Abstract
The clinical efficacy of adjuvant radiotherapy in sigmoid colon cancer remains questioned. To evaluate the clinical efficacy of adjuvant external beam radiotherapy (EBRT) for patients with pathologic stage T4b sigmoid colon cancer. Patients with stage pT4b sigmoid colon cancer receiving adjuvant EBRT or not followed by surgery between 2004 and 2016 were extracted from the Surveillance, Epidemiology, and End Results database. Analysis of overall survival (OS) was performed using Kaplan–Meier curves and prognostic factors were identified using Cox proportional hazards regression models with 95% confidence intervals within the entire cohort. A risk-stratification system was then developed based on the β regression coefficient. Among 2073 patients, 284 (13.7%) underwent adjuvant EBRT. The median OS in the group receiving adjuvant EBRT was significantly longer than that in the non-radiotherapy group (p < 0.001). Age, serum carcinoembryonic antigen (CEA) level, perineural invasion, lymph node dissection (LND) number, and adjuvant EBRT were independent factors associated with OS. A risk‐stratification system was generated, which showed that low‐risk patients had a higher 5-year survival rate than high-risk patients (75.6% vs. 42.3%, p < 0.001). Adjuvant EBRT significantly prolonged the 5-year survival rate of high-risk patients (62.6% vs. 38.3%, p = 0.009) but showed no survival benefit among low‐risk patients (87.7% vs. 73.2%, p = 0.100). Our risk‐stratification model comprising age, serum CEA, perineural invasion, and LND number predicted the outcomes of patients with stage pT4b sigmoid colon cancer based on which subgroup of high-risk patients should receive adjuvant EBRT.
Similar content being viewed by others
Introduction
While the incidence of colon cancer is decreasing steadily worldwide, over 100,000 newly diagnosed colon cancer patients are reported annually1. Radical surgery followed by adjuvant chemotherapy is still the preferred curative treatment for locally advanced colon cancer; however, the prognosis remains unsatisfactory, with a 5-year overall survival (OS) rate of 52–64%2. Although distant metastasis is decreasing with the development of modern systemic therapy in recent decades, the reported morbidity of local recurrence ranges from 10 to 40%3,4,5,6, underscoring the role of adjuvant radiotherapy. However, the clinical efficacy of adjuvant radiotherapy, as well as its safety and feasibility, have long been questioned7,8.
The radical resection of locally advanced sigmoid cancer is sometimes much more difficult mainly due to anatomical features9,10,11, with a reported proportion of R1/R2 of 15–35%5,8,12,13. The incidence of postoperative recurrence for left colon cancer is higher than that of the right14,15. However, the sigmoid colon has a relatively fixed location compared with other sites of the colon, which makes adjuvant external beam radiotherapy (EBRT) for sigmoid colon cancer much more acceptable16,17. The current study explored the potential role of adjuvant radiotherapy for patients with pathological T4b (T4b: tumor directly invades or adheres to adjacent organs or structures) sigmoid colon cancer in the Surveillance, Epidemiology, and End Results (SEER) database and then established an easily performed model to identify selected patients expected to show more benefits from adjuvant radiotherapy.
Methods
Study design and ethics statement
This retrospective study analyzed data from the publicly accessible SEER database. Before the study, we obtained an official permit for the research purpose (ID: 12284-Nov2019). Informed consent or ethical approval was not required for this study.
Patient selection and data extraction
Cases of stage pT4b sigmoid colon cancer were screened in the SEER database by SEER‐Stat software (SEER*Stat 8.3.8). Cases of sigmoid colon cancer were retrieved based on the International Classification of Diseases for Oncology (ICD-O-3) code; namely, “Site recode ICD-O-3/WHO 2008" (Sigmoid colon). A total of 5953 patients with pT4b sigmoid colon cancer between 2004 and 2016 were initially identified as eligible for this study. Among these, 3880 patients were excluded as follows: (1) 1297 patients had multiple primary tumors; (2) 1187 patients did not undergo surgery or local tumor excision; (3) 83 patients received preoperative radiotherapy or intraoperative radiotherapy or both preoperative and postoperative radiotherapy or an unknown sequence; and (4) 1313 patients were with M1 or unknown. All patients underwent active follow-up. Finally, 2073 patients, including 284 (13.7%) patients receiving radiotherapy and 1789 (86.3%) patients without radiotherapy, were further analyzed (Fig. 1). The T classification was restaged according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging classification system based on the following codes: derived AJCC T, 6th (2004+); derived AJCC, 7th (2010+); and Collaborative Stage (CS) tumor size (2004+)18.
The following data were also collected and categorized as follows: insurance (insured, uninsured, or unknown), age at diagnosis (< 50 years, 50–69 years, or ≥ 70 years), sex (male or female), race (white, black, others, or unknown), marital status (married, others, or unknown), differentiation status (well, moderate, poor, undifferentiated, or unknown), tumor size (< 3 cm, 3–4.9 cm, ≥ 5 cm, or unknown)3,19, serum carcinoembryonic antigen (CEA) level (elevated, normal, or unknown), perineural invasion (PNI) status (yes, no, or unknown), N classification (N0, N1, N2, or unknown), lymph node dissection (LND) number (< 12, ≥ 12, or unknown)20, adjuvant EBRT (yes or no), and survival (months). Data on adjuvant chemotherapy were not extracted in this study, mainly because it was hard to distinguish unknown chemotherapy from no chemotherapy in the SEER database19,21, although adjuvant chemotherapy is an important prognostic factor of colon cancer.
Statistical analyses
The primary endpoint in this study was OS. Kaplan–Meier (K–M) survival curves were determined using log-rank tests. Univariate analysis was conducted on all variables in this study; those with p < 0.05 were included in the multivariate Cox regression models to estimate the potential predictors associated with OS.
An easy risk score model was established using the β regression coefficient to predict the prognosis of patients with stage pT4b sigmoid colon cancer, which was assessed by receiver operating characteristic (ROC) curve analysis22. The cutoff value affecting OS was determined using the Youden index22. K–M survival curves were then used to evaluate the role of adjuvant radiotherapy in the treatment of patients with stage pT4b sigmoid colon cancer stratified according to risk scores.
Statistical tests were conducted using RStudio, including the Table 1, survminer, and survival packages, or IBM SPSS Statistics for Windows, version 24.0. A p value < 0.05 was considered statistically significant.
Ethical approval
For this type of study formal consent is not required.
Informed consent
As the data used was from SEER dataset (public). Consent to participate could be checked in SEER.
Results
Patient characteristics
The clinicopathological characteristics of the 2073 patients eligible for inclusion in this study are summarized in Table 1. The proportions of patients aged ≥ 70 years, tumor size ≥ 5 cm, and LND number ≥ 12 were 39.1%, 72.9%, and 77.0%, respectively; however, only 13.7% of patients received adjuvant EBRT.
Prognostic factors affecting OS
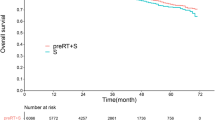
The median OS times of the adjuvant EBRT and non-radiotherapy cohorts were 84 months and 51 months, respectively, with 1-, 3-, and 5-year survival rates of 94.5% vs. 79.8%, 70.9% vs. 59.8%, and 59.4% vs. 45.5%, respectively (p < 0.001, Fig. 2). Univariate analysis showed that age (p < 0.001), race (p = 0.023), marital status (p < 0.001), differentiation (p < 0.001), tumor size (p = 0.027), serum CEA (p < 0.001), PNI (p < 0.001), N classification (p < 0.001), LND number (p < 0.001), and adjuvant radiotherapy (p < 0.001) were associated with OS (Table 2). Age ≥ 70 years (p < 0.001), elevated serum CEA (p = 0.012), PNI (p = 0.025), LND number < 12 (p < 0.001), and no radiotherapy (p = 0.016) were independently associated with worse OS (Table 2).
Establishment of the risk-stratification model
A risk score model was established according to the β regression coefficient and Exp (B) derived from the Cox model (Table 3). Briefly, age ≥ 70 years was scored as three points, elevated serum CEA as two, PNI as two, and LND number < 12 as three.
After excluding patients with missing information for any of the four variables, only 672 (32.4%) patients remained for further analysis. These remaining patients were scored according to the new risk score model. The area under the ROC curve was 0.703, and the optimal cutoff value was 2.5 points (Fig. 3a). According to this cutoff value, 354 (52.7%) patients were classified as having a low risk of poor prognosis (total score < 2.5), while 318 (47.3%) patients had a high risk of poor prognosis (total score ≥ 2.5). The median OS was significantly longer in the low-risk group than in the high-risk group, with 5-year survival rates of 75.6% and 42.3%, respectively (p < 0.001, Fig. 3b).
Distribution‐based cutoff optimization for risk score. (a) Receiver operating characteristic curve of risk scores; the optimal cutoff was assessed for the event of death. (b) Overall survival (OS) rates according to risk stratifications (low risk vs high risk, P < 0.001). Pictures show the number of subjects at risk in each group at 20-month increments. Pictures show the number of censoring in each group at 20-month increments.
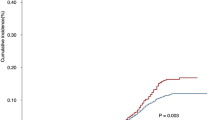
Furthermore, there was no significant difference in the median OS between low-risk patients with and without adjuvant radiotherapy (p = 0.100, Fig. 4a). Nevertheless, the median OS in high-risk patients receiving adjuvant radiotherapy was significantly longer than that in patients who underwent surgery alone (not reached vs. 44 months, p = 0.009, Fig. 4b), with 5-year survival rates of 62.6% vs. 38.8% (Table 4).
Overall survival (OS) rates according to (a) radiotherapy for low‐risk patients (radiotherapy vs. non-radiotherapy, P = 0.100) and (b) radiotherapy for high‐risk patients (radiotherapy vs. non-radiotherapy, P = 0.009). Pictures show the number of subjects at risk in each group at 20-month increments. Pictures show the number of censoring in each group at 20-month increments.
Discussion and conclusions
To the best of our knowledge, this is the first study to establish an easily assessed risk-stratification model to identify a subgroup of patients with stage pT4b sigmoid colon cancer who might benefit from adjuvant EBRT. According to the new risk stratification model, patients with a total score ≥ 2.5 would benefit more from adjuvant EBRT (p = 0.009), whereas patients with a total score < 2.5 would not show a survival benefit from adjuvant EBRT (p = 0.100).
Adjuvant EBRT is not routinely used in the treatment of colon cancer. Retrospective studies in the 1980s and the 1990s originally reported improved local control (LC) and disease-free survival (DFS) by adjuvant EBRT12,23,24; however, these findings were refuted by a subsequent randomized controlled trial (RCT, Intergroup-0130). No differences in DFS or OS were observed between arms of the trial; however, the results in this trial have always been challenging because of the high ineligibility rates and inclusion of T3 patients7. Considering that the patterns of treatment failure have changed in the era of systemic therapy, including targeted therapy and immune therapy25, the role of adjuvant EBRT has been re-recognized along with the exploration of preoperative neoadjuvant chemoradiation for colon cancer and decreased toxicity associated with radiation. In 2016, a retrospective single-institution study5 reported that adjuvant EBRT could enhance LC and DFS (hazard ratio [HR] 0.044, p < 0.05; HR 0.145, p < 0.05, respectively) of patients with colon cancer, specifically those with T4b and/or residual tumors, findings that were confirmed using external data or national cancer database8,26.
Compared with other sites of colon cancer, adjuvant EBRT for sigmoid colon cancer has several advantages. First, the sigmoid colon is in a relatively fixed anatomical location, which facilitates the delineation of the gross target volume16,17. Second, the organs at risk of sigmoid colon cancer are generally fewer than those of other sites of colon cancer. Third, the radiation exposure dose limit for the colon is lower than that for the small intestine. In this study, among patients in the crude cohort, 284 (13.7%) patients with stage pT4b sigmoid colon cancer who received postoperative radiotherapy showed prolonged OS compared with that in patients who did not receive postoperative radiotherapy (p < 0.001). As is well known, one treatment size does not fit all. We successfully identified a subgroup of high-risk patients with stage pT4b sigmoid colon cancer who could benefit from adjuvant EBRT based on our newly developed risk-stratification model.
Whether age affects LC and DFS remains controversial3,27,28; however, aging is an independent risk factor for poor prognosis of colon cancer, with different aging cutoff values4,8,21,29. In this study, these cutoff values were 50 and 70 years and the proportion of patients aged ≥ 70 years was as high as 39.1%. Aging was an independent risk factor for OS, with patients aged ≥ 70 years showing a two-fold increase in poor prognosis, indicating that aging patients, specifically those aged ≥ 70 years, urgently required adjuvant treatment. Moreover, pT4b sigmoid colon cancer typically requires extensive colectomy that aging patients cannot generally tolerate; thus, adjuvant EBRT might be an option for aging patients with stage pT4b disease.
CEA is a routine index used for colon cancer diagnosis and surveillance30,31; however, it remains controversial whether elevated CEA levels are an independent risk factor for poor prognosis14,19,32. In this study, serum CEA was an independent risk factor for OS. Moreover, elevated serum CEA doubled the risk of death in patients with stage pT4b sigmoid colon compared with that in patients with normal serum CEA levels.
Lymph node evaluation is often a critical factor in predicting the prognosis of colon cancer and is also a deciding factor for postoperative treatment2,33,34. However, LND number other than lymph node classification was independently associated with OS, as confirmed in this study, although the cutoff values for LND number varied in previous reports3,35,36. In this study, the cutoff value of LND number was 12, as recommended by the National Comprehensive Cancer Network (NCCN) guideline; based on this threshold, LND number < 12 increased the risk of death by nearly two-fold.
PNI is an aggressive characteristic of colon cancer, with incidence rates of 15–32%14,37. In a retrospective study of 269 patients with colorectal cancer, Liebig et al.38 reported that patients with PNI had a four-fold worse 5-year survival compared with that in patients without PNI, a finding confirmed by several other studies20,35,39. However, PNI was also reported to be not associated with poor prognosis in cecum adenocarcinoma or colon cancer3,14. Nonetheless, a meta-analysis of 58 studies confirmed the association of PNI with both decreased 5-year OS (relative risk [RR] 2.09, 95% confidence interval [95% CI] [1.68; 2.61]) and DFS (RR 2.35, 95% CI [1.66; 3.31])40. In our study, PNI increased the risk of worse OS in patients with sigmoid colon cancer compared with the risk in those without PNI.
This study has some limitations. First, this was a retrospective analysis and selection bias was difficult to avoid. Second, data on local recurrence, a critical endpoint of adjuvant EBRT, were unavailable in the SEER database41. Third, while recommended by the NCCN guidelines, this study did not analyze adjuvant chemotherapy, which may have weakened our conclusions. Fourth, data on surgical margin status, microsatellite status, and Ki-67% were not obtained from the current SEER database18,42; hence, the newly developed risk model did not include these variables. Finally, details on radiotherapy including clinical tumor volume, radiation technique, dose/fraction, and acute/late toxicity were not recorded43,44; thus, the feasibility of adjuvant EBRT requires further study.
In conclusion, the results of this study confirmed the survival benefit of postoperative radiotherapy for pT4b sigmoid colon cancer. A risk-stratification model including the easily measured factors of age, serum CEA, PNI, and LND number was then successfully established. In this model, patients with pT4b sigmoid colon cancer with scores ≥ 2.5 were recommended to receive postoperative radiotherapy.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel, R. L. et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70, 145–164 (2020).
Agas, R. A. F. et al. Assessing the effect of radiotherapy in addition to surgery in colon adenocarcinomas: a systematic review and meta-analysis of contemporary evidence. J. Gastrointest. Cancer 51, 445–460 (2019).
Hosseini, S., Bananzadeh, A. M., Mohammadianpanah, M., Salek, R. & Taghizadeh-Kermani, A. Prognostic significance of adjuvant radiation therapy in adenocarcinoma of the cecum. Radiat. Oncol. J. 36, 45–53 (2018).
Krishnamurty, D. M. et al. Neoadjuvant radiation therapy in locally advanced colon cancer: a cohort analysis. J. Gastrointest. Surg. 22, 906–912 (2018).
Ludmir, E. B. et al. Role of adjuvant radiotherapy in locally advanced colonic carcinoma in the modern chemotherapy era. Ann. Surg. Oncol. 23, 856–862 (2016).
Deijen, C. L. et al. Ten-year outcomes of a randomised trial of laparoscopic versus open surgery for colon cancer. Surg. Endosc. 31, 2607–2615 (2017).
Martenson, J. A. Jr. et al. Phase III study of adjuvant chemotherapy and radiation therapy compared with chemotherapy alone in the surgical adjuvant treatment of colon cancer: results of intergroup protocol 0130. J. Clin. Oncol. 22, 3277–3283 (2004).
Wegner, R. E. et al. Utilization of adjuvant radiotherapy for resected colon cancer and its effect on outcome. Ann. Surg. Oncol. 27, 825–832 (2020).
Willett, C. G. et al. Adjuvant postoperative radiation therapy for colonic carcinoma. Ann. Surg. 206, 694–698 (1987).
Duttenhaver, J. R., Hoskins, R. B., Gunderson, L. L. & Tepper, J. E. Adjuvant postoperative radiation therapy in the management of adenocarcinoma of the colon. Cancer 57, 955–963 (1986).
Gunderson, L. L., Sosin, H. & Levitt, S. Extrapelvic colon–areas of failure in a reoperation series: implications for adjuvant therapy. Int. J. Radiat. Oncol. Biol. Phys. 11, 731–741 (1985).
Willett, C. G., Fung, C. Y., Kaufman, D. S., Efird, J. & Shellito, P. C. Postoperative radiation therapy for high-risk colon carcinoma. J. Clin. Oncol. 11, 1112–1117 (1993).
Margalit, O. et al. Postoperative radiation for pathologic stage T4 colon cancers receiving adjuvant chemotherapy. Clin. Colorectal. Cancer 18, 226-230.e2 (2019).
Wang, L. et al. Left colon as a novel high-risk factor for postoperative recurrence of stage II colon cancer. World J. Surg. Oncol. 18, 54 (2020).
Leijssen, L. G. J., Dinaux, A. M., Kunitake, H., Bordeianou, L. G. & Berger, D. L. Pathologic factors are more important than tumor location in long-term survival in colon cancer. Int. J. Colorectal. Dis. 33, 709–717 (2018).
Szeto, A., Chin, L., Whelan, P., Wilson, J. & Lee, J. Image-guided radiation therapy using surgical clips for localization of colonic metastasis from thyroid cancer. Radiat. Oncol. 9, 298 (2014).
Chang, H. et al. Neoadjuvant chemoradiotherapy followed by surgery in patients with unresectable locally advanced colon cancer: a prospective observational study. Onco Targets Ther. 11, 409–418 (2018).
Fang, C. et al. Nomogram individually predicts the overall survival of patients with gastroenteropancreatic neuroendocrine neoplasms. Br. J. Cancer 117, 1544–1550 (2017).
Huang, Y. et al. The survival benefit of adjuvant radiotherapy for pathological T4N2M0 colon cancer in the Modern Chemotherapy Era: evidence from the SEER database 2004–2015. Artif. Cells Nanomed. Biotechnol. 48, 834–840 (2020).
Achilli, P. et al. Survival impact of adjuvant chemotherapy in patients with stage IIA colon cancer: analysis of the National Cancer Database. Int. J. Cancer 148, 161–169 (2020).
McLaughlin, C. et al. Adjuvant radiation therapy for T4 non-rectal colon adenocarcinoma provides a cause-specific survival advantage: a SEER database analysis. Radiother. Oncol. 133, 50–53 (2019).
Jima, B. R., Hassen, H. Y., Getnet, Y., Bahwere, P. & Gebreyesus, S. H. Diagnostic performance of midupper arm circumference for detecting severe wasting among infants aged 1–6 months in Ethiopia. Am. J. Clin. Nutr. https://doi.org/10.1093/ajcn/nqaa294 (2020).
Amos, E. H. et al. Postoperative radiotherapy for locally advanced colon cancer. Ann. Surg. Oncol. 3, 431–436 (1996).
Willett, C. G. et al. Does postoperative irradiation play a role in the adjuvant therapy of stage T4 colon cancer?. Cancer J. Sci. Am. 5, 242–247 (1999).
Hellman, S. & Weichselbaum, R. R. Importance of local control in an era of systemic therapy. Nat. Clin. Pract. Oncol. 2, 60–61 (2005).
Niloofar, A., Mosalaei, A., Shapour, O. & Mohammadianpanah, M. Role of external irradiation in high-risk resected colon cancer. Indian J. Cancer 42, 133–137 (2005).
Elias, A. W. et al. Recurrence and long-term survival following segmental colectomy for right-sided colon cancer in 813 patients: a single-institution study. J. Gastrointest. Surg. 24, 1648–1654 (2020).
Sebastian, N. T., Tan, Y., Miller, E. D., Williams, T. M. & Diaz, D. A. Surgery with and without adjuvant radiotherapy is associated with similar survival in T4 colon cancer. Colorectal. Dis. 22, 779–789 (2020).
Hawkins, A. T. et al. Neoadjuvant radiation for clinical T4 colon cancer: a potential improvement to overall survival. Surgery 165, 469–475 (2019).
Gold, P. & Freedman, S. O. Specific carcinoembryonic antigens of the human digestive system. J. Exp. Med. 122, 467–481 (1965).
Mach, J. P. et al. Long-term follow-up of colorectal carcinoma patients by repeated CEA radioimmunoassay. Cancer 42, 1439–1447 (1978).
Magaji, B. A., Moy, F. M., Roslani, A. C. & Law, C. W. Survival rates and predictors of survival among colorectal cancer patients in a Malaysian tertiary hospital. BMC Cancer 17, 339 (2017).
Gill, S. et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much?. J. Clin. Oncol. 22, 1797–1806 (2004).
Figueredo, A., Charette, M. L., Maroun, J., Brouwers, M. C. & Zuraw, L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care’s gastrointestinal cancer disease site group. J. Clin. Oncol. 22, 3395–3407 (2004).
Vergara-Fernandez, O. et al. Oncological implications of lymph nodes retrieval and perineural invasion in colorectal cancer: outcomes from a referral center. Rev. Investig. Clin. 70, 291–300 (2018).
Orsenigo, E., Gasparini, G. & Carlucci, M. Clinicopathological factors influencing lymph node yield in colorectal cancer: a retrospective study. Gastroenterol. Res. Pract. 2019, 5197914 (2019).
Fujita, S. et al. Prospective evaluation of prognostic factors in patients with colorectal cancer undergoing curative resection. J. Surg. Oncol. 84, 127–131 (2003).
Liebig, C. et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol. 27, 5131–5137 (2009).
Quah, H. M. et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis. Colon Rectum. 51, 503–507 (2008).
Knijn, N., Mogk, S. C., Teerenstra, S., Simmer, F. & Nagtegaal, I. D. Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am. J. Surg. Pathol. 40, 103–112 (2016).
Huang, Y. X. et al. Role of postoperative radiotherapy in pT3N0 rectal cancer: a risk-stratification system based on population analyses. Cancer Med. 8, 1024–1033 (2019).
Li, J. et al. Prognostic nomogram based on the metastatic lymph node ratio for gastric neuroendocrine tumour: SEER database analysis. ESMO Open 5, e000632 (2020).
Zhang, C. H. et al. The prognostic impact of the metastatic lymph nodes ratio in colorectal cancer. Front. Oncol. 8, 628 (2018).
McLaughlin, C. et al. Adjuvant radiation therapy for T4 non-rectal colon adenocarcinoma provides a cause-specific survival advantage: A SEER database analysis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 133, 50–53 (2019).
Acknowledgements
The authors sincerely thank the Surveillance, Epidemiology, and End Results (SEER) program for the efforts in establishing the SEER database.
Funding
This research was funded by the Fujian Province Natural Science Foundation (Grant Number 2017J01260) and the Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant Number 2017Y9074).
Author information
Authors and Affiliations
Contributions
L.Y.B., W.L., and S.L.D. conceived and designed the study. S.L.D., Z.X.Q., and L.H.Q. collected the data. L.Y.B. and W.Y.J. analyzed the data. W.J.X. supervised the study. L.Y.B. and W.L. wrote the manuscript and L.Y.B. prepared Figs. 1, 2, 3 and 4. All authors read and approved the final manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, Y., Wang, L., Shao, L. et al. Prognostic analysis and beneficiary identification of adjuvant external beam radiotherapy for stage pT4b sigmoid colon cancer. Sci Rep 11, 11782 (2021). https://doi.org/10.1038/s41598-021-91172-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91172-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.