Abstract
Pollen storage belongs among the most important activities associated with pollen handling. It overcomes the differences in pollen shedding and ovule receptivity during controlled pollination experiments. It is especially important for species like common juniper (Juniperus communis L.) with an extremely low quality of seeds due to pollination failure. Additionally, it is a substantial part of germplasm preservation programmes in pollen banks. In the present paper, the effect of short-term storage of pollen was studied using pollen samples from five shrubs in an in vitro germination test. Two temperature regimes were tested. The pollen viability of freshly collected pollen varied considerably between individual shrubs, exhibiting 67.3–88.6% germination rate and 248.0–367.3 µm of pollen tubes. Storage at + 4 °C for four months was accompanied by a profound decline in pollen viability. The germination percentage was reduced to 49.2–75.2% and the pollen tube length to 32.5–69.0%, depending on individual shrubs. The corresponding decline in pollen viability characteristics during storage at − 20 °C was only negligible in two of the tested shrubs. In the remaining three shrub samples, an increase in germination percentage was observed. Pollen tube growth responded more sensitively to freezing, but, on average, the decrease in length was lower than that at + 4 °C. The rate of reduction in pollen tube length varied between 11.5 and 45.4%. Cytological events accompanying in vitro germination of freezer-stored pollen exhibited some delay in releasing the exine from pollen grains during the early stages of germination as compared with freshly collected pollen. In conclusion, short-term storage of the common juniper pollen in a freezer is better for the preservation of its viability than storage at + 4 °C.
Similar content being viewed by others
Introduction
Common juniper (Juniperus communis L.) is an obligatory dioecious species of the family Cupressaceae with a wide distribution in the Northern Hemisphere. It may be found in North America, Europe, Asia, and in some areas of North Africa1. In Europe, this species extends from southern Spain to the arctic circle in Norway2. As a species with a broad ecological amplitude, the common juniper is spread across all of Slovakia with an altitude range extending from the lowlands up to 1495 m above sea level (a.s.l.)3,4. The common juniper is an anemophilous species, but a certain degree of entomophily is also quite common5. The transformation from entomo- to anemophily during the evolution of pollination mechanisms in gymnosperms has left some traces in the structure and function of ovules and pollen grains in conifers6. A distinctive feature of common juniper pollen grains is the mechanism of their germination, including the mechanism of its initiation. Hydration, as a prerequisite for metabolic activation of pollen grains and subsequent pollen tube formation, is accompanied by shedding of the exine, which is a phenomenon unique to gymnosperms7. After restructuring, pollen grains begin to germinate when in culture. Cytologically, the initial stages of the process share little analogy with other species of gymnosperms. Additionally, the duration of pollen germination on artificial media differs profoundly between J. communis of the family Cupressaceae and conifers of the family Pinaceae. The pollen grains of the Cupressaceae start to germinate in 4–5 days, and the pollen of Pinaceae needs 24–48 h to germinate with the pollen tubes reaching full growth within 3–4 days8. The other deviating feature that discriminates between species of these families is the potential of their pollen for long-term storage. Mature pollen of the Pinaceae dehydrates to less than 10% water content before being shed, resulting in remarkable aerial buoyancy. This allows the dehydrated pollen to be collected and stored at low temperatures (< − 20 °C) for several years. In contrast, mature pollen of the Cupressaceae has about 30% water content, making it difficult to store for a long period of time9. Unfortunately, this aspect of pollen biology has been neglected in the entire family. No study has appeared yet referring to the behaviour of pollen under storage. Preference has been given to pollen characteristics, such as the quantitative aspects of pollen production in Olea europaea10, pollen morphology and pollen surface structure in some Juniperus species11, and aerobiological and allergenic properties of the cypress pollen12. In common juniper, few studies have reported on the cytological mechanism of in vitro pollen germination7,13. Although common juniper is generally not regarded as a commercially important species, its fruits are broadly used in culinary and pharmacy. As such, it may become an object of breeding14,15. This may require artificial crossing, for which efficient pollen storage is indispensable. In the present paper, the effect of storage on pollen viability under two temperature regimes was studied along with the dynamics of cytological events accompanying in vitro germination of freezer-stored juniper pollen grains to obtain the first data of the kind in the common juniper.
Results
A uniform response of the pollen grains towards storage conditions was registered in all five shrubs investigated with a conspicuous decline in germination percentage and pollen tube length after storage. Pollen tube growth reacted more sensitively to storage than germination. The most profound reductions in pollen viability traits were observed in samples stored at + 4 °C. The germination percentage of freshly collected pollen of individual shrubs ranged between 67.3 and 88.6%, whereas that in stored pollen was between 18.0 and 39.6%. In relative terms, storage represented a 49.3–73.2% decline in germination (Fig. 1). The same tendency was also observed in pollen tube growth, when freshly collected pollen possessed 248.0–367.3 µm long pollen tubes, and pollen stored at + 4 °C was characterised by 93.9–218.5 µm long pollen tubes. The corresponding decline reached 32.5–68.7%.
Graphical illustrations of variation in pollen germination percentage (a) and pollen tube length (b) of individual shrubs revealed in fresh pollen and in pollen under storage. Different letters refer to the statistical significance of the differences between tested individuals and storage variants, resulting from Duncan's pairwise tests.
Contrary to storage at + 4 °C, pollen stored at − 20 °C had an increased germination by 0.3% in shrub no. 1 and 0.6% in shrub no. 5 as compared with fresh pollen. A more conspicuous increase in pollen germinability was registered in individual no. 4, exhibiting 70.0% germination in fresh pollen and 93.6% in pollen stored at − 20 °C. In the remaining two shrubs (no. 2, 3), only a negligible decline in pollen germination was recorded. The deviation from freshly collected pollen varied within 0.5–16.8%. In general, the germination characteristics of pollen stored at − 20 °C were comparable with those of the fresh pollen and varied between 67.6 and 93.6%. As a second viability trait, pollen tube growth deviated more profoundly from that of fresh pollen than germination. On average, the pollen tube length of pollen stored at − 20 °C ranged from 163.0 to 286.6 µm, which represents a 11.4–45.7% decline compared to fresh pollen (Figs. 1, S1). ANOVA and Duncan`s grouping confirmed the highly significant differences between tested shrubs in both pollen germination percentage (P < 0.019) and pollen tube length (P < 0.0001) of the freshly collected pollen and the two storage conditions. Summarily, the pollen viability data of these conditions is illustrated in Fig. 2.
Average values of pollen germination percentage (a) and pollen tube length (b) of five shrubs of each fresh pollen and pollen stored at + 4 °C and − 20 °C. Different letters refer to the statistical significance of the differences between three groups of tested pollen, as revealed by Duncan's pairwise tests.
Based on Duncan`s test, the freshly collected pollen and pollen under − 20 °C storage had a comparable germination percentage. Statistically significant differences between the three pollen samples were due to the low quality of pollen stored at + 4 °C. Pollen tube growth was more sensitive to storage temperature as evidenced by a profound difference in pollen tube length under the tested storage conditions, which was confirmed by the ANOVA test (Analysis of variance). It follows from the data presented in Table 1 that except for the interaction between storage temperature and individual shrubs, the differences in pollen viability traits were highly significant between storage temperature variants, individual tested shrubs, repetitions, storage temperature, and tested individuals.
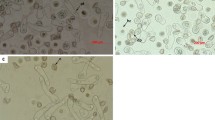
Cytological investigation of the pollen germination mechanism in freezer-stored samples indicated some deviations from the process involved in fresh pollen. Immediately after transfer from the storage container to cultivation media, the dormant pollen grains appeared as contracted structures of spherical and rectangular shapes with a conspicuously structuralised exine surface (Fig. 3a).
Dynamics of pollen hydrophilic capsule formation in pollen grains immediately after sowing on media (a) and subsequent changes after 10 (b), 50 (c), 55 (d), 60 (e), and 90 min (f) of cultivation; e-exine, m -hydrophilic capsule membrane, n-pollen nucleus, c-hydrophilic capsule. The photos were taken using Axioplan 2 microscope with built in AxioVision 4 software (Carl Zeiss Vision Gmb H).
The sequence of events accompanying the early stages of pollen germination were cytologically followed for 90 min after plating. Ten minutes after sowing pollen on the nutrient media, the prevailing amount of pollen grains remaining in a shrivelled stage indicated delayed imbibition (Fig. 3b). A massive detaching of the exine from pollen grains took place 50–55 min after pollen sowing (Fig. 3c,d). This unique phenomenon was caused by pollen hydration due to intense water penetration through the functional pore of the exine. The mucilaginous substance of the intine, containing abundant polysaccharides, swelled and caused the exine to burst, which was left on the media in the form of two half-opened valves with their edges curved inwards13. In some pollen grains, the exine had not yet been detached from the pollen body at this stage of pollen imbibition (Fig. 3c,d). As a result of continuing hydration, the hydrophilic capsule of the pollen grains became rather distinct, enlarging its volume during the 60–90 min period of pollen incubation. Externally, the capsule was bordered by a semipermeable membrane of the endoexine and internally by the intine (Fig. 3d–f). After 2–3 days of cultivation, the intine began to elongate, giving rise to the pollen tube. The entire process of pollen tube formation took place within a hydrophilic capsule and was rather asynchronous among pollen grains (Fig. 4a,b). The asynchronous
nature of pollen tube growth became more apparent after 5–6 days of pollen cultivation on the media, when more advanced stages of the process were observed, involving an elongated intine, shoe-like pollen tubes characteristic of the early stages of pollen tube growth, and elongated linear pollen tubes (Fig. 4c,d). Pollen germination took place on the background of contaminated nutrient media with numerous bacteria and fungi spreading over the surface. The germination potential of pollen grains was fully expressed on the seventh day of cultivation. The viable pollen grains with elongated pollen tubes appeared as very compact structures (Fig. 4e). Feulgen staining revealed that the haploid nucleus of a microspore had divided, giving rise to the pollen tube and vegetative cell (Fig. 4f).
Discussion
Short- and long-term storage are among the most important activities associated with pollen handling. It enables breeders to overcome differences in pollen shedding and ovule receptivity during controlled pollination of trees on the same locality or trees in widely separated regions16. Storage temperature, moisture content of the pollen, and relative humidity during storage play a decisive role in retaining the viability of stored pollen17. Optimal values of these factors and their combination may differ in individual groups of plants, depending on the structure and chemistry of pollen. In the present study, the effort was made to standardise the relative humidity within desiccators used for + 4 °C and − 20 °C storage. In both containers, the relative humidity was maintained at 27–30%, approaching the humidity level suggested for pollen storage in conifers (10–22%)18,19. Moisture content of the pollen was not determined during the study, but all five pollen lots sampled were uniformly dried under laboratory temperatures before storage. As for the temperature variable, the storage temperature for pollen with a high moisture content has been recommended to be around 0°C20. Conversely, our results showed unequivocally that storage at − 20 °C was a better alternative for the storage of juniper pollen. This conclusion was supported by a profound decline in pollen viability parameters in pollen stored at + 4 °C compared with the viability of fresh pollen. In contrast, only a slight decline in pollen viability after storage at − 20 °C was observed, and in some samples, a stimulatory effect of this temperature on pollen germination occurred. This may be taken as confirmation that temperatures around − 18 °C and − 20 °C, which are recommended for the storage of pine pollen9,21, are equally suitable for storage of J. communis pollen. Although there are several reports on the complete retention of pollen germination and pollen tube length in pine (Pinus mugo) pollen after one year of storage in the refrigerator22 or of a negligible decrease in germination in Pinus radiata and P. banksiana after one or two years of storage at + 4 °C8,23, the response of juniper pollen to short-term storage under similar temperatures was much more severe. This applies primarily to pollen tube growth, while the effect of cold storage on germination rate was less pronounced, especially at − 20 °C. Apparently, even when the capacity of stored pollen grains to germinate was less affected compared to fresh pollen, recovery from low temperatures may have partly exhausted the energetic resources of a pollen grain, resulting in a certain loss of vigour. The apertures on the exine have a high hydrophilicity that explains the death of pollen when it is stored for a few months at > 10% relative humidity13. Obviously, further research is needed to elucidate the conditions necessary for juniper pollen germination to occur after a longer period of storage. The approach based on in vitro germination shed some light on the germination mechanism, contributing simultaneously to characterisation of the pollen viability potential in quantitative terms. The ascertained germination of freshly harvested juniper pollen ranged between 67.3 and 88.6%, which was lower than that of fresh pollen in Pinus sylvestris, P. mugo, and their hybrids (88.2–95.4%)24. In a study on the effect of nitrogen fertilisation on the quality of J. communis pollen, a relatively low germination rate in pollen collected from fertilised plants (1.36%) was reported compared with non-fertilised plants (24.9%)25. The low pollen quality was considered one of the factors responsible for reproductive failure in natural populations of common juniper. A direct transfer of freezer-stored pollen onto a nutrient media surface, without previous preconditioning, adversely affected some processes associated with in vitro germination. During the taxoid type of pollen hydration, the exine is released from a pollen grain within 3–4 min.7. In our study, the entire process was completed within 60–90 min, during which an overwhelming majority of the pollen grains formed their hydrophilic capsules. The process was originally reported to take place in both viable and dead pollen grains, but recent data indicated that only 75% of incubated pollen grains released their exines after 96 h of incubation25. This is an additional reason for the continuation of pollen germination experiments aimed at elucidating aspects of the pollen germination mechanism in common juniper.
Summarily, the presented results on pollen storage in common juniper may be taken as preliminary studies because of the limited period of storage. Nevertheless, the experiment showed a higher sensitivity of juniper pollen to storage at + 4 °C compared, for example, to pine pollen. Conversely, freezer-storage at − 20 °C seemed more promising for retaining pollen viability, as a prerequisite for overcoming differences in the receptivity of juniper reproductive organs over different seasons. This is a necessary step in carrying out artificial pollination experiments with juniper oriented toward improving the poor quality of seeds. The latter is believed to be caused by reproductive failure in natural populations, causing the species to become endangered26. Only a 1.94% yield of fully developed seeds was postulated for juniper under natural conditions26, which is a big challenge to a better understanding of the events involved in the species’ reproductive cycle. In a broader sense, seed abortion was ascribed to pollination failure and to the factors related to physiology and nutrition of the female shrubs during the long maturation of the cones26. However, a more detailed specification of the factors responsible for this phenomenon was not provided. Therefore, it is reasonable to expect that intensive study on pollen quality, dispersion of pollen during the flowering period, and efficient pollination of the ovules, which ensures undisturbed embryogeny, might shed more light on the intricate mechanism of sexual reproduction in J. communis.
Conclusions
The presented results on longevity of stored common juniper pollen may be taken as a preliminary study. Nevertheless, the experiments demonstrated the higher sensitivity of juniper pollen to storage at + 4 °C compared, for example, to pine pollen. Conversely, freezer-storage at − 20 °C seemed more promising for retaining pollen viability, as a prerequisite for carrying out a supplementary pollination of the species to improve the quality of its seeds. The latter is believed to be caused by reproductive failure in natural populations, causing the species to become endangered. Regarding a critically endangered status of several Juniperus species around the world and expected similarity in pollen properties, the presented data may be of interest also for the conservation programmes, especially in establishing the pollen banks of the species.
Materials and methods
Plant material and short-term storage conditions
Male shrubs of the common juniper (Juniperus communis L.) growing in Dubniky of Western Slovakia (340 m a.s.l.) were used for study. The locality is situated near the village Bukova on a foot of the Little Carpatian Mountain Ridge and represents a public pasture land with advanced secondary succession. As common juniper is not a protected species in Slovakia, and the locality represents public land, collection of material for research does not require any specific permission, and was done in accordance with the national legislation (Act No. 543/2002 Z. z. on conservation of nature and landscape, §§ 4 and 47, Act No. 220/2004 Z. z. on protection and use of agricultural land). Twigs with mature microstrobili were cut separately from 5 shrubs and transferred to vessels with water and taken to the laboratory 2 days before discharging the pollen from microsporangia. Released pollen grains were collected on a large sheet beneath the vessels and sieved. The pollen grains of each individual were, after sieving, left in the laboratory for one additional day to dry more completely and used immediately in a germination test. This portion of samples was attributed to fresh pollen. The remaining dried pollen was divided into two parts in each tested shrub. One part of the pollen was stored at + 4 °C, the other at − 20 °C. The pollen was stored in glass test tubes that were plugged loosely with cotton-wool caps and placed into a desiccator with calcium chloride. The two temperature regimes included refrigerator storage at + 4 °C and freeze-storage at − 20 °C. The relative humidity inside the desiccators was 27–30%. The pollen was stored for a period of four months.
In vitro germination
Pollen germination was carried out at 25 °C in the dark with a separate evaluation of pollen viability in each shrub under study. The cultivation media consisted of 1.5% agar and 5% sucrose27; the latter has been reported to be optimal for common juniper pollen germination in culture5,7. The pollen was evenly dusted over the gel surface in small Petri dishes (47 mm diameter) by blowing it from a soft-hair brush. Each dish was placed in a larger Petri dish (70 mm diameter) containing 2 ml H2O and covered with a lid. Each sample was tested in triplicate. Immediately after sowing, the dynamics of pollen hydrophilic capsule formation was followed by 10, 50, 55, 60, and 90 min cultivation. Plates were incubated for 7 days, which was necessary for juniper pollen to fully express the growth potential of pollen tubes5. During the second half of the culture period, contamination of the cultivation media occurred by fungal spores and bacteria on the pollen surface and from the air8. The number of germinated pollen grains was recorded from a random sample of 100 pollen grains, and the pollen tube length was measured on a sample of 30 pollen grains on each Petri dish (total of 300 pollen grains and 90 pollen tubes per shrub). The evaluation was made with a NU 2 microscope (Carl Zeiss Jena) using a 10× eyepiece with a scale and 25× objective. A pollen grain was considered viable when its pollen tube reached a length comparable to the diameter of the pollen grain body28. The presence of clearly differentiated pollen tubes in individual pollen grains served as a main criterion of pollen viability. Another viability criterion was mitotic division of the microspore nucleus, giving rise to the vegetative and generative cells, both of which were visualised in growing pollen tubes by Feulgen staining. When measuring the length of a pollen tube, the distance from a zone where the round shape of the pollen grain body extended into the pollen tube was considered to the tip of the pollen tube. In total, the germination percentage evaluation was based on 3000 counts involving three repetitions per individual and 5 shrubs with freshly collected and stored pollen. Pollen from each of the shrubs was evaluated separately. Likewise, the pollen tube length data included 900 measurements. Differences in both viability traits were tested regarding the storage temperature and shrubs using two-way analysis of variance (ANOVA). Germination percentages were arcsine transformed prior to analysis. Calculations were performed using the GLM (General linear model) procedure in the statistical package SAS, version 9.4. Pairwise differences between individual shrubs were tested by Duncan`s test.
Feulgen staining of microspore nuclei
Germinating pollen grains were washed out from the cultivation medium surface with 1 N HCl into a thin-walled glass test tube using a pipette. The test tube containing pollen was plugged with a rubber stopper and incubated in a water bath (+ 60 °C) for 5 min. The hydrochloric acid was subsequently drained carefully from each tube. Feulgen solution was added to the pollen grains stuck on the tube wall. The test tube was plugged with a stopper and left at room temperature in the dark for 1 h. In the same way as with hydrochloric acid, Feulgen solution was discharged from the tube to which 7% acetic acid was added. A few drops of the mixture containing pollen grains were placed on a microscopic glass, covered with a cover glass, and examined under a microscope.
References
Thomas, P., El-Barghati, M. & Polwart, A. Biological flora of the British Isles: Juniperus communis L. J. Ecol. 95, 1404–1440 (2007).
Van Der Merwe, M., Winfield, M. O., Arnold, G. M. & Parker, J. S. Spatial and temporal aspects of the genetic structure of Juniperus communis populations. Mol. Ecol. 9, 379–386 (2000).
Blatny, T. & Stastny, T. Natural distribution of forest trees in Slovakia (Slovak Publisher of Agriculture Literature, 1959).
Futak, J., Jasicova, M. & Schidlay, E. Flora of Slovakia II (Slovak Academy of Sciences Publisher, 1966).
Surso, M. V. Pollination and pollen germination in common juniper (Juniperus communis: Cupressaceae). Arctic Environ. Res. 18, 162–174 (2018).
Labandeira, C. C., Kvacek, J. & Mostovski, M. H. Pollination drops, pollen and insect pollination of Mesozoic gymnosperms. Taxon 56, 663–695 (2007).
Duhoux, E. Evolution structurale de la paroi du grain de pollen du Juniperus communis L. (Cuppressacés), cultive in vitro au cours de la phase d`hydratation. C. R. Acad. Sci. 274, 2767–2770 (1972).
Razmologov, V. P. About germination and storage of pollen in some gymnosperms. Bull. Glav. Bot. Sada Mosc. 52, 79–87 (1964).
Fernando, D. D., Lazzaro, M. D. & Owens, J. N. Growth and development of conifer pollen tubes. Sex. Plant Reprod. 18, 149–162 (2005).
Rojo, J., Salido, P. & Pérez-Badia, R. Flower and pollen production in the ‘Corniabra’’ olive (Olea europaea L.) cultivar and the influence of environmental factors’. Trees 29, 1235–1245 (2015).
Bortenschlager, S. Aspects of pollen morphology in the Cupressaceae. Grana 29, 129–138 (1990).
de la Guardia, C. D., Alba, F., de Linares, C., Nieto-Lugilde, D. & López Caballero, J. Aerobiological and allergenic analysis of Cupressaceae pollen in Granada (Southern Spain). J. Investig. Allergel. Clin. Immunol. 16, 24–33 (2006).
Duhoux, E. Mechanism of exine rupture in hydrated taxoid type of pollen. Grana 21, 1–7 (1982).
Hazubska-Przybyl, T. Propagation of Juniper species by plant tissue culture: a mini-review. Forests 10, 1028. https://doi.org/10.3390/f10111028 (2019).
Feng, X. P. et al. Podophyllotoxin profiles combined with SRAP molecular markers in Juniperus rigida: a promising alternative source of podophyllotoxin. Ind. Crop. Prod. 153, 112547. https://doi.org/10.1016/j.indcrop.2020.112547 (2020).
Binder, W. D., Mitchell, G. M. & Ballantyne, D. J. Pollen Viability Testing Storage and Related Physiology (Canadian Forestry Service, Pacific Forest Research Centre, 1974).
Ehrenberg, C. E. Studies on the longevity of stored pine pollen (Pinus sylvestris L. and P. contorta var Murrayanna Engelm.). Medd. Skogsforskn. Inst. Stockh. 49, 1–31 (1960).
Snyder, E. B. Extracting, processing and storing southern pine pollen. Southern forest experimental station U.S. Forest Service. Occas. Pap. 191, 1–14 (1961).
Stanley, R. G. Viable pine (Pinus ponderosa) pollen stored 15 years produced unsound seed. Silvae Genet. 11, 1–3 (1962).
Stanley, R. G. & Linskens, H. F. Pollen. Biology, Biochemistry, Management (Springer, 1974).
Surso, M. V. Effect of prolong storage of pollen on seed formation in Scots pine. Acad. Sci. USSR Nautschnije Dokl. 214, 1–18 (1989).
Ostrolucka, M. G., Cicova, L. & Bolvansky, M. Influence of temperature and storage on pollen viability in Pinus mugo Turra. Folia Oecol. 30, 157–162 (2003).
Siregar, I. Z. & Sweet, G. B. The impact of extraction and storage on the viability of radiata pine pollen. Silvae Genet. 49, 10–14 (2000).
Kormutak, A., Galgoci, M., Bolecek, P. & Gömöry, D. Effect of storage on pollen viability in Pinus sylvestris L. Pinus mugo Turra and their hybrid swarms. Dendrobiology 82, 43–51 (2019).
Pers-Kamczyc, E., Tyrala-Wierucka, Z., Rabska, M., Wrońska-Pilarek, D. & Kamczyc, J. Higher nutritional availability improves productivity but reduces the quality of pollen grains of the Juniperus communis L. J. Plant Physiol. https://doi.org/10.1016/j.jplph.2020.153156 (2020).
Ward, L. K. & Shellswell, C. H. Looking After Juniper, Ecology, Conservation and Folklore (Plantlife, 2017).
Brewbaker, J. L. & Kwack, B. H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 50, 859–865 (1963).
Lindgren, K. & Lindgren, D. Germinability of Norway spruce and Scots pine pollen exposed to open air. Silva Fenn. 30, 3–9 (1996).
Acknowledgements
We thank the VEGA Grant Agency (project no. 2/0022/20) for financial support. The contribution of the reviewers in improving the manuscript is highly appreciated. Thanks to Proof-Reading-Service com Ltd., Devonshire Business Centre, UK for English correction.
Author information
Authors and Affiliations
Contributions
B.P. and G.M.—pollen collection, K.A.—laboratory processing, G.D.—statistics.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kormuťák, A., Bolecek, P., Galgóci, M. et al. Longevity and germination of Juniperus communis L. pollen after storage. Sci Rep 11, 12755 (2021). https://doi.org/10.1038/s41598-021-90942-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90942-9
This article is cited by
-
Pollen viability and longevity in Juniperus taxa native to Slovakia
Scientific Reports (2024)
-
Characterization of pollen tube development in distant hybridization of Chinese cork oak (Quercus variabilis L.)
Planta (2023)
-
X-ray computed tomography (CT) and ESEM-EDS investigations of unusual subfossilized juniper cones
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.