Abstract
Nontyphoidal Salmonella, an important zoonotic pathogen and a major cause of foodborne illnesses, could be a potential reservoir of plasmids harbouring mobile colistin resistance gene (mcr). This study reported, for the first time, a high rate of mcr-carrying Salmonella clinical isolates (3.3%, 24/724) in Thailand, associated with mcr-3 gene (3.0%, 22/724) in S. 4,[5],12:i:-(15.4%, 4/26), S. Typhimurium (8.8%, 5/57), and S. Choleraesuis (5.6%, 13/231). Remarkably, the increasing trends of colistin and extended-spectrum cephalosporin resistances have displayed a high agreement over the years, with a dramatic rise in the mcr-carrying Salmonella from 1.1% (6/563) during 2005–2007 to 11.2% (18/161) during 2014–2018 when CTX-M-55 became abundant. Clonal and plasmid analysis revealed that the self-transferable IncA/C and a novel hybrid IncA/C-FIIs MDR plasmids were the major vehicles to disseminate both mcr-3 and blaCTX-M55 genes among diverse Salmonella strains, from as early as 2007. To our knowledge the occurrence of mcr-3 and the co-existence of it with blaCTX-M-55 in S. Choleraesuis are reported here for the first time, leading to clinical concern over the treatment of the invasive salmonellosis. This study provides evidence of the potential reservoirs and vectors in the dissemination of the mcr and highlights the co-selection by colistin and/or cephalosporins.
Similar content being viewed by others
Introduction
Antimicrobial resistance (AMR) is the greatest public health threat that can cross between human and other animal populations. This problem requires action at global, regional, and national levels to tackle it. Recently, the World Health Organisation published a catalogue of 12 families of antimicrobial-resistant bacteria that pose the greatest threat to human health1. These bacteria are resistant to a large number of antimicrobials, especially to carbapenems and extended-spectrum cephalosporins (ESCs) that are the best available antibiotics for treating multi-drug resistant (MDR) Gram-negative bacteria. This then leads to the use of colistin, a widely used antimicrobial in animal production for decades, as one of the last-resort therapeutic options2. Currently, colistin resistance has become of significant concern, due to the identification of plasmid-mediated colistin resistance, conferred by the mobile colistin resistance (mcr) gene.
The mcr-1 gene was first identified in Escherichia coli mainly in swine isolates (> 20%) during 2011 to 20143. Nowadays, ten mcr-like genes (mcr-1 to mcr-10) have been reported among human and animal bacterial isolates3,4,5,6,7,8,9,10,11,12. Recently, a high frequency of mcr genes was reported in nontyphoidal Salmonella, an important zoonotic pathogen and a major cause of foodborne illnesses in many countries, including China13,14,15, Taiwan16, France17, Germany17, and Portugal18,19. Interestingly, at least six types of mcr genes (mcr-1 to -5 and mcr-9)4,6,7,16,20,21 have been identified in many serotypes of S. enterica, including S. Typhimurium16,22, S. Rissen20, S. 1,4,[5,12:i:-20, S. Anatum16, S. Albany16, S. Newport16, S. Derby22, S. Indiana22, and S. London22. In addition, at least three types of mcr genes (mcr-4, -5, and -9) had their first discovery in a S. enterica strain6,7,11. However, in contrast to the extensive studies focused on E. coli, there is only limited data available for mcr-harbouring Salmonella isolates and data on the plasmids carrying mcr genes responsible for the spread of colistin resistance.
Moreover, the mcr genes have been described in diverse genetic environments, on a wide variety of plasmid types, including the IncI2, IncHI2, and IncX4 plasmids13,14,15,16,18,19, especially in animal isolates. Notably, the co-occurrence of mcr genes and extended-spectrum beta-lactamases (ESBLs) have been reported since 2016 from E. coli calf isolates in France23 and continuously recognised among both E. coli and Salmonella isolates, leading to the assumed relationship between the ESBLs and mcr gene21,23,24,25,26. Considering that the combination of these genes could speed up the dissemination of MDR and extensively drug resistance (XDR) among Gram-negative bacteria, information regarding the co-occurrence and emergence of mcr and ESBL genes among the isolates are needed.
Here, we aimed to assess the dissemination mechanism of colistin resistance among Salmonella isolates, and the co-occurrence of mcr and ESBL genes in Salmonella clinical isolates in Thailand, the AMR mechanisms, clonality, and plasmid profiles of the isolates, as well as the transferability and characteristics of the resistance plasmids were investigated.
Results
Bacterial strains and antimicrobial susceptibility
A total of 724 nontyphoidal Salmonella isolates were recovered from the clinical samples collected from 32 out of 77 provinces in Thailand during the two periods of 2005–2007 (n = 563) and 2014–2018 (n = 161). More than 40 serotypes were identified from these isolates, most of which were S. Enteritidis (43.0%, 311/724), followed by S. Choleraesuis (31.9%, 231/724), S. Typhimurium (7.9%, 57/724), and S. 4,5,12:I:-(3.6%, 26/724). A total of 422 isolates (58.3%) were from bacteremic patients, most of which were S. Choleraesuis (46.2%, 195/422) and S. Enteritidis (43.4%, 183/422). Overall, a high rate of AMR was found among 724 nontyphoidal Salmonella isolates from patients in Thailand. Most isolates (75.3%, 545/724) were resistant to at least three classes of antimicrobial agents, whereas less than 5% of isolates (35/724) were susceptible to all antimicrobial agents tested. The most common resistance was to nalidixic acid (84.9%, 615/724), followed by ampicillin (77.4%, 560/724), tetracycline (50.6%, 366/724), chloramphenicol (32.0%, 232/724), gentamicin (26.9%, 195/724), trimethoprim-sulfamethoxazole (20.9%, 151/724), and ciprofloxacin (10.9%, 79/724). A high frequency of ESC-resistant Salmonella was observed in 22.9% of isolates (166/724), most of which were S. Choleraesuis (78.9%, 131/166). Of the 166 ESC-resistant isolates, 125 isolates (75.3%) were isolated from bacteremic patients. Resistance to ertapenem was not found in this study. Colistin susceptibility of nontyphoidal Salmonella clinical isolates in Thailand revealed a minimum inhibitory concentration (MIC) of colistin ranging from 0.25–32 µg/mL. Of note, a high rate of colistin resistance (18.8%, 136/724) with MIC range of 4–32 µg/mL was identified among the isolates.
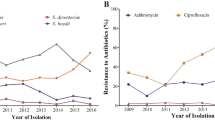
The AMR frequency in Salmonella isolates was considerably higher in those isolated during 2014–2018 than in 2005–2007 for all tested antimicrobial agents except for nalidixic acid and gentamicin (Fig. 1a). Notably, during those periods, a significant increase in the frequency of colistin resistance from 12.8% (72/563) to 39.8% (64/161) of isolates (p < 0.0001) was found between these two time periods. Over 2005–2018, the frequency of colistin resistance increased as follows: 2005 (6.7%, 5/74), 2006 (3.1%, 1/32), 2007 (14.7%, 67/457), 2014 (54.2%, 13/24), 2015 (75.0%, 3/4), 2016 (33.3%, 1/3), 2017 (34.3%, 25/73), and 2018 (40.4%, 23/57). We found that the increasing trend of colistin resistance and ESC resistance frequencies displayed a high agreement with each other over those time periods (Fig. 1b). Remarkably, an increasing frequency of mcr-carrying isolates was found from 1.1% (6/563) in 2005–2007 to 11.2% (18/161) in 2014–2018 (Supplementary Fig. S1).
The AMR among 724 nontyphoidal Salmonella isolated from humans in Thailand during 2005–2007 and 2014–2018. (a) Comparison of AMR frequency in nontyphoidal Salmonella isolates between 2005–2007 and 2014–2018. (b) Frequency of ESC resistance and colistin resistance over time for nontyphoidal Salmonella isolated from patients from 2005–2016. AMP ampicillin, CRO ceftriaxone, CAZ ceftazidime, GEN gentamicin, TE tetracycline, CIP ciprofloxacin, NAL nalidixic acid, CHL chloramphenicol, SXT trimethoprim-sulfamethoxazole, ERT ertapenem, COL colistin, ESCs extended-spectrum cephalosporins.
Comparison of the AMR between ESC-resistant Salmonella and non-ESC-resistant Salmonella isolates from the whole strain collection revealed that ESC-resistant Salmonella isolates displayed significantly higher resistance frequencies in all the tested antimicrobial agents (p < 0.0001) than in the non-ESC-resistant Salmonella isolates (Fig. 2a). Notably, we found that ESC-resistant Salmonella isolates had significantly higher rates of colistin resistance (35.5% (59/166) vs. 13.8% (77/558), p < 0.0001) and the presence of mcr genes (10.2% (17/166) vs. 1.3% (7/558), p < 0.0001) than in the non-ESC-resistant Salmonella isolates (Fig. 2a). In addition, among the ESC-resistant Salmonella isolates, a significant increase in the frequency of mcr-harbouring isolates from 5% of the isolates (6/120) in 2005–2007 to 23.9% (11/46) in 2014–2017 (p < 0.0001) was detected. During 2005–2007, CMY-2 (55.8%, 67/120) was the most common mechanism of ESC resistance detected among the ESC-resistant isolates. The emergence of CTX-M-55 was detected in all 46 ESC-resistant Salmonella isolates during 2014–2018, one of which displayed both CTX-M-55 and TEM-135 (Fig. 2b).
(a) Comparison of the AMR observed between ESC-resistant Salmonella and non ESC-resistant counterparts from the 724 nontyphoidal Salmonella isolated from patients in Thailand during 2005–2007 and 2012–2016. (b) Resistance mechanisms detected among ESC-resistant Salmonella isolates in 2005–2007 and 2014–2018. AMP ampicillin, GEN gentamicin, TE tetracycline, CIP ciprofloxacin, NAL nalidixic acid, CHL chloramphenicol, SXT trimethoprim-sulfamethoxazole, ERT ertapenem, COL colistin, ESCs extended-spectrum cephalosporins; *, p < 0.05; and **, p < 0.01.
Of the 24 mcr-harbouring Salmonella isolates, 22 isolates carried mcr-3 variant genes (21 for mcr-3.1 and one for mcr-3.2) with a colistin MIC range from 4–32 μg/mL, and two isolates carried the mcr-1.9 gene with a colistin MIC of 8 μg/mL. Markedly, of the 166 ESC-resistant Salmonella isolates, 17 carried the mcr-3 gene, and 15 and two isolates co-harboured the mcr-3 and blaCTX-M55 or blaCMY-2 gene, respectively. The high prevalence of mcr-3 genes was found in S. Typhimurium and S. 4,5,12:I:-, a monophasic variant of S. Typhimurium, with 8.8% (5/57) and 15.4% (4/26) of isolates, respectively. Moreover, the mcr-3.1 gene was detected in 5.6% of S. Choleraesuis isolates (13/231), all of which were from bacteremic patients and most of which co-harboured both blaCTX-M55 and qnrS1 genes.
Clonal relatedness
All 24 mcr-harbouring Salmonella isolates (13 S. Choleraesuis, 5 S. Typhimurium, and 6 S. 4,5,12:I:-) were subtyped by PFGE, revealing that they formed six PFGE clusters (designated A to F) and 17 different pulsotypes, when using a cut-off of 80% and 95% genetic similarity, respectively (Fig. 3). The most common pulsotype (A1) contained four mcr3.1-carrying S. Choleraesuis isolates obtained from four different bacteremic patients in Bangkok during 2014–2015, two of which displayed a high-level colistin MIC (16 and 32 μg/mL, respectively) and were resistant to all antimicrobial agents tested except for ertapenem. These isolates had the D87G amino acid substitution in GyrA and carried the blaCTX-M55, blaTEM-1, and qnrS1 genes. Pulsotype E1, a major pulsotype among the S. 4,5,12:I:- isolates, contained three mcr-harbouring S. 4,5,12:I:- isolates (two of mcr-3.1 and one of mcr-1.9) from two different provinces (Bangkok and Chiang Mai) in 2015 and 2017. One mcr3.1-carrying S. 4,5,12:I:- blood isolate was resistant to all antimicrobial agents tested except for ertapenem. In addition, this isolate also carried some other plasmid-borne AMR determinants, including blaCTX-M55, blaTEM-135, and qnrS1 genes. The most common pulsotype among the mcr-harbouring S. Typhimurium isolates, pulsotype E5, contained three indistinguishable mcr-3.1-carrying S. Typhimurium isolates obtained from stool and rectal swab from two different provinces (Ratchaburi and Nonthaburi) in Thailand in 2007. These isolates displayed an ESC-resistance phenotype that was mediated by the blaCTX-M55 and blaTEM-1 genes.
Dendrogram generated by PFGE-XbaI of 24 mcr-harbouring Salmonella isolates from humans in Thailand. Isolate summary information showing antimicrobial susceptibility profiles, colistin MIC, AMR mechanisms, plasmid profiles, and pulsotypes. Black squares represent isolates that were resistant to antimicrobial agents. Antimicrobial agents are abbreviated as follows: AMP ampicillin, CRO ceftriaxone, CAZ ceftazidime, GEN gentamicin, TE tetracycline, CIP ciprofloxacin, NAL nalidixic acid, CHL chloramphenicol, SXT trimethoprim-sulfamethoxazole, ERT ertapenem, COL colistin, BKK Bangkok, CBR Chonburi, ST Satun, CHM Chiang Mai, RBR Ratchaburi, NTB Nonthaburi, PMQR plasmid-mediated quinolone resistance, ND not determined; and -, not found. *Plasmid sizes and AMR mechanisms confirmed for the location on the plasmid by Southern blot and hybridisation are underlined by a single line or a double line.
Plasmid characterisation
The 22 mcr-3-carrying Salmonella isolates and two mcr-1.9-carrying Salmonella isolates were characterised for plasmid profiles by S1-PFGE and the locations of the AMR genes were identified by Southern blot hybridisation using the specific probes for the respective genes. The transferability of mcr-carrying plasmid was determined in all mcr-carrying Salmonella isolates using transconjugation experiment. Of 24 mcr-carrying Salmonella isolates, 14 isolates successfully transferred mcr gene to the recipient, seven of which co-transferred mcr-3.1 and blaCTX-M-55 genes. Remarkably, the self-transferable mcr-1.9-carrying plasmid, 33 kb-IncX4, was spread among two different clones (E1 and E2) of S. 4,5,12:I:- isolated from a stool sample from a patient in Chiang Mai in 2017 (Fig. 3). In addition, the mcr-3.1 gene was disseminated among at least five genetically unrelated clones (cluster A, C, D, E, and F) via various plasmids of IncA/C, IncA/C-IncFIIs, IncFIIs, and IncFII, with a size ranging from 40–283 kb (Fig. 3). The plasmids displayed high conjugation efficiencies, ranging from 2.3 × 10–5 to 1.3 × 10–2 colony forming units (CFU) per recipient cell. Of particular note, probe hybridisation revealed that the mcr-3.1 and blaCTX-M-55 genes were co-located on the same plasmid in seven of the mcr-3.1-carrying Salmonella isolates, three of which also co-existed with the qnrS1 gene (Figs. 3 and 4). The co-dissemination of both mcr-3.1 and blaCTX-M-55 genes in S. Typhimurium isolates in all cases was mediated by the ~ 170-kb conjugative plasmid IncA/C, which was spread among two different clones (E5 and E6) of S. Typhimurium isolated from a stool and a rectal swab in 2007 (Fig. 4). Moreover, the co-dissemination of these genes in S. Choleraesuis isolates was mediated by two multi-replicon plasmids (IncA/C and IncFIIs), which had self-transferable plasmid sizes of 66-kb, 83-kb, and 168-kb (Fig. 4). The dendrogram revealed that these conjugative plasmids were disseminated among at least two genetically unrelated clones (cluster A and F) of S. Choleraesuis, isolated from three different bacteremic patients in Chiang Mai during 2017 to 2018 (Fig. 3). Interestingly, the co-transference of colistin and ESC resistance was explored in seven Salmonella isolates by conjugation, using the azide-resistant E. coli K12 as a recipient. High conjugation efficiencies were demonstrated, ranging from 3.3 × 10–4 to 5.0 × 10–4 CFU per recipient cell in these resistance plasmids (Table 1), while their transconjugants showed a colistin MIC ranging from 4–8 μg/mL and a ceftriaxone MIC ranging from 64–256 μg/mL. The transconjugants exhibited 16- to 32-fold and 64- to 256-fold increases in the MICs of colistin and ceftriaxone, respectively, compared with those in a recipient (Table 1). Remarkably, most isolates were also co-transferred with the resistance to tetracycline, gentamicin, and chloramphenicol (Table 1). In addition, the recombinant plasmid of MCR-3.1 displayed a slight colistin effect, with a 16-fold increase in the MIC of colistin from 0.25 to 4 μg/mL, compared with those in E. coli DH5α containing pBK-CMV alone. The recombinant CTX-M-55 plasmid resulted in a 32-fold increase in the MIC of ceftriaxone from 1 to 32 μg/mL compared with those in E. coli DH5α containing pBK-CMV alone (Table 1).
Plasmid profile analysis of nontyphoidal Salmonella isolates with conjugative plasmid carrying the mcr-3 gene together with blaCTX-M-55 gene by S1-PFGE and Southern blot hybridisation. Representative (a) PFGE profiles of total DNA digestion with S1 nuclease, and (b,c) relative hybridisation with the (b) mcr-3 probe and (c) blaCTX-M-55 probe. Lane 1, plasmid profile analysis of S. Choleraesuis strain H-17–072; lane 2, S. Choleraesuis strain H-17–073; lane 3, S. Choleraesuis strain H-17–053; lane 4, S. Typhimurium strain H-07–020; lane 5, S. Typhimurium strain H-07–021; lane 6, S. Typhimurium strain H-07–037; lane 7, S. Typhimurium strain H-07–038; lane 8, S. Typhimurium strain H-07–385; lane 9, S. Typhimurium strain H-07–388; lane M, CHEF DNA Size Standard-Lambda Ladder (#170–3635), marker labels are in kilo-bases. Arrows indicate the locations of AMR plasmids.
Discussion
Since the first discovery of the mcr gene in China in late 20153, the mcr-1 gene has rapidly spread globally within Enterobacteriales across the 30 countries in five continents27. To date, nine other mcr genes have been identified (mcr-24, mcr-35, mcr-46, mcr-57, mcr-68, mcr-79, mcr-810, mcr-911, and mcr-1012). In Thailand, a few studies have investigated the mcr genes, mostly mcr-1, and only in E. coli and Klebsiella pneumoniae isolates, which found approximately 2% and 1% of isolates during 2014–2018, respectively28,29,30. The mcr gene was first recognised in Thailand in three of 179 E. coli clinical isolates in Chachoengsao province, Central Thailand, in as early as 201430.
As far as we are aware, this study reports here for the first time a high prevalence of colistin resistance and mcr genes in nontyphoidal Salmonella isolated from patients in Thailand. The occurrence of mcr genes might be more common in Salmonella with 11.2% of isolates during 2014–2018 than those previously recognised by other studies, which reported only a low frequency of mcr-positive Salmonella (about 1%) among clinical isolates during 2012–201531. Remarkably, the current high frequency of mcr genes in Salmonella isolates was attributed to the dissemination of the mcr-3 gene. Despite the mcr-3 gene was first discovered in porcine E. coli isolates in China in 20155, it was detected in Salmonella isolated from patients in 2007 from our retrospective study of Salmonella clinical isolates during 2005–2007 in Thailand. Thus, it should be noted that the mcr gene, especially mcr-3.1, has already spread in Thailand since at least 2007, the earliest isolation time point of the mcr-harbouring isolates among our collection of Salmonella clinical isolates. Currently, the mcr-3 gene has become more widespread and prevalent in Salmonella, S. Typhimurium, and a monophasic variant of S. Typhimurium in particular21,24,25. The clonal spread of the MDR ST34 S. Typhimurium (including monophasic variants) was observed in China during 2014–2019, during which time the global distribution was confirmed by the high similarity between the genomes of mcr-3-harboring Salmonella isolated from across four continents25. Interestingly, most patients with mcr-3-positive Salmonella infections from Denmark, Canada, USA, and Australia had a travel history to Thailand, Vietnam, or China, during 2013–201625.
Moreover, we found some AMR plasmids supporting the dissemination of the mcr-3 gene among diverse genetic backgrounds, including the IncA/C, IncHI2, IncFII, and IncA/C-IncFIIs plasmids. These plasmids have been described in E. coli and in few serotypes of Salmonella that have mostly been recovered from food animal isolates25. Remarkably, two distinct monophasic variants of S. Typhimurium isolates carried the mcr-1.9 gene on IncX4 plasmids with an identical size of 33 kb. In accordance with previous studies, the 33-kb IncX4 plasmid carrying the mcr-1.9 gene has been identified in unrelated E. coli and K. pneumoniae isolates from humans and food-producing animals in many countries, including China, Italy, United States, Brazil, and Portugal32,33.
The present study reported an increasing frequency of AMR among nontyphoidal Salmonella clinical isolates from all six regions of Thailand over the last 8 years, in which more than three-quarters of the isolates showed a MDR phenotype. Remarkably, a significantly higher proportion of mcr gene positive isolates were recognised in the ESC-resistant Salmonella isolates than in the non-ESC-resistant counterparts. More worrisome, the frequency of colistin and ESC resistance have dramatically increased over recent years and a high proportion of MDR Salmonella co-harbouring mcr-3 and blaCTX-M-55 genes has been described in this study. Interestingly, the co-localisation of mcr-3 and blaCTX-M-55 genes on the same plasmid was recognised in a high proportion of these isolates (7/15, 46.7%), which were attributed to the self-transferable MDR plasmids of the ̴170-kb IncA/C (formerly known as IncC) and novel hybrid IncA/C-IncFIIs (size of 66-kb, 83-kb, and 168-kb) plasmids in nonclonal S. Typhimurium and S. Choleraesuis strains, respectively. The self-transferable IncA/C plasmid, a broad host range plasmid, has previously demonstrated an important role in the dissemination of the blaCTX-M-55 gene among S. Choleraesuis in blood isolates, leading to the high prevalence of ESC resistance in Thailand in recent years34. The hybrid IncA/C-IncFIIs plasmid shared the same backbone modules of both IncA/C and IncFIIs (species-specific FII replicons for Salmonella) plasmids, where the genetic plasticity of the plasmid probably shaped the dissemination of the resistance determinants, intensifying the spread of MDR and XDR in Salmonella. This is similar to a previous study, where an IncC-FII hybrid plasmid played a role in the widespread distribution of ST34 in S. Typhimurium and a monophasic variant co-carrying mcr-3 and blaCTX-M-55 genes in China or even globally, mediated by IS15DI25. The specific genetic background is required for acquisition and maintenance of mcr-carrying plasmids.
Our study demonstrates that the mcr genes were disseminated through neither plasmid nor clone specific where the clonality analysis revealed a polyclonal spread. However, three indistinguishable mcr-3.1-carrying S. Typhimurium were identified in two different provinces in 2007, probably a small outbreak at that time. The mcr-3 has rapidly disseminated and become the most common mcr gene among the isolates. The IncA/C plasmid could probably be the major vehicle of the spread of mcr-3 in Thailand from as early as 2007. Moreover, this study, taken together with previous studies25, indicates that the dissemination of the mcr-3-carrying MDR S. Typhimurium and monophasic serovar could potentially become globally spread and of great concern. Remarkably, to our knowledge the occurrence of mcr-3 and its co-existence with blaCTX-M-55 were described for the first time among S. Choleraesuis in the present study, all of which were from bacteremic patients. This becomes a clinical concern over the treatment of the invasive salmonellosis. With the highest ability to cause septicemia (OR 44.00; 95% CI 34.28–56.47), S. Choleraesuis was previously ranked the most common recovered serotype from bacteremic patients in Thailand34,35. Noticeably, a high proportion of colistin-resistant Salmonella without the mcr gene was observed in this study, underlining chromosomal mutations and other resistance mechanisms. The common chromosomal mutations that have been reported in Salmonella involve the PmrA/PmrB and PhoP/PhoQ two-component regulatory systems36.
Notably, both colistin and cephalosporins (more specifically ceftiofur) have been extensively used in food animal systems over the past few decades in many countries37. Considering that the frequencies of both mcr-3-positive Salmonella and Salmonella co-carrying mcr-3 and blaCTX-M-55 genes have arisen over those same time periods, this could be attributed to the co-selection by colistin and/or cephalosporins in both a veterinary field and human clinical setting. This notion was supported by the high agreement between the increasing trends of colistin resistance and ESC resistance over the past 8 years, as demonstrated in the present study. This highlights the urgent need to strengthen an AMR control strategy to prevent further spread, since this poses a threat to global health due to travel and trade in animal products.
Materials and methods
Bacterial strains
A total of 724 non-duplicate nontyphoidal Salmonella clinical isolates from various hospitals in 32 provinces from different regions of Thailand during 2005–2007 (n = 563) and 2014–2018 (n = 161) were characterised. Clinical isolates used in this study were obtained from a repository collection after standard characterisation and identification as part of the standard care of the patients that was unrelated to the present study. There were 620 isolates obtained from the Salmonella and Shigella Center, National Institute of Health, Department of Medical Sciences (Nonthaburi, Thailand) and 104 isolates from the Department of Microbiology, King Chulalongkorn Memorial Hospital (Bangkok, Thailand), Queen Savang Vadhana Memorial Hospital (Chonburi, Thailand), Phra Pok Klao Hospital (Chanthaburi, Thailand), and Surin Hospital (Surin, Thailand). Most samples (32.3%) were collected from patients in Bangkok. The common specimen isolated were from blood, stool, rectal swab, and urine, most of which were from blood (58.3% of isolates). The isolates were identified by biochemical characteristics and the serotyping of S. enterica was performed according to the Kauffman-White serotyping scheme38.
Antimicrobial susceptibility testing
The antimicrobials ampicillin, ceftriaxone, ceftazidime, gentamicin, tetracycline, ciprofloxacin, nalidixic acid, chloramphenicol, trimethoprim-sulfamethoxazole, ertapenem, and colistin were obtained from Sigma-Aldrich (St. Louis, MO, USA). MICs of the antimicrobial agents were determined by the agar-dilution technique, except the broth-microdilution technique was used for colistin. The Clinical and Laboratory Standards Institute criteria were used for interpreting the MICs39.
Detection of AMR determinants
A total of 724 Salmonella isolates were screened for the presence of mcr-1 to mcr-9 genes by multiplex PCR using both the primers designed in this study (Supplementary Table S1) and those previously described9,40. The DNA sequences of the entire mcr genes were determined by PCR amplification and DNA sequencing of the amplicons using the indicated primers (Supplementary Table S1).
All isolates for which MICs of ≥ 2 μg/mL for either ceftriaxone or ceftazidime were further confirmed for the presence of the blaOXA, blaTEM, blaSHV, blaCTX-M, and blaVEB genes by multiplex PCR as previously described41,42,43. Specific CTX-M groups (CTX-M-1, CTX-M-2, CTX-M-9, and CTX-M-8/25 groups) were also investigated by multiplex PCR44, as was the presence of plasmid-mediated ampC genes, as previously described45. The obtained DNA sequences of the entire bla genes were determined by PCR amplification and DNA sequencing as previously reported34.
A total of 79 ciprofloxacin-resistant Salmonella isolates were determined for the quinolone resistance-determining region (QRDR) mutations in the gyrA and parC genes, and plasmid-mediated quinolone resistance (PMQR) genes. The gyrA and parC genes were PCR-amplified and sequenced using the previously described primers and method46, and the nucleotide sequences were compared to those of S. Typhimurium LT246. The presence of the PMQR genes, including qnrA, qnrB, qnrC, qnrD, qnrS, aac(6’)-Ib-cr, and qepA, was screened by PCR as previously described47,48.
Clonal analysis
Pulsed-field gel electrophoresis (PFGE) was used to determine the clonal relatedness of the mcr-harbouring Salmonella isolates using the PulseNet International protocol 2017 from the Centers for Disease Control and Prevention. Total bacterial DNA, in the form of low-melt agarose plugs, was cut with XbaI (Fermentas, USA) and separated using a CHEF-Mapper XA PFGE system (Bio-Rad, Hercules, USA). The PFGE patterns were compared by InfoQuestTMFP Software, version 4.5 (Bio-Rad, Hercules, USA). The relatedness of the clones was determined using the Dice coefficient, unweighted pair group method with arithmetic means (UPGMA), 1.0% optimisation, and 1.0% band position tolerance, and interpreted based on the percentage similarities and Tenover criteria49.
Transfer of colistin resistance
Twenty-four mcr-carrying Salmonella isolates were determined for the transferability of colistin resistance by transconjugation experiment using the broth-mating technique and an azide-resistant E. coli K12 strain as the recipient, as previously described50. Transconjugants were selected from the MacConkey agar plate containing 150 μg/mL of sodium azide and 2 µg/mL of colistin and, confirmed by PCR. The MICs of antimicrobial agents for the donor, recipient, and transconjugant strains were determined by the agar-dilution technique, except the broth-microdilution technique was used for colistin. After that, their MICs were compared. The conjugation efficiencies of these mcr-carrying Salmonella isolates were then determined.
Plasmid analysis
Plasmid profiles of all mcr-carrying isolates were characterised by PFGE using S1 nuclease (S1-PFGE). The plasmid DNA was first linearised by S1 nuclease, where the total bacterial DNA in low-melt agarose plugs was digested with S1 nuclease (Fermentas, USA) and separated using a CHEF-Mapper XA PFGE system (Bio-Rad, Hercules, USA). A Lambda ladder (Bio-Rad, Hercules, USA) was used as the molecular-weight size marker and InfoQuestTMFP Software, version 4.5 was used to estimate the plasmid sizes. The locations of the mcr and bla genes in the plasmids were determined by Southern blot hybridisation using specific probes. Probe labelling, hybridisation, and detection were performed using the DIG DNA labelling and detection kit (Roche Diagnostics, Indianapolis, IN, USA), according to the manufacturer’s protocols. The major plasmid types found in Enterobacteriales, including FIA, FIB, FIC, HI1, HI2, I1-IƳ, L/M, N, P, W, T, A/C, K, B/O, X, Y, F, FIIA, and X4 replicon types, were detected by PCR-based replicon typing 51,52.
Cloning of AMR genes
The AMR genes, mcr-3.1 and blaCTX-M-55 were PCR amplified using the primers shown in Supplementary Table S1. These PCR products were cloned into the pTZ57R/T TA vector (Fermentas, UK) and subcloned into the pBK-CMV expression vector (Stratagene, La Jolla, CA) with EcoRI and ApaI digestion. The recombinant vectors were transformed into E. coli DH5α and then selected for transformants on LB agar plates containing 50 μg/mL of kanamycin using BlueWhite colony screening. The selected colonies were confirmed for correct inserts by PCR and DNA sequencing.
Statistical analysis
The significance of any differences in the AMR profile was determined by Fisher's exact test (two-tailed) using the GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA), accepting significance at the p < 0.05 level.
Ethical approval
The study protocol was approved by the Mahidol University Central Institutional Review Board (MU-CIRB), Mahidol University (Nakhon Pathom, Thailand) [MU-CIRB 2019/032.0402]. All experiments were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments and comparable ethical standards.
Informed consent
For this type of study of anonymised clinical isolates, the requirement for informed consent from patients was waived by the Mahidol University Central Institutional Review Board (MU-CIRB), Mahidol University (Nakhon Pathom, Thailand) [MU-CIRB 2019/032.0402].
Data availability
The data specific to the clinical isolates that support the findings of this study are not publicly available because of privacy/ethical restrictions. However, upon reasonable request, the data will be available from the corresponding author T.C.
References
El-Azizi, M. et al. Evaluating antibiograms to monitor drug resistance. Emerg. Infect. Dis. 11, 1301–1302. https://doi.org/10.3201/eid1108.050135 (2005).
Rhouma, M., Beaudry, F., Theriault, W. & Letellier, A. Colistin in pig production: Chemistry, mechanism of antibacterial action, microbial resistance emergence, and one health perspectives. Front. Microbiol. 7, 1789. https://doi.org/10.3389/fmicb.2016.01789 (2016).
Liu, Y. Y. et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. https://doi.org/10.1016/S1473-3099(15)00424-7 (2016).
Xavier, B. B. et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. https://doi.org/10.2807/1560-7917.ES.2016.21.27.30280 (2016).
Yin, W. et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio https://doi.org/10.1128/mBio.00543-17 (2017).
Carattoli, A. et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. https://doi.org/10.2807/1560-7917.ES.2017.22.31.30589 (2017).
Borowiak, M. et al. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 72, 3317–3324. https://doi.org/10.1093/jac/dkx327 (2017).
AbuOun, M. et al. mcr-1 and mcr-2 (mcr-6.1) variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J. Antimicrob. Chemother. 73, 2904. https://doi.org/10.1093/jac/dky272 (2018).
Yang, Y. Q., Li, Y. X., Lei, C. W., Zhang, A. Y. & Wang, H. N. Novel plasmid-mediated colistin resistance gene mcr-71 in Klebsiella pneumoniae. J. Antimicrob.. Chemother. https://doi.org/10.1093/jac/dky111 (2018).
Wang, X. et al. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg. Microbes Infect. 7, 122. https://doi.org/10.1038/s41426-018-0124-z (2018).
Carroll, L. M. et al. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype typhimurium isolate. MBio https://doi.org/10.1128/mBio.00853-19 (2019).
Wang, C. et al. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 9, 508–516. https://doi.org/10.1080/22221751.2020.1732231 (2020).
Yang, Y. Q. et al. Co-occurrence of mcr-1 and ESBL on a single plasmid in Salmonella enterica. J. Antimicrob. Chemother. 71, 2336–2338. https://doi.org/10.1093/jac/dkw243 (2016).
Li, X. P. et al. Clonal spread of mcr-1 in PMQR-carrying ST34 Salmonella isolates from animals in China. Sci. Rep. 6, 38511. https://doi.org/10.1038/srep38511 (2016).
Yi, L. et al. mcr-1-Harboring Salmonella enterica Serovar Typhimurium Sequence Type 34 in Pigs, China. Emerg. Infect. Dis. 23, 291–295. https://doi.org/10.3201/eid2302.161543 (2017).
Chiou, C. S. et al. Dissemination of mcr-1-carrying plasmids among colistin-resistant Salmonella strains from humans and food-producing animals in Taiwan. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.00338-17 (2017).
El Garch, F. et al. mcr-1 is borne by highly diverse Escherichia coli isolates since 2004 in food-producing animals in Europe. Clin. Microbiol. Infect. 23, 51. https://doi.org/10.1016/j.cmi.2016.08.033 (2017).
Figueiredo, R. et al. Detection of an mcr-1-encoding plasmid mediating colistin resistance in Salmonella enterica from retail meat in Portugal. J. Antimicrob. Chemother. 71, 2338–2340. https://doi.org/10.1093/jac/dkw240 (2016).
Hu, Y., Liu, F., Lin, I. Y., Gao, G. F. & Zhu, B. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect. Dis. 16, 146–147. https://doi.org/10.1016/S1473-3099(15)00533-2 (2016).
Campos, J., Cristino, L., Peixe, L. & Antunes, P. MCR-1 in multidrug-resistant and copper-tolerant clinically relevant Salmonella 1,4,[5],12:i:- and S. Rissen clones in Portugal, 2011 to 2015. Euro Surveill 21. https://doi.org/10.2807/1560-7917.ES.2016.21.26.30270 (2016).
Litrup, E. et al. Plasmid-borne colistin resistance gene mcr-3 in Salmonella isolates from human infections, Denmark, 2009–17. Euro Surveill. https://doi.org/10.2807/1560-7917.ES.2017.22.31.30587 (2017).
Hu, Y. et al. Salmonella harbouring the mcr-1 gene isolated from food in China between 2012 and 2016. J. Antimicrob. Chemother. 74, 826–828. https://doi.org/10.1093/jac/dky496 (2019).
Haenni, M. et al. Co-occurrence of extended spectrum beta lactamase and MCR-1 encoding genes on plasmids. Lancet Infect. Dis. 16, 281–282. https://doi.org/10.1016/S1473-3099(16)00007-4 (2016).
Mulvey, M. R., Bharat, A., Boyd, D. A., Irwin, R. J. & Wylie, J. Characterization of a colistin-resistant Salmonella enterica 4,[5],12:i:- harbouring mcr-3.2 on a variant IncHI-2 plasmid identified in Canada. J Med Microbiol 67, 1673–1675. https://doi.org/10.1099/jmm.0.000854 (2018).
Sun, R. Y. et al. Global clonal spread of mcr-3-carrying MDR ST34 Salmonella enterica serotype Typhimurium and monophasic 1,4,[5],12:i:- variants from clinical isolates. J. Antimicrob. Chemother. 75, 1756–1765. https://doi.org/10.1093/jac/dkaa115 (2020).
Fukuda, A. et al. Co-harboring of cephalosporin (bla)/colistin (mcr) resistance genes among Enterobacteriaceae from flies in Thailand. FEMS Microbiol Lett 365, https://doi.org/10.1093/femsle/fny178 (2018).
Schwarz, S. & Johnson, A. P. Transferable resistance to colistin: A new but old threat. J. Antimicrob. Chemother. 71, 2066–2070. https://doi.org/10.1093/jac/dkw274 (2016).
Srijan, A. et al. Genomic Characterization of Nonclonal mcr-1-Positive Multidrug-Resistant Klebsiella pneumoniae from Clinical Samples in Thailand. Microb Drug Resist 24, 403–410. https://doi.org/10.1089/mdr.2017.0400 (2018).
Eiamphungporn, W. et al. Prevalence of the colistin resistance gene mcr-1 in colistin-resistant Escherichia coli and Klebsiella pneumoniae isolated from humans in Thailand. J. Glob. Antimicrob. Resist. 15, 32–35. https://doi.org/10.1016/j.jgar.2018.06.007 (2018).
Runcharoen, C. et al. Whole genome sequencing of ESBL-producing Escherichia coli isolated from patients, farm waste and canals in Thailand. Genome Med. 9, 81. https://doi.org/10.1186/s13073-017-0471-8 (2017).
Cui, M. et al. Prevalence and molecular characterization of mcr-1-positive Salmonella strains recovered from clinical specimens in China. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.02471-16 (2017).
Manageiro, V. et al. IncX4 plasmid carrying the new mcr-1..9 Gene variant in a CTX-M-8-producing Escherichia coli isolate recovered from swine. Front. Microbiol. 10, 367. https://doi.org/10.3389/fmicb.2019.00367 (2019).
Nang, S. C., Li, J. & Velkov, T. The rise and spread of mcr plasmid-mediated polymyxin resistance. Crit. Rev. Microbiol. 45, 131–161. https://doi.org/10.1080/1040841X.2018.1492902 (2019).
Luk-In, S. et al. High prevalence of ceftriaxone resistance among invasive Salmonella enterica serotype Choleraesuis isolates in Thailand: The emergence and increase of CTX-M-55 in ciprofloxacin-resistant S Choleraesuis isolates. Int. J. Med. Microbiol.. 308, 447–453. https://doi.org/10.1016/j.ijmm.2018.03.008 (2018).
Hendriksen, R. S. et al. Risk factors and epidemiology of the ten most common Salmonella serovars from patients in Thailand: 2002–2007. Foodborne Pathog. Dis. 6, 1009–1019. https://doi.org/10.1089/fpd.2008.0245 (2009).
Olaitan, A. O., Morand, S. & Rolain, J. M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 5, 643. https://doi.org/10.3389/fmicb.2014.00643 (2014).
Kempf, I., Jouy, E. & Chauvin, C. Colistin use and colistin resistance in bacteria from animals. Int. J. Antimicrob. Agents 48, 598–606. https://doi.org/10.1016/j.ijantimicag.2016.09.016 (2016).
Popoff MY, L. M. L. Antigenic formulas of the Salmonella serovars. 7th revision ed, (Institut Pasteur: WHO Collaborating Centre for Reference Research on Salmonella, 1992).
The Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100 (Clinical and Laboratory Standards Institute, Wayne, PA, 2020).
Rebelo, A. R. et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. https://doi.org/10.2807/1560-7917.ES.2018.23.6.17-00672 (2018).
Bonnet, R. et al. A novel CTX-M beta-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceae isolated in Brazil. Antimicrob. Agents Chemother. 44, 1936–1942 (2000).
Colom, K. et al. Simple and reliable multiplex PCR assay for detection of blaTEM, blaSHV and blaOXA-1 genes in Enterobacteriaceae. FEMS Microbiol. Lett. 223, 147–151. https://doi.org/10.1016/S0378-1097(03)00306-9 (2003).
Mabilat, C. & Courvalin, P. Development of “oligotyping” for characterization and molecular epidemiology of TEM beta-lactamases in members of the family Enterobacteriaceae. Antimicrob. Agents Chemother. 34, 2210–2216 (1990).
Xu, L., Ensor, V., Gossain, S., Nye, K. & Hawkey, P. Rapid and simple detection of blaCTX-M genes by multiplex PCR assay. J. Med. Microbiol. 54, 1183–1187. https://doi.org/10.1099/jmm.0.46160-0 (2005).
Perez-Perez, F. J. & Hanson, N. D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40, 2153–2162 (2002).
Eaves, D. J. et al. Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob. Agents Chemother. 48, 4012–4015. https://doi.org/10.1128/AAC.48.10.4012-4015.2004 (2004).
Kang, H. Y., Tamang, M. D., Seol, S. Y., & Kim, J. Dissemination of Plasmid-mediated qnr, aac(6')-Ib-cr, and qepA Genes Among 16S rRNA Methylase Producing Enterobacteriaceae in Korea. J. Bacteriol. Virol. 39, 173–182. https://doi.org/10.4167/jbv.2009.39.3.173 (2009).
Kehrenberg, C., Friederichs, S., de Jong, A., Michael, G. B. & Schwarz, S. Identification of the plasmid-borne quinolone resistance gene qnrS in Salmonella enterica serovar Infantis. J. Antimicrob. Chemother. 58, 18–22. https://doi.org/10.1093/jac/dkl213 (2006).
Tenover, F. C. et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 33, 2233–2239 (1995).
Ceccarelli, D., Salvia, A. M., Sami, J., Cappuccinelli, P. & Colombo, M. M. New cluster of plasmid-located class 1 integrons in Vibrio cholerae O1 and a dfrA15 cassette-containing integron in Vibrio parahaemolyticus isolated in Angola. Antimicrob. Agents Chemother. 50, 2493–2499. https://doi.org/10.1128/AAC.01310-05 (2006).
Carattoli, A. et al. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63, 219–228. https://doi.org/10.1016/j.mimet.2005.03.018 (2005).
Poirel, L. et al. Genetic features of MCR-1-producing colistin-resistant Escherichia coli isolates in South Africa. Antimicrob. Agents Chemother. 60, 4394–4397. https://doi.org/10.1128/AAC.00444-16 (2016).
Acknowledgements
We are grateful to the staff at the Salmonella and Shigella Center, National Institute of Health, Department of Medical Sciences, Nonthaburi, Thailand and at the Department of Microbiology, Faculty of Medicine, Chulalongkorn University, for providing nontyphoidal Salmonella isolates and for their cooperation.
Funding
This research was supported by the Ratchadapisek Somphot Fund for Postdoctoral Fellowship, Chulalongkorn University; the Ratchadapiseksompotch Fund, Chulalongkorn University (grant No. CU_GR_63_150_30_57); the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University; Mahidol University (grant No. A37/2562); and the Ministry of Higher Education, Science, Research and Innovation (MHESI) and Thailand Research Fund (TRF).
Author information
Authors and Affiliations
Contributions
S.C., P.C., N.S., T.S., M.T., S.C. collected the specimen and performed identification methods; S.L., N.K., U.R. performed phenotypical and genotypical methods; S.L., D.L.W, R.P., R.L., C.P. analysed the results, statistical analysis, and discussion; S.L., T.C., W.K. were the leaders of the team, planned the experiments, supervised data analysis, writing, and preparing the manuscript. All authors contributed to the study conception and design. All authors read, reviewed, and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luk-in, S., Chatsuwan, T., Kueakulpattana, N. et al. Occurrence of mcr-mediated colistin resistance in Salmonella clinical isolates in Thailand. Sci Rep 11, 14170 (2021). https://doi.org/10.1038/s41598-021-93529-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93529-6
This article is cited by
-
Evidence of international transmission of mobile colistin resistant monophasic Salmonella Typhimurium ST34
Scientific Reports (2023)
-
Isolation, susceptibility profiles and genomic analysis of a colistin-resistant Salmonella enterica serovar Kentucky strain COL-R
3 Biotech (2023)
-
Overcoming addition of phosphoethanolamine to lipid A mediated colistin resistance in Acinetobacter baumannii clinical isolates with colistin–sulbactam combination therapy
Scientific Reports (2022)
-
Plasmidome in mcr-1 harboring carbapenem-resistant enterobacterales isolates from human in Thailand
Scientific Reports (2022)
-
High prevalence of mgrB-mediated colistin resistance among carbapenem-resistant Klebsiella pneumoniae is associated with biofilm formation, and can be overcome by colistin-EDTA combination therapy
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.