Abstract
Studies based on self-reported alcohol consumption and telomere length show inconsistent results. Therefore, we studied the association between gamma-glutamyl transferase (GGT), a widely used biomarker of alcohol intake, and telomere length. The possible health relevance in young adulthood was explored by investigating cardiometabolic risk factors. Mixed modelling was performed to examine GGT and alcohol consumption in association with telomere length in buccal cells of 211 adults between 18 and 30 years old of the East Flanders Prospective Twin Survey. In addition, we investigated the association between GGT and cardiometabolic risk factors; waist circumference, systolic blood pressure, fasting glucose, HDL cholesterol, and triglycerides. Although we did not observe an association between self-reported alcohol consumption and telomere length, our results show that a doubling in serum GGT is associated with 7.80% (95% CI − 13.9 to − 1.2%; p = 0.02) shorter buccal telomeres, independently from sex, chronological age, educational level, zygosity and chorionicity, waist-to-hip ratio and smoking. The association between GGT was significant for all five cardiometabolic risk factors, while adjusting for age. We show that GGT, a widely used biomarker of alcohol consumption, is associated with telomere length and with risk factors of cardiometabolic syndrome, despite the young age of this study population.
Similar content being viewed by others
Introduction
Telomeres are the ends of the chromosomes and consist of TTAGGG tandem DNA-sequence repeats. They form a nucleoprotein complex and protect the chromosomes from degradation, which would eventually lead to loss of genetic information1. Telomeres shorten due DNA replication and are suggested to be particularly sensitive to oxidative stress2, as this can cause cleavage specifically at the polyguanosine sequence in the telomeric region3. GGG‐specific DNA damage may play an important role in increasing the rate of telomere shortening, which might impact aging and chronic diseases4. Telomere length is associated with several life-style factors in adults, including smoking5,6, stress7,8, diet9, education10, obesity11, and air pollution12,13. Some of these factors, such as maternal pre-pregnancy BMI and prenatal air pollution exposure, even explain the variance of telomere length at birth14,15,16. Telomere length is proposed as a biomarker of ageing and implicated in the pathology of several age-related chronic diseases, such as cardiovascular disease, osteoporosis, Alzheimer’s disease, cancer, and mortality17,18.
Epidemiologic studies investigating the association between alcohol consumption and telomere length show inconsistent results. Although some studies found no association19,20,21,22,23, a few studies24,25 suggest that heavy drinking is associated with shorter telomeres. A limitation of these studies is that alcohol consumption is based on self-reported intake and respondents may overestimate or underestimate the amount of alcohol consumed. Therefore, it is interesting to explore the association between markers of alcohol intake and telomere length. Gamma-glutamyl transferase (GGT) is a widely used biomarker of alcohol intake26. GGT maintains adequate levels of intracellular glutathione, a major antioxidant, to protect cells against oxidants which are produced during normal alcohol metabolism26. The primary role of cellular GGT is to degrade extracellular glutathione at the cell membrane into amino acids which can be used for the synthesis of intracellular glutathione26. Hence, elevated serum GGT might be an marker for oxidative stress27.
Emerging evidence demonstrates that telomere biology is linked to numerous age-related diseases17,18. However, further research is needed to investigate the causal involvement of telomere biology and to expose the mechanisms contributing to the development of these diseases. As telomere length, GGT is also strongly associated with risk of cardiovascular disease28, metabolic syndrome29,30, and mortality31,32. Although the association between GGT and telomere length is barely studied33,34. Advanced understanding of their interrelation already in young adulthood may be relevant to provide new insights on the underlying pathways of disease development. We hypothesize that elevated serum GGT would identify individuals with shorter telomere length. Furthermore, to explore in young adults the possible health implications of alcohol consumption and oxidative stress, indicated by high serum GGT concentrations, we investigate the association between GGT and cardiometabolic risk factors and the role of biological ageing.
Results
Characteristics of the study population
Our study population comprises 211 individuals; 69.2% (n = 146) of the participants included both twins from each twin pair, whereas the remaining 30.8% (n = 65) only had one participating twin from each twin pair (Table 1). Our sample comprised of 104 (49.3%) men and 107 (50.7%) women with a mean (SD) age of 22.8 (3.2) years. Our analysis included 68 (32.2%) dizygotic twins, 69 (32.7%) monozygotic-dichorionic twins and 74 (35.1%) monozygotic-monochorionic twins. The average number of reported alcohol consumptions per week (SD) was 5.4 units (7.9) and the median (IQR) serum GGT concentration was 16 U/L (12–20) with a minimum of 7 U/L and a maximum of 110 U/L. The mean T/S (SD) was 1.04 (0.28) in adult buccal cells. We observed a significant intra-pair correlation in serum GGT between twin 1 and twin 2 (r = 0.68, p < 0.001, n = 73). The correlation was significantly stronger (p = 0.01) in monozygotic twins (r = 0.85, p < 0.001, n = 47) compared with dizygotic twins (r = 0.49, p = 0.01, n = 26), suggesting a role for genetic influences (heritability of 72%). The correlation in relative adult telomere length in buccal cells was similar (p = 0.99) in monozygotic and dizygotic twins (Supplement Fig. 1).
Gamma-glutamyl transferase and telomere length
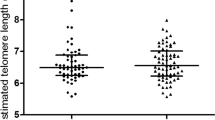
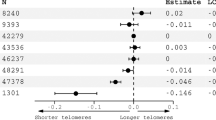
Serum GGT correlated inversely with telomere length in young adulthood (r = − 0.27, p < 0.0001) as shown in Fig. 1. A doubling in GGT is associated with 7.80% shorter telomeres (95% CI − 13.9 to − 1.2; p = 0.02) when adjusting for sex, age, zygosity and chorionicity, education twin, maternal education, waist-to-hip ratio and smoking (Fig. 2). As telomere length is highly variable at birth we additionally adjusted for telomere length at birth. In this analysis accounting for telomere length at birth, we show that a doubling in serum GGT was associated 11.86% shorter telomeres (95% CI − 17.7 to − 5.6; p = 0.001). The association between GGT and telomere length remained significant after adjusting for cardiometabolic risk factors. Finally, after additional adjustment for medication use possibly associated with GGT 14 days prior to examination, the association between GGT and telomere length remained significant (Fig. 2).
Gamma-glutamyl transferase in association with relative adult telomere length in buccal cells. The figure was plotted using GraphPad Prism version 5.00 (https://www.graphpad.com/).
Gamma-glutamyl transferase in association with relative adult telomere length in buccal cells. The main model is adjusted for sex, age (linear and quadratic), zygosity and chorionicity, education twin, maternal education, waist-to-hip ratio, and smoking (n = 200). The second model is additionally adjusted for telomere length at birth (n = 137). The third model is additionally adjusted for medication use, possibly associated with gamma-glutamyl transferase, during the last 14 days prior to the examination (n = 200). The figure was plotted using GraphPad Prism version 5.00 (https://www.graphpad.com/).
The number of alcohol consumptions per week (in units) was positively correlated with GGT (r = 0.29, p < 0.0001) (Supplement Fig. 2). However, we observed no correlation (r = − 0.03, p = 0.70) between the number of units alcohol consumptions per week and telomere length. Although we noted a negative correlation (r = − 0.15, p = 0.03) between the number of years of drinking and telomere length in young adulthood, this did not remain significant after adjusting for age.
Gamma-glutamyl transferase and telomere length in association cardiometabolic risk
We observed a significant association between GGT and cardiometabolic risk factors. After adjusting for age, a doubling in GGT is associated with a 0.10 mmol/l increase in fasting glucose (95% CI 0.01 to 0.18; p = 0.03), a 5.52 cm increase in waist circumference (95% CI 4.0 to 7.1; p ≤ 0.0001), a 11.11 mmol/l increase in triglycerides (95% CI 2.6 to 19.6; p = 0.01), a 4.91 mmol/l decrease in HDL cholesterol (95% CI − 8.7 to − 1.1; p = 0.01), a 5.03 mmHg increase systolic (95% CI 2.2 to 7.8; p = 0.0008) and a 2.37 increase diastolic blood pressure (95% CI 0.25 to 4.5; p = 0.03). We did not observe a significant association between telomere length and cardiometabolic risk factors after adjusting for age. Only a doubling in telomere length was significantly associated with a − 0.13 mmol/l decrease in glucose (95% CI − 0.25 to − 0.003; p = 0.05). These results are shown in Table 2.
Discussion
We investigated the association between serum gamma-glutamyl transferase, a biomarker of alcohol intake and cardiometabolic risk factors, and telomere length in 211 young adults. Although we did not observe an association between self-reported alcohol consumption and telomere length, we did observe that serum GGT has a negative association with telomere length in buccal cells. A doubling in serum GGT is associated with 7.80% shorter telomeres. This association remains significant after additional adjustment for telomere length at birth. Furthermore, adjusting for the use of specific medication35, which could potentially elevate GGT levels, did not influence the results.
Compared with our results, a recent study in 205 individuals in South Africa noted a significant negative correlation between leukocyte telomere length and GGT (r = − 0.16, p = 0.03)34. In contrast to our findings, no associations between telomere length and indicators of oxidative stress including GGT were observed in a study in 143 elderly Dutch men and 109 Greek elderly men33. Even though studies on GGT and telomere length are limited, a previous study in 372 persons already observed an association between the glutathione cycle and leukocyte telomere length36. They found a negative correlation between blood gamma-glutamyl amino acids and telomere length. These metabolites indicate increased oxidative stress due to alterations in the glutathione metabolism.
Therefore, oxidative stress may be a possible underlying mechanism between GGT and telomere length. GGT maintains the cytoplasmic homeostasis of glutathione, an important antioxidant which is crucial for detoxification of reactive oxygen species (ROS) as well as other toxic compounds27. Telomeres are especially sensitive to oxidative stress due to their triple-G containing structure and also have a low efficiency of DNA damage repair. As a result, telomeres containing oxidative damage will become successively shorter in each round of replication and the sequence beyond the damage will be lost2,37.
We show a positive correlation between GGT and the number of alcohol consumption per week. Our findings support GGT as a biomarker of alcohol use26. The well-known elevation of serum GGT after alcohol consumption could be a consequence of the increases the production of ROS after chronic and acute alcohol consumption27. Previous studies investigating the association between alcohol consumption, based on self-reported intake, and telomere length show inconsistent results. Although some studies found no association19,20,21,22,23, various other studies24,25 suggest that heavy drinking is associated with shorter telomeres. We did not observe an association between reported alcohol consumption and telomere length in our study population. This may be due to underreporting and biases regarding self-reported alcohol use, attenuating the true magnitude of the association.
Our findings have the potential to be relevant for public health since GGT is more than an indicator of alcohol consumption, GGT is strongly associated with risk of cardiovascular disease28 and metabolic syndrome29,30. A 2014 meta-analysis by Kunutsor et al. of 29 cohort studies showed an association between GGT and cardiovascular disease with an adjusted relative risk of 1.23 (1.16–1.29) per 1 standard deviation change in log GGT28, whereas a meta-analysis of 10 prospective cohort studies comprising 6595 incident metabolic syndrome cases, observed an association between GGT and metabolic syndrome with a relative risk of 1.88 (1.49–2.38) in the top versus the bottom thirds of baseline GGT29.
Although the number of metabolic syndrome cases is very low (n = 6) in our young study population (age between 18 and 30 years), we already observe that GGT is significantly associated with cardiometabolic risk factors defined by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) criteria for metabolic syndrome38. These include waist circumference, systolic blood pressure, fasting glucose, HDL cholesterol, and triglycerides38. Possible underlying processes of metabolic syndromes that have been postulated to be related to GGT are inflammation, oxidative stress, and insulin resistance30. Contrary to GGT, telomere length was not associated with cardiometabolic risk factors in this study population. A previous cross-sectional study including older participants, aged 18–65 years, showed that shorter telomeres are significantly associated with the presence of metabolic syndrome and all metabolic risk factors, except blood pressure39. Elucidating the underlying mechanism of both telomere length and GGT already in young adulthood will contribute to the understanding of the development of disease later in life. However, further research with a longitudinal study design, is needed to investigate whether GGT in young adulthood is a predictor of disease development later in life and to explore if GGT is directly associated with cardiometabolic risk or indirectly via telomere length. The answer to this question has the potential to provide opportunities in prevention of cardiovascular disease and metabolic syndrome.
It is an advantage that the results were obtained using mixed models since this method prevents false positive associations due relatedness structure between twin members and increases in power obtained through the application of a correction that is specific to this structure40. However, there are limitations to this study. First, DNA was collected via non-invasive buccal swabs. Although the absolute length of the telomeres might differ between tissues, within an individual telomere length is highly correlated between buccal cells and blood41,42. However, disadvantages include the lower quality of the DNA isolated from buccal swabs43 and the risk that poor oral hygiene or infection during collection might alter the oral cell composition due to infiltration of immune cells with a different telomere profile than buccal cells44. Compared to leukocyte telomeres which might be affected by a more diverse cell population in blood, buccal cell telomeres are less influenced by different types of cells44. A second limitation of our study is that no data is available on other markers of alcohol consumption, such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT). This would be an enhancement because GGT levels likely vary due to medications use, liver disease, and other etiological changes. Third, it is a limitation that our observations are made in a small study population as this may lead to a higher variability. Lastly, although we adjusted for potential major confounders that may affect both GGT and telomere length, we acknowledge that the main results cannot ascertain causality. Therefore, although we have hypothesised that variation in GGT influences telomere length, and undertaken the analyses accordingly, we cannot exclude the possibility that the association is confounded by factors, including genetic variants, that independently influence both telomere length and GGT, nor can we exclude reverse causation occurring when people with short telomeres are more prone to consuming alcohol and have higher GGT levels than people with longer telomeres.
Conclusions
We observed a strongly negative association between serum GGT and telomere length in buccal cells. Our results suggest that GGT, a biomarker of alcohol consumption, is a better predictor of telomere length than self-reported alcohol consumption. We suggest that the underlying mechanism may be oxidative stress. Despite the young age of our study population, we observed an association between GGT and indicators of cardiometabolic disease.
Materials and methods
Subjects
The twins in our study participated in a prenatal programming study45 comprising 424 twin pairs (804 individuals), part of The East Flanders Prospective Twin Study (EFPTS)46. The EFPTS, a population based register of multiple births in the province of East Flanders (Belgium), collects perinatal data and examines the placenta at birth since 1964. Telomere length in buccal cells was available for 214 individuals as described previously47. We excluded 3 participants because missing data on GGT (2) and on alcohol consumption (1). The present study sample consist of 211 young adults, born between 1969 and 1981. Written informed consent was obtained from all participants, and ethical approval was given by the Ethics Committee of the Faculty of Medicine of the Katholieke Universiteit Leuven. This study has been carried out according to the Helsinki declaration.
Data collection
Biometric and laboratory measurements of adult twins were obtained at the research centre during a 2-h morning session between February 1997 and April 200045. All methods were performed in accordance with relevant regulations and all measurements were carried out following standardized guidelines. In fasting blood samples, gamma-glutamyl transferase was measured using an Olympus AU600 Auto-Analyzer (Kyoto, Japan) and plasma glucose, HDL cholesterol, and triglycerides were measured using an auto‐analyzer (AU600; Olympus). Anthropometric measurements were performed by two trained researchers according to standardized procedures. Body mass index was calculated as body mass (in kg) divided by squared height (in m). Standing height was measured with a Harpenden fixed stadiometer (Holtain Ltd, United Kingdom) to the nearest 0.1 cm and body mass on a balance scale (SECA, Germany) to the nearest 0.1 kg. Waist and hip circumference were measured with a flexible steel tape to the nearest 0.1 cm. Waist circumference was taken between the costal margin and the iliac crest, and hip circumference at the widest part of the hips, generally at the level of the greater trochanters. The waist-to-hip ratio was calculated as the ratio of the circumference of the waist to that of the hips. Blood pressure was measured on the right arm in triplicate by sphygmomanometry and auscultation (Korotkoff phases I and V) in sitting position. The reported blood pressure is the average of the 3 measurements. Cardiometabolic risk factors were based on the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) criteria for metabolic syndrome and include waist circumference, systolic blood pressure, fasting glucose, HDL cholesterol, and triglycerides38.
The twins completed questionnaires to obtain information on smoking status, alcohol use, disease and medication use. Current smoking was expressed as yes/no and current alcohol use was analyzed as units per week. Each unit was equivalent to approximately 10–12 g alcohol intake. We obtained the international classification of disease, 10th revision (ICD10) codes for the disease status of the participants but no one was diagnosed with liver disease. Medication use possibly associated with increased GGT (such as anticonvulsants, antacids, retinoids, and antidepressants) during the last 14 days prior to the examination was expressed as yes/no. Zygosity was determined at birth by sequential analysis based on sex, choriontype, blood group determined on umbilical cord blood, placental alkaline phosphatase and, since 1982, DNA fingerprints48. After DNA-fingerprinting, a zygosity probability of 99.9% was reached.
Parental educational level as a proxy of socioeconomic status was categorized into three groups according to the Belgian education system, namely as no education or primary school, lower secondary education, and higher secondary education and tertiary education. Education level of the twin was categorized into two groups, higher secondary education or lower and tertiary education.
Telomere length measurements
Mouth swabs were taken and DNA was extracted with the QIAamp DNA micro kit (Qiagen, Venlo, The Netherlands). DNA purity and concentration were assessed using the Nanodrop 1000 spectrophotometer (Isogen Life Science, Belgium). The methods for telomere length measurement in buccal cells have been previously described47. In brief, using an adapted quantitative real-time PCR method, relative telomere length was determined as the ratio of telomere sequence repeats to a single copy nuclear control gene, 36B4 (acidic ribosomal phosphoprotein P0). Each PCR reaction was performed in triplicate and three non-template controls as well as six inter-run calibrators were included on each 384-well plate. All samples were analyzed with the 7900HT Fast Real-Time PCR system (Life Technologies). After thermal cycling, raw data was collected and processed. The relative average telomere length was calculated as the ratio of the cycle threshold value of telomere sequence repeat to single copy gene (T/S) in the study participants compared with that of the averaged T/S value for the study population using the qBase software (Biogazelle, Zwijnaarde, Belgium). The program uses modified software based on the classic comparative CT method and takes the reference gene into account and uses inter-run calibration algorithms to correct for run-to-run differences49. All samples were analyzed in triplicate and included in the study when the difference in quantification cycle (Cq) value was less than 0.50. The coefficients of variation within triplicates of the telomere and single copy gene assay were 0.48% and 0.31%, respectively. The inter-assay coefficient of variation was 7.38%. We observed no correlation between the coefficient of variation with the mean buccal telomere length of the triplicates (r = 0.10; P = 0.17). The methods for telomere length measurement in placental tissue is similar to the method described above. Except HBG (human beta-globin) is used as single copy control gene instead of 36B4 and triplicate values were only included when the difference in Cq was less than 0.30.
Statistical analyses
We used SAS software, version 9.4 (SAS Institute, Cary, NC), for data management and statistical analyses. All reported P values are two-sided and were considered statistically significant when P < 0.05. After inspection of the distribution of all variables, measures of GGT and telomere length were log10-transformed. To assess the unadjusted relations between GGT and telomere length and alcohol consumption, the Pearson correlation coefficients were determined. Mixed modelling was performed to investigate buccal telomere length in young adulthood in association with GGT. The twins were analyzed as individuals using a multilevel regression, which accounts for relatedness between members of a twin pair by adding a random intercept to the model. The variance–covariance structure was allowed to differ between the three zygosity and chorionicity groups, including dizygotic dichorionic, monozygotic dichorionic and monozygotic monochorionic. Mixed modelling was performed adjusted for covariates selected a priori, namely sex, age (linear and quadratic), zygosity and chorionicity, education twin, maternal education, waist-to-hip ratio and smoking. To capture potential non-linear effects of year of age, the quadratic terms of this variable was added. In addition, we performed sensitivity analyses with additional adjustment for telomere length at birth and the use of medication possibly associated with elevated GGT. In addition, we also investigated the association between GGT and risk factors of cardiometabolic syndrome, while adjusting for age.
Finally, twins were studied as pairs, we analysed the correlation between GGT in twin 1 and twin 2 for monozygotic and dizygotic twins separately. This was also repeated for telomere length. Since monozygotic twins are genetically identical, whereas DZ twins share half of their genes, a greater within-pair similarity in MZ twins than in DZ twins reflects genetic influences. If this is the case, the heritability (h) of the GGT and telomere length can be estimated form twice the difference between MZ and DZ correlations. Using the twin design, the relative influence of genetic and environmental factors on birth can be estimated. The significance level for the difference in intra-pair correlation coefficient between groups is tested with a Fisher Z transformation.
References
Blackburn, E. H. Switching and signaling at the telomere. Cell 106, 661–673 (2001).
von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (2002).
Oikawa, S. & Kawanishi, S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 453, 365–368 (1999).
Kawanishi, S. & Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 1019, 278–284 (2004).
Astuti, Y., Wardhana, A., Watkins, J. & Wulaningsih, W. Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ. Res. 158, 480–489 (2017).
Nawrot, T. S. et al. Telomere length and its associations with oxidized-LDL, carotid artery distensibility and smoking. Front. Biosci. 2, 1164–1168 (2010).
Shalev, I. et al. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology 38, 1835–1842 (2013).
Entringer, S. et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl. Acad. Sci. USA. 108, E513–E518 (2011).
Freitas-Simoes, T. M., Ros, E. & Sala-Vila, A. Nutrients, foods, dietary patterns and telomere length: Update of epidemiological studies and randomized trials. Metabolism 65, 406–415 (2016).
Adler, N. et al. Educational attainment and late life telomere length in the health, aging and body composition study. Brain Behav. Immun. 27, 15–21 (2013).
Mundstock, E. et al. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity 23, 2165–2174 (2015).
Martens, D. S. & Nawrot, T. S. Air pollution stress and the aging phenotype: The telomere connection. Curr. Envrion. Health Rep. 3, 258–269 (2016).
Pieters, N. et al. Biomolecular markers within the core axis of aging and particulate air pollution exposure in the elderly: A cross-sectional study. Environ. Health Perspect. 124, 943–950 (2015).
Bijnens, E. et al. Lower placental telomere length may be attributed to maternal residential traffic exposure: A twin study. Environ. Int. 79, 1–7 (2015).
Martens, D. S., Plusquin, M., Gyselaers, W., De Vivo, I. & Nawrot, T. S. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 14, 148 (2016).
Martens, D. S. et al. Prenatal air pollution and newborns’ predisposition to accelerated biological aging. JAMA Pediatr. 171, 1160–1167 (2017).
Herrmann, M., Pusceddu, I., Marz, W. & Herrmann, W. Telomere biology and age-related diseases. Clin. Chem. Lab. Med. 56, 1210–1222 (2018).
De Meyer, T. et al. Telomere length as cardiovascular aging biomarker: JACC review topic of the week. J. Am. Coll. Cardiol. 72, 805–813 (2018).
Weischer, M., Bojesen, S. E. & Nordestgaard, B. G. Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS Genet. 10, e1004191. https://doi.org/10.1371/journal.pgen.1004191 (2014).
Needham, B. L. et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc. Sci. Med. 85, 1–8 (2013).
Sun, Q. et al. Healthy lifestyle and leukocyte telomere length in U.S. women. PLoS ONE 7, e38374. https://doi.org/10.1371/journal.pone.0038374 (2012).
Mirabello, L. et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell 8, 405–413 (2009).
Latifovic, L., Peacock, S. D., Massey, T. E. & King, W. D. The influence of alcohol consumption, cigarette smoking, and physical activity on leukocyte telomere length. Cancer Epidemiol. Biomarkers Prev. 25, 374–380 (2016).
Pavanello, S. et al. Shortened telomeres in individuals with abuse in alcohol consumption. Int. J. Cancer 129, 983–992 (2011).
Shin, C. & Baik, I. Associations between alcohol consumption and leukocyte telomere length modified by a common polymorphism of ALDH2. Alcohol. Clin. Exp. Res. 40, 765–771 (2016).
Whitfield, J. B. Gamma glutamyl transferase. Crit. Rev. Clin. Lab. Sci. 38, 263–355 (2001).
Lee, D. H., Blomhoff, R. & Jacobs, D. R. Jr. Is serum gamma glutamyltransferase a marker of oxidative stress?. Free Radic. Res. 38, 535–539 (2004).
Kunutsor, S. K., Apekey, T. A. & Khan, H. Liver enzymes and risk of cardiovascular disease in the general population: A meta-analysis of prospective cohort studies. Atherosclerosis 236, 7–17 (2014).
Kunutsor, S. K., Apekey, T. A. & Seddoh, D. Gamma glutamyltransferase and metabolic syndrome risk: A systematic review and dose-response meta-analysis. Int. J. Clin. Pract. 69, 136–144 (2015).
Liu, C. F., Zhou, W. N. & Fang, N. Y. Gamma-glutamyltransferase levels and risk of metabolic syndrome: A meta-analysis of prospective cohort studies. Int. J. Clin. Pract. 66, 692–698 (2012).
Choi, K. M. et al. Implication of liver enzymes on incident cardiovascular diseases and mortality: A nationwide population-based cohort study. Sci. Rep. 8, 3764 (2018).
Chung, H. S. et al. Gamma-glutamyltransferase variability and the risk of mortality, myocardial infarction, and stroke: A nationwide population-based cohort study. J. Clin. Med. 8, 832 (2019).
de Vos-Houben, J. M. et al. Telomere length, oxidative stress, and antioxidant status in elderly men in Zutphen and Crete. Mech. Ageing Dev. 133, 373–377 (2012).
Weale, C. J. et al. Leucocyte telomere length and glucose tolerance status in mixed-ancestry South Africans. Cells 8, 464 (2019).
Hadzagic-Catibusic, F., Hasanbegovic, E., Melunovic, M., Zubcevic, S. & Uzicanin, S. Effects of carbamazepine and valproate on serum aspartate aminotransferase, alanine aminotransferase and gamma: Glutamyltransferase in children. Med. Arch. 71, 239–242 (2017).
Zierer, J. et al. Metabolomics profiling reveals novel markers for leukocyte telomere length. Aging 8, 77–94 (2016).
Reichert, S. & Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 13, 20170463. https://doi.org/10.1098/rsbl.2017.0463 (2017).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 (2009).
Revesz, D., Milaneschi, Y., Verhoeven, J. E. & Penninx, B. W. Telomere length as a marker of cellular aging is associated with prevalence and progression of metabolic syndrome. J. Clin. Endocrinol. Metab. 99, 4607–4615 (2014).
Yang, J., Zaitlen, N. A., Goddard, M. E., Visscher, P. M. & Price, A. L. Advantages and pitfalls in the application of mixed-model association methods. Nat. Genet. 46, 100–106 (2014).
Gadalla, S. M., Cawthon, R., Giri, N., Alter, B. P. & Savage, S. A. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging 2, 867–874 (2010).
Finnicum, C. T. et al. Relative telomere repeat mass in buccal and leukocyte-derived DNA. PLoS ONE 12, e0170765. https://doi.org/10.1371/journal.pone.0170765 (2017).
Hansen, T. V., Simonsen, M. K., Nielsen, F. C. & Hundrup, Y. A. Collection of blood, saliva, and buccal cell samples in a pilot study on the Danish nurse cohort: comparison of the response rate and quality of genomic DNA. Cancer Epidemiol. Biomark. Prev. 16, 2072–2076 (2007).
Shalev, I. Early life stress and telomere length: Investigating the connection and possible mechanisms: A critical survey of the evidence base, research methodology and basic biology. BioEssays 34, 943–952 (2012).
Loos, R. J., Beunen, G., Fagard, R., Derom, C. & Vlietinck, R. The influence of zygosity and chorion type on fat distribution in young adult twins consequences for twin studies. Twin Res. 4, 356–364 (2001).
Derom, C. et al. The east flanders prospective twin survey (EFPTS): 55 years later. Twin Res. Hum. Genet. 22, 454–459 (2019).
Bijnens, E. M. et al. Telomere tracking from birth to adulthood and residential traffic exposure. BMC Med. 15, 205 (2017).
Vlietinck, R. Determination of the Zygosity of Twins (KU Leuven, 1986).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19 (2007).
Acknowledgements
This investigation is supported by the EU research council “project ENVIRONAGE” (ERC-2012-StG 310890), and the Flemish Scientific Fund (FWO, G073315N). Dr. Bijnens holds a fellow-ship from the Marguerite-Marie Delacroix foundation. Dr. Martens is a postdoctoral fellow of the FWO (12X9620N). Since its start, the East Flanders Prospective Twin Survey has been partly supported by grants from the Fund of Scientific Research Flanders and Twins, a non-profit Association for Scientific Research in Multiple Births (Belgium).
Author information
Authors and Affiliations
Contributions
T.S.N., C.D., E.T., S.W. and E.M.B. designed the study; C.D. and R.J.F.L did data collection; DSM optimized the telomere length protocol. EMB performed the telomere length measurements in buccal cells. T.S.N. and E.M.B. analyzed the data; T.S.N. and E.M.B. wrote the first draft of the paper. All authors critically revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bijnens, E.M., Derom, C., Thiery, E. et al. Serum gamma-glutamyl transferase, a marker of alcohol intake, is associated with telomere length and cardiometabolic risk in young adulthood. Sci Rep 11, 12407 (2021). https://doi.org/10.1038/s41598-021-91987-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91987-6
This article is cited by
-
Smoking and diabetes cause telomere shortening among alcohol use disorder patients
Scientific Reports (2024)
-
Persistent dyslipidemia increases the longitudinal changes in telomere length
Lipids in Health and Disease (2023)
-
Does gamma-glutamyltransferase correlate with liver tumor burden in neuroendocrine tumors?
Endocrine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.