Abstract
This study reports for the first time on the synthesis of novel resin@P-Ag2O material and its application for reducing the chloride effect on COD determination of high salinity water. This engineered core–shell nanomaterial with cationic ion exchange resin core and porous Ag2O shell was prepared by facile ion exchange and silver oxidation method at ambient temperature without using toxic chemicals. The material was characterized by FTIR, XRD, SEM, and SEM–EDX mapping. In the chloride removal test, this material gave a high adsorption capacity of ca. 244 mgCl/gAg at the mild condition with high durability after several adsorption–desorption cycles. Moreover, resin@P-Ag2O was applied for removing chloride in water to improve the accuracy of the SMEWW 5220C:2012 method for COD determination of high salinity water. The result showed that the COD of a water sample with salt content after being treated by the material had a low error (≤ 10%) as compared to the sample without salt. Meanwhile, the COD of salty water measured by the dilution method had an error of around 15%. These results indicate that resin@P-Ag2O material has a very potential application for chloride removal and COD determination of high salinity water.
Similar content being viewed by others
Introduction
Chemical oxidation demand (COD) is a very popular and important parameter for evaluating the organic pollution level of water sources as well as for designing and constructing wastewater treatment systems. COD value of a water sample is determined by the SMEWW 5220C:2012 method1, where the sample was treated with dichromate under the acid condition and Ag+ catalyst at 150 °C for 2 h. The reactions during COD determination using potassium hydrogen phthalate (KHP, used as a COD standard) follow Eqs. (1) and (2), where one mole of Cr2O72− consumed for the oxidation of KHP corresponds to 1.5 mol of oxygen1,2.
For water samples (both surface water and wastewater) with high chloride (i.e. > 1.0 g/L), the SMEWW 5220C:2012 method has a positive deviation due to the reactions presented in Eqs. (3) and (4)1,2. In order to reduce the error, a certain amount of HgSO4 is added for forming the HgCl2 complex. However, due to its high toxicity, the use of mercury is applied with caution.
Three different approaches (i.e. new, corrected, and modified methods) have been proposed for improving the COD measurement of highly salty water3. The new methods were developed to reduce the time for determination such as the chemiluminescence system to analyze Cr3+ instead of Cr6+4 and the flow-injection method using KMnO4 and ion exchange column to collect and analyze Mn2+5 or Ce(SO4)2 and analyze Ce4+6. Other techniques used new digestion methods such as microwave- and ultrasound-assisted digestions7,8 and their combination with using Mn(III)9 and Ce(IV)6 as oxidants for faster and more complete oxidation. Although these new methods have a short determination time with high sensitivity, they require new instruments and new techniques. In correction methods, the real COD from organics was determined by the subtraction of apparent COD to the COD by chloride. This method is labor-intensive with several steps of (i) measure the chloride concentration, (ii) prepare the synthetic water sample with the same chloride but without organics content, (iii) measure the COD values of both actual and synthetic samples, and (iv) subtraction for obtaining the real COD value. Therefore, modification based on the current method is considered a preferable choice for practical applications among the three approaches.
Several modification methods have been proposed for reducing the error of COD determination of highly salty water such as sample dilution, increase of HgSO4/Cl− ratio, using masking agents, decrease of K2Cr2O7 amount, applying of new digestion methods3. In the dilution method, a water sample is added with water to decrease the chloride concentration to under 2.0 g/L without masking agents and the deviation of this method is in the range of 0.15–5.8% due to dilution error10. This method is suitable for water samples with high contents of both organic compounds and chloride. However, it has low accuracy for water with high chloride but low COD value. The use of a high HgSO4:Cl− ratio (e.g. 20:1) is also applied for the sample with high chloride but low organic content with a deviation of < 10% and the deviation could reach 12% for the sample with a NaCl concentration of 40 g/L11. However, this method is used with caution due to the high toxicity of mercury compounds as well as this method does not completely eliminate the effect of chloride. In the masking method, masking agents such as Ag+12, Cr3+13, and Al3+14 are added for reducing free chloride, where silver nitrate is considered as the most suitable one for precipitation of free chloride ion in AgCl form in water with Cl−:COD ratio of ≤ 3:1. However, this method is not suitable for the water sample with Cl−:COD ratio of > 3:1. Besides, the pollution of the masking agents in water or its recovery has not been addressed. Since the reaction of K2Cr2O7 with organics is faster than that with chloride, decrease in K2Cr2O7 content11 and/or combine it with the increase of HgSO4:Cl− ratio15 could reduce the effect of chloride. This method is complex since the suitable content of K2Cr2O7 and ratio of HgSO4:Cl− need to be determined. Another method is utilizing Bi-based adsorbent to adsorb the HCl produced during the acidification of chloride by 18 N H2SO4 solution to obtain a chloride-free sample before COD analysis2. The process uses concentrate H2SO4 solution at 150 °C as well as BiOOH material to absorb HCl, which seems not to be an environmental-friendly method. Although proposed and tested by several studies, these approaches have their disadvantages, thus finding a new, effective, cheap, and facile method for improving the accuracy of COD determination is necessary for water samples with a sodium chloride concentration of over 2 g/L.
This study proposes a new and facile solution by using a specially designed porous Ag2O shell on cationic exchange resin core (resin@P-Ag2O) for selective removal of the chloride content before COD measurement of the salty water sample. The material was synthesized by simple ion exchange and silver oxidation method at room temperature without using toxic chemicals. By using this material, the determination of COD for salty water is simple, highly accurate, environmental-friendly, and low-cost due to the recyclability of the material.
Experimental section
Chemicals and materials
All analytical grade chemicals such as AgNO3, NaOH, NaCl, K2Cr2O7, and Fe2(SO4)3 reagents were from Merck (Germany) and directly used without further purification. Standard potassium hydrogen phthalate (KHP) solution was received from Hach (USA). Deionized (DI) water was from a Millipore Milli-Q water system in the laboratory using double-distilled water as the inlet water source. Macroporous Purolite C145 cation exchanger with sulfonic acid (SO3−) functional groups shipped in Na+ form was kindly obtained from Purolite (China) with properties as described in Table S1 of Supplementary Information.
Generation of resin@P-Ag2O material
The synthesis of resin@P-Ag2O material was conducted in 2 steps. At first, polymeric macroporous cation exchange resin with strong acid functional groups was preloaded with Ag+ cation that is substantially insoluble (Eq. 5). After that, Ag2O within the pore phase of macroporous cation exchanger and the porous shell was synthesized by soaking of \(\overline{\left(RS{O}_{3}^{-}\right){Ag}^{+}}\) in NaOH solution (Eq. 6).
In a typical procedure, 30 g of ion exchange resin was added into 200 mL of 4% AgNO3 solution at pH 6 and the mixture was stirred for 3 h. The exchanged resin was then collected and preliminarily washed with DI water. After that, the washed resin was added into 200 mL of 10% NaOH solution and stirred for 4 h in order to form Ag2O nanoparticles in the pore and the porous shell on the resin. The material (resin@P-Ag2O) was then collected and washed with DI water several times and finally dried at ambient temperature naturally. Materials with different Ag loadings were synthesized by using AgNO3 solution with different concentrations.
Material characterization
Silver loading was determined by using an inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 20000DV, Perkin Elmer). Before analysis, the sample was treated with a 2% HNO3 solution to ensure all non-particle forms of silver are dissolved and removed. The surface chemical property of resin@P-Ag2O was carried out using Fourier transform infrared spectroscopy (Bruker-FTIR, 500–4000 cm−1). Wide-angle X-ray diffraction (WAXRD) patterns were recorded using a D2 Phaser XRD 300 W diffractometer equipped with CuKα radiation (λ = 1.5406 Å) at step size and step time of 0.01° and 30 s, respectively. The morphology, particle size, and elemental dispersion of the resin@P-Ag2O sample were evaluated by scanning electron microscopy (SEM) and SEM–EDX mapping techniques. Additionally, the average composition of the resin@P-Ag2O sample was also determined by energy-dispersive X-ray spectroscopy (EDX).
Batch adsorption experiment
In the chloride adsorption test, 0.5 g of the adsorbent was added into 50 mL of 4 g/L chloride solution and the mixture was shaken in a thermostatic water-bath shaker operated at speed of 120 rpm. After reached equilibrium condition, the adsorbent was collected by filtration, and chloride concentration in the supernatant was analyzed by conductivity determination of chloride solution (SPECORD 210 Plus, Analytik Jena, Germany). The used resin@P-Ag2O material was then regenerated by dipping into a 5% NaOH solution for 45 min. After regenerated, the adsorbent was rinsed with DI water several times before applying for chloride removal in the next test cycle. All experiments were replicated three times and the average results were reported in this study. The adsorption removal efficiency and adsorption capacity (defined as the amount of chloride adsorbed per mass unit of adsorbent) were calculated from the following equations:
where: qe (mg/g) is the adsorption capacity and H (%) is the removal efficiency. Co, Ct, and Ce (mg/L) are the initial, at time t, and equilibrium chloride concentrations in the aqueous solution, respectively. V (L) is the volume of the solution and m (g) is the weight of silver content.
Application of resin@P-Ag2O for COD analysis
KHP stock solution was diluted with DI water to form a COD standard solution of 120 mgCOD/L. NaCl was then added into the standard solution to form a series of 100-mL samples with NaCl concentration in a range of 0, 1.0, 1.5, 2.0, and 2.5 g/L. In the modified COD test, before obtaining the suitable condition, preliminary experiments were done by adding 2.0 g of resin@P-Ag2O into 100 mL of a standard solution containing NaCl for 30 min of adsorption. The material was then separated and the solution was taken for COD analysis followed the conventional SMEWW 5220C:2012 method.
Results and discussion
Material synthesis and characterization
The photos of resin@P-Ag2O samples are shown in Fig. 1, where the particle size of the synthesized material is determined to be around 0.5 mm. It can be observed that colors of fresh (Fig. 1A), chloride-adsorbed (Fig. 1B), and regenerated (Fig. 1C) resin@P-Ag2O are black, grey, and black colors, respectively. The chloride adsorption turns the black color of resin@P-Ag2O into a grey color, indicating the adsorption of chloride on the surface of Ag2O particles. Later, treatment with NaOH recovers chloride-adsorbed resin@P-Ag2O in grey color back to its fresh condition with black color, implying the successful regeneration of the material.
FTIR was applied for identifying the chemical composition in the structure of cationic exchange resin (Purolite C145) and resin@P-Ag2O, as shown in Fig. 2. For cationic exchange resin, the FTIR peaks in the wavenumber range of 3360–3590 cm–1 are simple O–H bond with the highest peak at 3457 cm–1 while the wavenumber range of 2800–3060 cm–1 is specific to various bonds in polystyrene structure with a peak at 2922 cm–1. The peak at 1601 cm–1 is specific for the C–C simple bond of the styrene ring while peaks at 1039, 1128, and 1181 cm–1 are attributed to the sulfonic group of SO3. The peak at 835 cm–1 confirms the substitution at the benzene ring level by sulfonic groups and divinylbenzene cross-linking. For FITR of resin@P-Ag2O material, its FTIR pattern is similar to that of cationic exchange resin except for a high-intensity peak at 1300 cm−1 of Ag2O vibration.
XRD patterns of fresh resin@P-Ag2O and resin@P-Ag2O after chloride adsorption (Cl-resin@P-Ag2O) are plotted in Fig. 3. For resin@P-Ag2O material, the highest peak of Ag2O observed at 2θ of 33.5° is attributed to as-synthesized Ag2O at the facet of (111). This confirms the successful synthesis of resin@P-Ag2O by the proposed protocol of the facile ion exchange and silver oxidation method. In Cl-resin@P-Ag2O material, the XRD peaks of 28°, 32°, and 46° are attributed to the presence of AgCl (111), AgCl (200), and AgCl (220), respectively, implying that crystalline AgCl formed with the grey color is the result from chloride adsorption using resin@P-Ag2O.
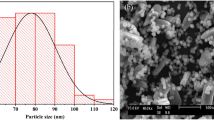
The SEM images of resin@P-Ag2O material at different resolutions and its SEM–EDX mapping results are displayed in Figs. 4 and 5, respectively. Obviously, resin@P-Ag2O has a spherical shape from the hard framework of the cationic exchange resin core. Additionally, the results from Fig. 5 confirm that the shell of the material is mainly composed of Ag2O with an atomic percentage of 72.28%. As also seen in Fig. 4, SEM images with high resolution confirm that as-synthesized Ag2O on the cationic resin surface is in an array form and a porous structure with nanosize Ag2O particles. Based on the material characteristics, the mechanism for the formation of resin@P-Ag2O material and its application for chloride removal is summarized and illustrated in Fig. 6.
Chloride removal experiments
The chloride adsorption of resin@P-Ag2O during 120 min of the experiment is illustrated in Fig. 7a, proving the rapid adsorption of chloride was obtained with fast equilibrium reached after 20 min. The experimental condition was then chosen at 20 min of equilibrium time and 4.0 gCl/L of chloride concentration (i.e. similar to brackish water). The effect of solution pH (adjusted by using HNO3 or NaOH solution) was investigated in a range of 5 to 9 (as found in natural surface water) and the result is presented in Fig. 7b. It is noted that the adsorption capacity is relatively similar at 230 to 244 mgCl/gAg in the pH range of 6 to 8, which is very suitable for the practical surface water sample application. However, the capacity was lower at pH 5 and pH 9 with values of 192 and 168 mgCl/gAg, respectively, suggesting low or high pH value is not favorable for chloride adsorption. At pH 5, a part of dissociated chloride ions (Cl−) could be acidified in the form of HCl, and its potential to form AgCl on the resin@P-Ag2O surface is slightly reduced. At pH 9, hydroxide ion (OH−) competes with Cl− for adsorption on the surface of resin@P-Ag2O. Figure 7c describes the effect of adsorbent dosage on the chloride removal in a range of 0.5–2.0 g in 50 mL of 4.0 g/L of chloride solution. As expected, the adsorption capacity decreased with the increase of adsorbent dosage, and the dosage was chosen at 1.0 g/50 mL, which is suitable for practical applications. In summary, the suitable condition for chloride adsorption in 50 mL of 4 g/L was pH 6–8, adsorbent amount of 1.0 g, and after 20 min of equilibrium time.
It is widely accepted that the presence of anions such as sulfate, nitrate, and phosphate may have strong effects on the adsorption of chloride ions using cation exchange resin16. In this study, the effect of these anions was examined at a concentration of 500 mg/L for each anion. The real salinity water (taken at 3 km from the Co Chien river estuary) was also tested for investigation of the simultaneous presence of these ions in the actual water environment. Results in Fig. 8a showed a very little effect of nitrate and phosphate on the chloride adsorption using resin@P-Ag2O, possibly due to the highly selective adsorption of chloride on Ag2O in terms of thermodynamic and kinetic. However, the chloride adsorption capacity decreased around 8% under the presence of sulfate, which could attribute to the competition of sulfate for forming Ag2SO4 precipitate on the surface of resin@P-Ag2O material. Interestingly, the adsorption capacity increased around 3% in the case of a real water sample via an unclear mechanism that needs further in-depth investigation.
The effect of Ag loading on the chloride adsorption is demonstrated in Fig. 8b, where Ag content (wt.%) increases from 0 (original resin) to 2.1, 3.7, and 4.3 (resin@P-Ag2O). As seen in Fig. 8b, pure resin does not adsorb any chloride due to its cationic characteristics. The adsorption capacity increased when Ag loading increased from 2.1 to 3.7, where it reaches the highest capacity due to the sufficient amount and effective dispersion of Ag on the surface of the resin. The capacity then decreased with a further increase of Ag loading to 4.3, possibly because of the formation of large Ag2O particles with lower surface area in the excess of Ag content.
In order to test the potential of resin@P-Ag2O for practical application, the adsorption—regeneration experiment was conducted 10 times and the result is presented in Fig. 8c. It is obvious that resin@P-Ag2O material has a high durability with stable chloride removal after at least 10 cycles of adsorption—regeneration, which is suitable for practical application.
Application of resin@P-Ag2O for COD determination of salinity water
In the environmental analysis field, COD determination of salinity water using the SMEWW 5220C:2012 method faces many difficulties due to the error from undesirable oxidation of chloride by K2Cr2O7 under concentrate acidic environment. Although several techniques have been proposed for improving the accuracy of the method as discussed previously, the application of these techniques is still very limited due to their own disadvantages. In this study, we proposed a new technique as an add-on but a simple step for COD measurement, and the results are displayed in Fig. 9. Results from conventional analysis (without pretreated with resin@P-Ag2O) showed that the COD value of solution increased with the increase of NaCl content, and therefore the COD analysis error. However, the obtained COD values for the sample pretreated with resin@P-Ag2O were more closed to the standard solution (without adding NaCl) with an acceptable error of ≤ 10% (positive error). For water without NaCl content, the COD value of resin@P-Ag2O treated sample was lower than that of the untreated one due to the adsorption of organic compounds on the resin@P-Ag2O, suggesting that resin@P-Ag2O should not be used for water sample with a salinity of < 1 g/L. For samples with salinity ≥ 1 g/L, this adsorption effect was reduced, possibly because of the predominant adsorption of chloride and the formation of AgCl on the resin. Interestingly, the COD value of sample containing 2.5 g/L of NaCl after treated with resin@P-Ag2O in combination with dilution method (in an analysis lab with Vilas certification) was 102 mg/L with an error of 15% (negative error, as compared to standard sample), which is probably from the dilution for reducing the salinity of water sample. These results indicate that the removal of chloride by resin@P-Ag2O before COD analysis is a reliable method for COD determination of salinity water samples. The effects of other ions such as iron, manganese, and bromide on the COD measurement are presented in Fig. 10, where the effects of resin@P-Ag2O dosage on corrected COD measurement are also provided. The effects of 1000 mgNaCl/L with 1000 mgNaBr/L or with 500 mgFeCl2/L + 500 mgMnCl2/L can be suppressed by using of 2 g of resin@P-Ag2O for 100 mL of water sample before doing the COD measurement. For water samples with high COD (e.g. 1000 mg/L) and chloride (e.g. 3000 mgNaCl/L), the results in Fig. S1 of Supplementary Information show that the COD could be measured with acceptable accuracy by choosing an appropriate dosage of resin@P-Ag2O. Although further intensive investigations are needed, this study suggests a way of using resin@P-Ag2O for improving the accuracy of COD measurement of salinity water, as presented in Table 1 for the suitable dosage of material for water sample with different salinities.
Conclusion
Engineered resin@P-Ag2O material with strong acidic ion exchange resin core and Ag2O shell was successfully synthesized and applied for removal of chloride in water. This novel material had high chloride adsorption capacity of 244 mgCl/gAg with high durability during cycling tests. The application of this material for improving the analysis accuracy of COD for salinity water is very potential and promising with a low error of ≤ 10%. The use of resin@P-Ag2O in terms of dosage was also proposed base on the salinity of the water sample. This study opens a new way in the application of nanomaterial for chloride adsorption to improve the accuracy of COD measurement for high salinity water and wastewater.
References
Clesceri, L. S. et al. Standard Methods for the Examination of Water and Wastewater (American Public Health Association, London, 1998).
Vaidya, B., Watson, S. W., Coldiron, S. J. & Porter, M. D. Reduction of chloride ion interference in chemical oxygen demand (COD) determinations using bismuth-based adsorbents. Anal. Chim. Acta 357, 167–175. https://doi.org/10.1016/S0003-2670(97)00541-2 (1997).
Ma, X. & Gao, Y. in 2010 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE). 1–4 (IEEE).
Hu, Y. & Yang, Z. A simple chemiluminescence method for determination of chemical oxygen demand values in water. Talanta 63, 521–526. https://doi.org/10.1016/j.talanta.2003.11.037 (2004).
Li, B., Zhang, Z., Wang, J. & Xu, C. Chemiluminescence system for automatic determination of chemical oxygen demand using flow injection analysis. Talanta 61, 651–658. https://doi.org/10.1016/S0039-9140(03)00326-6 (2003).
Korenaga, T., Zhou, X., Okada, K., Moriwake, T. & Shinoda, S. Determination of chemical oxygen demand by a flow-injection method using cerium(IV) sulphate as oxidizing agent. Anal. Chim. Acta 272, 237–244. https://doi.org/10.1016/0003-2670(93)80575-6 (1993).
Domini, C. E., Hidalgo, M., Marken, F. & Canals, A. Comparison of three optimized digestion methods for rapid determination of chemical oxygen demand: Closed microwaves, open microwaves and ultrasound irradiation. Anal. Chim. Acta 561, 210–217. https://doi.org/10.1016/j.aca.2006.01.022 (2006).
Cuesta, A., Todoli, J. L., Mora, J. & Canals, A. Rapid determination of chemical oxygen demand by a semi-automated method based on microwave sample digestion, chromium(VI) organic solvent extraction and flame atomic absorption spectrometry. Anal. Chim. Acta 372, 399–409. https://doi.org/10.1016/S0003-2670(98)00366-3 (1998).
Domini, C. E., Vidal, L. & Canals, A. Trivalent manganese as an environmentally friendly oxidizing reagent for microwave- and ultrasound-assisted chemical oxygen demand determination. Ultrason. Sonochem. 16, 686–691. https://doi.org/10.1016/j.ultsonch.2009.01.008 (2009).
Jianhua, M., Ma, J. & Yan, Z. Determination of COD in high salinity organic liquid wastes by high-speed catalytic method. Environ. Eng., 21 (2006).
Vyrides, I. & Stuckey, D. A modified method for the determination of chemical oxygen demand (COD) for samples with high salinity and low organics. Bioresour. Technol. 100, 979–982. https://doi.org/10.1016/j.biortech.2008.06.038 (2009).
Ballinger, D., Lloyd, A. & Morrish, A. Determination of chemical oxygen demand of wastewaters without the use of mercury salts. Analyst 107, 1047–1053. https://doi.org/10.1039/AN9820701047 (1982).
Thompson, K. C., Mendham, D., Best, D. & de Casseres, K. E. Communication. Simple method for minimising the effect of chloride on the chemical oxygen demand test without the use of mercury salts. Analyst 111, 483–485. https://doi.org/10.1039/AN9861100483 (1986).
Fah, H. C. Minimizing the use of mercuric salts for chloride and bromide corrections in chemical oxygen demand test. Malay. J. Chem. (MJChem) 5, 67–72 (2003).
Freire, D. D. C. & Sant’Anna, G. L. A proposed method modification for the determination of COD in saline waters. Environ. Technol. 19, 1243–1247. https://doi.org/10.1080/09593331908616784 (1998).
Nguyen, T. T., Padungthon, S. & Nguyen, N. H. Activated rice husk ash-supported silver nanoparticles as a novel adsorbent toward chloride removal. Desalin. Water Treat. 160, 308–315. https://doi.org/10.5004/dwt.2019.24239 (2019).
Acknowledgements
This research is funded by Vietnam National University–Ho Chi Minh City under Grant Number A2020-16-01. The authors also gratefully acknowledge technical support from An Giang University, VNU-HCM.
Author information
Authors and Affiliations
Contributions
T.T.N conceived of the idea, designed the experiments, and supervised the study. Q.A.N.T. and N.H.L. carried out the experiments and data processing. N.N.H. drafted and revised the manuscript. T.T.N. takes responsibility for the content of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, T.T., Nguyen Thi, Q.A., Le, N.H. et al. Synthesis of a novel porous Ag2O nanomaterial on ion exchange resin and its application for COD determination of high salinity water. Sci Rep 11, 11487 (2021). https://doi.org/10.1038/s41598-021-91004-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91004-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.