Curr. Allergy Asthma Rep. 14, 435 (2014).
Mitsias, D. I., Savvatianos, S. & Papadopoulos, N. G. An insight into the early mechanisms of allergen-specific immunotherapy. Immunotherapy 3, 333–336 (2011).
Gould, H. J. & Sutton, B. J. IgE in allergy and asthma today. Nat. Rev. Immunol. 8, 205–217 (2008).
Stokes, J. Anti-IgE treatment for disorders other than asthma. Front. Med. 4, 152 (2017).
Navinés-Ferrer, A., Serrano-Candelas, E., Molina-Molina, G.-J. & Martín, M. IgE-related chronic diseases and anti-IgE-based treatments. J. Immunol. Res. 2016, e8163803 (2016).
Kerzel, S. et al. Composition of the immunoglobulin classic antigen-binding site regulates allergic airway inflammation in a murine model of experimental asthma. Clin. Exp. Allergy 39, 591–601 (2009).
Snow, R. E., Chapman, C. J., Holgate, S. T. & Stevenson, F. K. Clonally related IgE and IgG4 transcripts in blood lymphocytes of patients with asthma reveal differing patterns of somatic mutation. Eur. J. Immunol. 28, 3354–3361 (1998).
Dahlke, I., Nott, D. J., Ruhno, J., Sewell, W. A. & Collins, A. M. Antigen selection in the IgE response of allergic and nonallergic individuals. J. Allergy Clin. Immunol. 117, 1477–1483 (2006).
Kerzel, S., Rogosch, T., Struecker, B., Maier, R. F. & Zemlin, M. IgE transcripts in the circulation of allergic children reflect a classical antigen-driven B cell response and not a superantigen-like activation. J. Immunol. 185, 2253–2260 (2010).
Kerzel, S. et al. Unlike in children with allergic asthma, IgE transcripts from preschool children with atopic dermatitis display signs of superantigen-driven activation. J. Immunol. 196, 4885–4892 (2016).
Bogue, M., Candéias, S., Benoist, C. & Mathis, D. A special repertoire of alpha:beta T cells in neonatal mice. EMBO J. 10, 3647–3654 (1991).
George, J. F. & Schroeder, H. W. Developmental regulation of D beta reading frame and junctional diversity in T cell receptor-beta transcripts from human thymus. J. Immunol. 148, 1230–1239 (1992).
Mortari, F., Wang, J. Y. & Schroeder, H. W. Human cord blood antibody repertoire. Mixed population of VH gene segments and CDR3 distribution in the expressed C alpha and C gamma repertoires. J. Immunol. 150, 1348–1357 (1993).
Schroeder, H. W., Zhang, L. & Philips, J. B. Slow, programmed maturation of the immunoglobulin HCDR3 repertoire during the third trimester of fetal life. Blood 98, 2745–2751 (2001).
Bauer, K. et al. Diversification of Ig heavy chain genes in human preterm neonates prematurely exposed to environmental antigens. J. Immunol. 169, 1349–1356 (2002).
Zemlin, M. et al. The postnatal maturation of the immunoglobulin heavy chain IgG repertoire in human preterm neonates is slower than in term neonates. J. Immunol. 178, 1180–1188 (2007).
Rogosch, T. et al. IgA response in preterm neonates shows little evidence of antigen-driven selection. J. Immunol. 189, 5449–5456 (2012).
Kerzel, S. et al. A single DH gene segment is sufficient for the establishment of an asthma phenotype in a murine model of allergic airway inflammation. Int. Arch. Allergy Immunol. 156, 247–258 (2011).
Gilfillan, S., Dierich, A., Lemeur, M., Benoist, C. & Mathis, D. Mice lacking TdT: Mature animals with an immature lymphocyte repertoire. Science 261, 1175–1178 (1993).
Trowsdale, J. & Betz, A. G. Mother’s little helpers: Mechanisms of maternal-fetal tolerance. Nat. Immunol. 7, 241–246 (2006).
Schroeder, H. W. Similarity and divergence in the development and expression of the mouse and human antibody repertoires. Dev. Comp. Immunol. 30, 119–135 (2006).
Silverstein, A. M. Ontogeny of the immune response. Science 144, 1423–1428 (1964).
Rajewsky, K. Clonal selection and learning in the antibody system. Nature 381, 751–758 (1996).
Davis, M. M. The evolutionary and structural ‘logic’ of antigen receptor diversity. Semin. Immunol. 16, 239–243 (2004).
Schroeder, H. W. & Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 125, S41-52 (2010).
Feeney, A. Comparison of junctional diversity in the neonatal and adult immunoglobulin repertoires. Int. Rev. Immunol. 8, 113–122 (1992).
Perlmutter, R. M., Kearney, J. F., Chang, S. P. & Hood, L. E. Developmentally controlled expression of immunoglobulin VH genes. Science 227, 1597–1601 (1985).
Schelonka, R. L. et al. DH and JH usage in murine fetal liver mirrors that of human fetal liver. Immunogenetics 62, 653–666 (2010).
Gilfillan, S., Benoist, C. & Mathis, D. Mice lacking terminal deoxynucleotidyl transferase: Adult mice with a fetal antigen receptor repertoire. Immunol. Rev. 148, 201–219 (1995).
Schelonka, R. L. et al. Absence of N addition facilitates B cell development, but impairs immune responses. Immunogenetics 63, 599–609 (2011).
Gilfillan, S. et al. Efficient immune responses in mice lacking N-region diversity. Eur. J. Immunol. 25, 3115–3122 (1995).
Nguyen, H. H. et al. Heterosubtypic immunity to influenza A virus infection requires a properly diversified antibody repertoire. J. Virol. 81, 9331–9338 (2007).
Rudd, B. D. et al. Acute neonatal infections ‘lock-in’ a suboptimal CD8+ T cell repertoire with impaired recall responses. PLoS Pathog. 9, e1003572 (2013).
Haeryfar, S. M. M. et al. Terminal deoxynucleotidyl transferase establishes and broadens antiviral CD8+ T cell immunodominance hierarchies. J. Immunol. 181, 649–659 (2008).
Komori, T., Okada, A., Stewart, V. & Alt, F. W. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science 261, 1171–1175 (1993).
Weller, S. et al. Autoantibodies in mice lacking terminal deoxynucleotidyl transferase: Evidence for a role of N region addition in the polyreactivity and in the affinities of anti-DNA antibodies. J. Immunol. 159, 3890–3898 (1997).
Benedict, C. L., Gilfillan, S., Thai, T. H. & Kearney, J. F. Terminal deoxynucleotidyl transferase and repertoire development. Immunol. Rev. 175, 150–157 (2000).
Glaab, T. et al. Tidal midexpiratory flow as a measure of airway hyperresponsiveness in allergic mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 280, L565–L573 (2001).
Shiokawa, S. et al. IgM heavy chain complementarity-determining region 3 diversity is constrained by genetic and somatic mechanisms until two months after birth. J. Immunol. 162, 6060–6070 (1999).
Holgate, S. T. & Polosa, R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 8, 218–230 (2008).
Punnonen, J., Yssel, H. & de Vries, J. E. The relative contribution of IL-4 and IL-13 to human IgE synthesis induced by activated CD4+ or CD8+ T cells. J. Allergy Clin. Immunol. 100, 792–801 (1997).
Smirnov, D. V., Smirnova, M. G., Korobko, V. G. & Frolova, E. I. Tandem arrangement of human genes for interleukin-4 and interleukin-13: Resemblance in their organization. Gene 155, 277–281 (1995).
Akdis, M. & Akdis, C. A. Therapeutic manipulation of immune tolerance in allergic disease. Nat. Rev. Drug Discov. 8, 645–660 (2009).
Mine, Y. & Yang, M. Epitope characterization of ovalbumin in BALB/c mice using different entry routes. Biochim. Biophys. Acta 1774, 200–212 (2007).
Ramsland, P. A., Kaushik, A., Marchalonis, J. J. & Edmundson, A. B. Incorporation of long CDR3s into V domains: Implications for the structural evolution of the antibody-combining site. Exp. Clin. Immunogenet. 18, 176–198 (2001).
Collis, A. V. J., Brouwer, A. P. & Martin, A. C. R. Analysis of the antigen combining site: correlations between length and sequence composition of the hypervariable loops and the nature of the antigen. J. Mol. Biol. 325, 337–354 (2003).
Pasha, M. A., Patel, G., Hopp, R. & Yang, Q. Role of innate lymphoid cells in allergic diseases. Allergy Asthma Proc. 40, 138–145 (2019).
Kim, H. Y., DeKruyff, R. H. & Umetsu, D. T. The many paths to asthma: Phenotype shaped by innate and adaptive immunity. Nat. Immunol. 11, 577–584 (2010).
Walker, J. A., Barlow, J. L. & McKenzie, A. N. J. Innate lymphoid cells—How did we miss them?. Nat. Rev. Immunol. 13, 75–87 (2013).
Bartemes, K. R. et al. IL-33–responsive lineage−CD25+CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. Immunol. 188, 1503–1513 (2012).
Roufosse, F. Targeting the interleukin-5 pathway for treatment of eosinophilic conditions other than asthma. Front. Med. 5, 49 (2018).
Yamaguchi, Y. et al. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J. Exp. Med. 167, 1737–1742 (1988).
Zemlin, M., Schelonka, R. L., Bauer, K. & Schroeder, H. W. Regulation and chance in the ontogeny of B and T cell antigen receptor repertoires. Immunol. Res. 26, 265–278 (2002).
Zemlin, M. et al. Expressed murine and human CDR-H3 intervals of equal length exhibit distinct repertoires that differ in their amino acid composition and predicted range of structures. J. Mol. Biol. 334, 733–749 (2003).
Rechavi, E. & Somech, R. Survival of the fetus: Fetal B and T cell receptor repertoire development. Semin. Immunopathol. 39, 577–583 (2017).
Fouda, G. G., Martinez, D. R., Swamy, G. K. & Permar, S. R. The impact of IgG transplacental transfer on early life immunity. Immunohorizons 2, 14–25 (2018).
Leon-Ponte, M., Kasprzyski, T., Mannik, L. A. & Haeryfar, S. M. M. Altered immunodominance hierarchies of influenza A virus-specific H-2(b)-restricted CD8+ T cells in the absence of terminal deoxynucleotidyl transferase. Immunol. Invest. 37, 714–725 (2008).
Kedzierska, K. et al. Terminal deoxynucleotidyltransferase is required for the establishment of private virus-specific CD8+ TCR repertoires and facilitates optimal CTL responses. J. Immunol. 181, 2556–2562 (2008).
Fazilleau, N. et al. Valpha and Vbeta public repertoires are highly conserved in terminal deoxynucleotidyl transferase-deficient mice. J. Immunol. 174, 345–355 (2005).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Huus, K. E., Petersen, C. & Finlay, B. B. Diversity and dynamism of IgA–microbiota interactions. Nat. Rev. Immunol. 1, 12. https://doi.org/10.1038/s41577-021-00506-1 (2021).
Zhao, Q. & Elson, C. O. Adaptive immune education by gut microbiota antigens. Immunology 154, 28–37 (2018).
Kwon, O., Lee, S., Kim, J.-H., Kim, H. & Lee, S.-W. Altered gut microbiota composition in Rag1-deficient mice contributes to modulating homeostasis of hematopoietic stem and progenitor cells. Immune Netw. 15, 252–259 (2015).
Thoene-Reineke, C. et al. Composition of intestinal microbiota in immune-deficient mice kept in three different housing conditions. PLoS ONE 9, e113406 (2014).
Gálvez, E. J. C., Iljazovic, A., Gronow, A., Flavell, R. & Strowig, T. Shaping of intestinal microbiota in Nlrp6- and Rag2-deficient mice depends on community structure. Cell Rep. 21, 3914–3926 (2017).
Lindner, C. et al. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J. Exp. Med. 209, 365–377 (2012).
Zhang, H., Sparks, J. B., Karyala, S. V., Settlage, R. & Luo, X. M. Host adaptive immunity alters gut microbiota. ISME J. 9, 770–781 (2015).
Acknowledgements
We thank Regina Stoehr and Sabine Jennemann for excellent technical assistance. The study was supported by German Research Council (DFG) SFB/TR22, TP A17 (MZ) and BMBF PRIMAL study (01GL1746D).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
R.S. wrote the first draft of the manuscript and contributed to data evaluation. C.M. performed the data evaluation and statistical analysis. T.R., S.K., M.Z. designed the study. E.K. supported organizational and coordinative tasks. All authors contributed to writing the manuscript, read and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stutz, R., Meyer, C., Kaiser, E. et al. Attenuated asthma phenotype in mice with a fetal-like antigen receptor repertoire. Sci Rep 11, 14199 (2021). https://doi.org/10.1038/s41598-021-93553-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93553-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.