Abstract
Complications such as slow flow are frequently observed in percutaneous coronary intervention (PCI) with rotational atherectomy (RA). However, it remains unclear whether the high incidence of slow flow results in the high incidence of periprocedural myocardial infarction (PMI), reflecting real myocardial damage. The aim of this study was to compare the incidence of PMI between PCI with versus without RA using propensity score-matching. We included 1350 elective PCI cases, which were divided into the RA group (n = 203) and the non-RA group (n = 1147). After propensity score matching, the matched RA group (n = 190) and the matched non-RA group (n = 190) were generated. The primary interest was to compare the incidence of PMI between the matched RA and non-RA groups. Before propensity score matching, the incidence of slow flow and PMI was greater in the RA group than in the non-RA group. After matching, the incidence of slow flow was still greater in the matched RA group than in the matched non-RA group (16.8% vs. 9.5%, p = 0.048). However, the incidence of PMI was similar between the matched RA and matched non-RA group (7.4% vs. 5.3%, p = 0.528, standardized difference: 0.086). In conclusion, although use of RA was associated with greater risk of slow flow, use of RA was not associated with PMI after a propensity score-matched analysis. The fact that RA did not increase the risk of myocardial damage in complex lesions would have an impact on revascularization strategy for severely calcified coronary lesions.
Similar content being viewed by others
Introduction
Rotational atherectomy (RA) is an essential procedure for the treatment of heavily calcified coronary lesions in percutaneous coronary intervention (PCI)1,2. However, the incidence of some complications, especially coronary perforation, is reported to be greater in PCI with RA than without3,4,5. Of those complications, slow flow is the most common complications following RA6,7, suggesting the incidence of slow flow would be higher in PCI with RA than without. However, it remains unclear whether the higher incidence of slow flow results in the higher incidence of periprocedural myocardial infarction (PMI), which reflects real myocardial damage and is closely associated with future cardiovascular events including cardiac death8,9,10,11,12. It is of importance to confirm whether RA increases the incidence of PMI, because interventional cardiologists would hesitate to select RA among several specific procedures including orbital atherectomy and intravascular lithotripsy for severely calcified lesions if RA increases the incidence of PMI considerably.
Although a few studies suggested the greater incidence of PMI in PCI with RA than without, the lesion characteristics such as the degree of calcification were more complex in PCI with RA than without3,13,14, which would make it difficult to compare the incidence of PMI between PCI with and without RA. Even randomized studies may not answer clearly whether the incidence of PMI was greater in PCI with RA, because the prevalence of crossover from PCI without RA to PCI with RA was quite high (12.5–16.0%) in recent randomized trials comparing PCI with versus without RA15,16, which is much higher rates of crossover compared to other randomized studies in the field of PCI17,18. Considering these specific background regarding PCI with RA, we hypothesized that a propensity score-matched analysis would work for adjusting clinical and lesion characteristics between PCI with versus without RA. The aim of this study was to compare the incidence of PMI between PCI with versus without RA using a propensity score-matched analysis.
Methods
Study design
This was a retrospective, single-center study. We reviewed consecutive PCI cases in the Saitama Medical Center, Jichi Medical University from January 2018 to March 2020. The inclusion criterion was (1) elective PCI cases that were performed during the study period. Elective PCI cases included the culprit lesions of acute myocardial infarction as long as PCI was planned as elective PCI. The exclusion criteria were (1) cases which did not have either creatine kinase (CK) or CK-myocardial band (MB) values at the next day of PCI, (2) cases in which ≥ 2 vessels were treated simultaneously, and (3) saphenous vein graft lesions. We divided the study cases into the lesions that were treated with RA (RA group) and the lesions that were treated without RA (non-RA group). Propensity score matching was performed to match the background characteristics between the 2 groups. The details of the propensity score-matched analysis are described in the statistical analysis section. Clinical characteristics and outcomes were compared between the 2 groups both before and after propensity score-matching. The primary interest in the present study was the incidence of PMI after a propensity score-matching. The secondary interests included the incidence of slow flow and other periprocedural complications. This study was approved by the Institutional Review Board of Saitama Medical Center, Jichi Medical University (S20-152), and written informed consent was waived by the institutional review board of Saitama Medical Center, Jichi Medical University, because of the retrospective study design. All methods were performed in accordance with the relevant guidelines and regulations.
Definition
The definition of overweight, hypertension, dyslipidemia, diabetes mellitus, and chronic renal failure was described in elsewhere19,20,21,22. We also calculated the estimated glomerular filtration rate (eGFR) from the serum creatinine level, age, weight, and gender using the following formula: eGFR = 194 × Cr-1.094 × age-0.287 (male), eGFR = 194 × Cr-1.094 × age-0.287 × 0.739 (female)20. In patients whose baseline CK levels were normal, PMI was defined as an elevation of CK levels ≥ 2 times of the upper limit of normal (ULN) with an elevation of CK-MB levels above the ULN at the next day of PCI23,24,25,26. If baseline CK levels were already elevated, PMI was defined as further increase of CK levels at the next day of PCI than CK level at baseline26. We also checked PMI defined by the Society for Cardiovascular Angiography and Interventions (SCAI), and PMI defined by the EXCEL (Evaluation of XIENCE vs. Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization)8,27.
PCI procedures
The choice of PCI devices such as guide wire, balloon, rotational atherectomy and stent was left at the discretion of interventional cardiologists at our cardiology center. IVUS or OCT were routinely used for almost all lesions. In bifurcation lesions, we usually insert a conventional guidewire to a side branch before stenting to the main vessel, and occasionally perform jailed balloon technique/jailed corsair technique. We conducted a single stent technique, and seldom selected two-stent technique, especially in elective PCI. Successful PCI was defined as angiographical residual diameter stenosis < 50% with decrease in minimum stenosis with TIMI flow grade ≥ 228,29.
Rotational atherectomy was performed to heavily calcified lesions, diffuse lesions expected to be difficult to stent, and ostial lesions22. We used the nicorandil based drug cocktail (nicorandil 12 mg, isosorbide dinitrate 2.5 mg, heparin 10,000 units, and normal saline 500 mL) for all RA cases. The lesion was crossed with a 0.014-inch conventional guidewire, which was exchanged with a 0.009-inch RotaWire floppy or RotaWire extra support guidewire (Boston Scientific, Natick, MA, USA) using a microcatheter. The RA burr was subsequently advanced over the wire to a position proximal to the lesion. The rotational speed was set at the conventional range (140,000–190,000 rpm) with the burr proximal to the lesion30. The burr was activated and moved forward with a slow pecking motion. Each run time was < 30 s, and care was taken to avoid a decrease in rotational speed > 5000 rpm. The presence of coronary flow was confirmed by injecting sufficient contrast medium immediately after the burr was pulled out.
Our university hospital had many operators including residents with different background. However, each PCI was strictly supervised by staff operator, and all PCI with RA were supervised by a senior staff operator (K. Sakakura). Our techniques regarding RA was almost consistent to those that were recommended by the clinical expert consensus document on RA from the Japanese association of cardiovascular intervention and therapeutics (CVIT)2. Staff operators did not hesitate to take over procedures, when residents felt any difficulties in procedures. Activated coagulation time was maintained over 250 s during procedures.
Angiographical analysis
Quantitative coronary angiography parameters were measured using a cardiovascular angiography analysis system (QAngio XA 7.3, MEDIS Imaging Systems, Leiden, Netherlands). The lesion length and reference diameter were measured. Bifurcation lesions were divided into seven categories according to the Medina classification system31. In this system, we recorded any narrowing ≥ 50% in angiography in each of the three arterial segments of the bifurcation in the following order: proximal main vessel, distal main branch, and side branch: 1 is used to indicate the presence of a significant stenosis and 0 was used to indicate the absence of stenosis31. Calcification was identified as readily apparent radiopacities within the vascular wall at the site of the stenosis, and was classified as none/mild, moderate (radiopacities noted only during the cardiac cycle before contrast injection), and severe (radiopacities noted without cardiac motion before contrast injection generally compromising both sides of the arterial lumen)32. Slow flow was defined as slow or absent distal runoff (TIMI flow grade ≤ 2) just after RA, balloon dilation or deployment of stent33.
Statistical analysis
Data are presented as a percentage for categorical variables, a mean ± standard deviation (SD) for normally distributed continuous variables, and median (quartile 1–quartile 3) for nonparametric variables. The Wilk-Shapiro test was performed to determine if the continuous variables were normally distributed. Normally distributed continuous variables were compared between the 2 groups using a Student’s t test. Otherwise, continuous variables were compared using a Mann–Whitney U test. Categorical variables were compared using a Fisher’s exact test. One-to-one propensity score matching was used to match the clinical background between the 2 groups. RA was set as a dependent variable, whereas parameters that were clinically relevant for complications in RA, such as age, male sex, overweight, hypertension, dyslipidemia, diabetes mellitus, estimated GFR, PCI indication for acute myocardial infarction, lesions at left main trunk and/or left anterior descending artery, chronic total occlusion, reference diameter, lesion length, lesion angle, calcification and bifurcation were set as independent variables5. For matching, the match tolerance was initially set as a width of 0.25 multiplied by the SD of the propensity score distribution34,35. However, the generated sample size was only 358 lesions (179 lesions in each group). In order to assess an appropriate sample size, we additionally performed the power analysis using the estimated incidences of PMI in PCI with RA as 9.7% and PCI without RA as 2.8%14. The required sample size was 384 lesions (192 lesions in each group) assuming as statistical power (1-β) of 80% and a sensitivity (α) of 5%. Therefore, we performed another matching using the more liberal match tolerance that was set as a width of 0.30 multiplied by the SD of the propensity score distribution, and the generated sample size was 380 lesions (190 lesions in each group). We adopted these 380 lesions (190 lesions in each group) as main analysis, and used 358 lesions (179 lesions in each group) as supplemental analysis. Balance between matched RA group and matched non-RA group after propensity score matching was assessed by using standardized difference of each covariate where a standardized difference < 0.1 was considered a negligible difference in the mean or prevalence of a covariate between RA group and non-RA group36,37.
Multivariate logistic regression analysis was performed to investigate associations between clinical variables including RA and PMI after propensity score matching analysis. In this model, PMI was used as the dependent variable. Variables that had a significant association (p < 0.05) between the matched 2 groups and use of RA were used as independent variables. The odds ratio (OR) and the 95% confidence interval (CI) were calculated. A p value < 0.05 was considered statistically significant. We analyzed all data by IBM SPSS statistics version 24 (Chicago, IL, USA).
Results
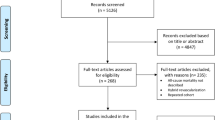
Overall, 1377 elective PCI cases were performed in our hospital from January 2018 to March 2020, and 27 PCI cases were excluded. Our final study population was 1350 PCI cases, which were divided into in the RA group (n = 203) and the non-RA group (n = 1147). After a propensity score-matched analysis, study population was divided into the matched RA group (n = 190) and matched non-RA group (n = 190). The study flowchart is shown as the Fig. 1.
Patient, lesion and procedural characteristics between the 2 groups before and after propensity score matching are summarized in Table 1. Although several patient’s characteristics were significantly different between the RA and non-RA groups before matching, all patient’s characteristics except diabetes mellitus were similar between the matched RA and matched non-RA groups.
Lesion characteristics were also similar between the matched RA and matched non-RA groups after matching. In procedural characteristics, the size of guiding catheter was larger in the matched RA group than in the matched non-RA group (p < 0.001). Orbital atherectomy was more frequently used in the matched non-RA group than in the matched RA group (p < 0.001). Furthermore, patient, lesion and procedural characteristics between the 2 groups after propensity score matching using the match tolerance that was set as a width of 0.25 multiplied by the SD of the propensity score distribution are summarized in Supplemental Table 1.
Complications between the 2 groups is shown in Table 2. The incidence of slow flow was significantly greater in the matched RA group (16.8%) than in the matched non-RA group (9.5%) (p = 0.048), whereas the incidence of PMI was similar between the 2 matched groups (7.4% vs. 5.3%, p = 0.528). Standardized difference of PMI in the 2 matched groups was 0.086. Furthermore, complications between the 2 groups after propensity score matching using the match tolerance that was set as a width of 0.25 multiplied by the SD of the propensity score distribution are shown in Supplemental Table 2.
We performed a multivariate logistic regression analysis to confirm the relationship between use of RA and PMI in the matched study population. Other than RA, we included diabetes mellitus and guide catheter size, which were significantly different between the 2 matched groups, as independent variables. Although the use of orbital atherectomy was significantly different between the matched group, there was no periprocedural myocardial infarction in lesions required orbital atherectomy, which makes impossible to calculate a odds ratio. Therefore, orbital atherectomy was not included in the variable. Table 3 shows use of RA (OR 1.229; 95% CI 0.479 − 3.153; p = 0.667) was not significantly associated with PMI after controlling confounding factors (diabetes mellitus and guide catheter size).
Discussion
We included 1350 elective PCI cases, which were divided into the RA group (n = 203) and the non-RA group (n = 1147). After propensity score matching, the matched RA group (n = 190) and the matched non-RA group (n = 190) were generated. The main findings of our study were as follows: (1) although the incidence of slow flow was greater in the matched RA group than in the matched non-RA group, the incidence of PMI was similar between the matched RA and matched non-RA group, suggesting RA would not increase the risk of PMI; and (2) other complications such as side branch occlusion, vessel perforation and device stuck were not different between the matched 2 groups.
We should discuss the discrepancy that the incidence of PMI was greater in the RA than in the non-RA group, whereas the incidence of PMI was similar between the matched RA and matched non-RA groups. Our group recently reported the determinants of PMI were complex lesion features such as diffuse long lesion, larger lesion angle or true bifurcation lesions14. The results before matching would suggest that the target lesions had more complex features such as calcification in PCI with RA than in PCI without RA. In fact, lesion length was significantly longer, lesion angle was significantly severer, and calcification was significantly severer in the RA group than in the non-RA group. Earlier retrospective studies comparing the incidence of complications between PCI with versus without RA could not adjust clinical background between PCI with and without RA3,13. Our propensity-score matching adjusted clinical and lesion characteristics well, and allowed us to compare the real additional risk of RA for complex lesions. Our results suggest that use of RA itself would not increase the risk of complications during PCI to complex lesions.
Of complications, only slow flow was more frequently observed in the matched RA group than in the matched non-RA group. There are several explanations why the incidence of slow flow was greater in the matched RA group. First, our group has been very careful about picking up slow flow during PCI with RA since November 2014, when our group started a randomized study regarding slow flow during RA(trial registration: UMIN 000015702). Indeed, our group has published several literatures regarding slow flow during RA30,38,39. Thus, we picked up mild slow flow (e.g. transient TIMI-2 flow only just after RA) as well as severe slow flow. The incidence of slow flow during RA varies widely from 2.7 to over 20%, depending on the definition, timing of judgement, and the length of target lesion40,41,42. Our incidence of slow flow in PCI with RA was relatively high as compared to literatures. Second, although we reviewed all cine-angiogram and catheter reports, we might not pick up slow flow in PCI without RA as we picked up slow flow in PCI with RA. Therefore, we might miss transient slow flow and underestimate the incidence of slow flow in PCI without RA.
Clinical implications of the present study should be noted. First, the greater incidence of PMI after RA was not attributed to use of RA itself, but to lesion complexity. We do not need to hesitate to use RA for complex lesions in fear of PMI or myocardial damage. The incidence of slow flow was greater in PCI with RA than in PCI without even after propensity score matching. RA operators should recognize the greater risk of slow flow during RA. However, if transient slow flow just after RA was treated adequately, most slow flow would not result in myocardial damage expressed as PMI. RA operators should be familiar with the prevention and bailout for slow flow to avoid myocardial damage that affects patient’s clinical outcomes2. Furthermore, the prevalence of chronic kidney disease was relatively high in our study population. Since earlier studies reported the high percentage of advanced atherosclerotic plaques such as large lipid volume or dense calcification in patients with chronic kidney disease43,44,45, those patients would have a greater risk of PMI. Thus, our study population was the high risk population for PMI. It is noteworthy that the incidence of PMI was comparable between the RA and non-RA groups in these high risk population.
Study limitations
First, this study was as single-center retrospective observational study, there is a risk of patient selection bias and group selection bias. Furthermore, some important variables such as the duration of diabetes mellitus were not available because of the retrospective nature of this study. The frequency of RA usage was greater in our catheter laboratory than Japanese national PCI registry5, which could be an institutional bias. Furthermore, the incident of coronary perforation or device entrapment was 0% in the RA group, which was low as compared to literatures3,15,46. We might be familiar with various techniques such as halfway RA to prevent severe complications47. Second, our study population was not a patient-level database, but a PCI-level database. Therefore, we could not exclude the effect of the clustered nature of one or more individual measurements from one patient. Third, we did not adopt the definition of PMI by the universal definition [cardiac troponin > 5 times upper limit of normal and PCI-related clinical or angiographic complications], but adopted the definition of PMI by the rise of CK/CK-MB, because the definition of PMI by the universal definition might be influenced by the subjective judgment11. Fourth, we used lesion characteristics such as PCI indication for acute myocardial infarction, lesions at left main trunk and/or left anterior descending artery, chronic total occlusion, reference diameter, lesion length, lesion angle, calcification and bifurcation, which were drawn from angiographical findings. The matching of lesion characteristics might be insufficient by such angiographical findings, and might be improved by IVUS findings. However, it was difficult to use IVUS findings for matching, because pre-procedural IVUS could not cross the calcified lesion before RA in approximately half of cases. Finally, there was a possibility of a Type II error (ß error) in the comparison of complications between the 2 matched groups. However, the required sample size would be 384 lesions (192 lesions in each group) in a powered analysis, while our propensity score matching generated 380 lesions (190 lesions in each group), which was almost similar to the estimated sample size. Therefore, the possibility of a Type II error (ß error) might be minimum. Moreover, the standardized difference of PMI between the 2 matched groups was 0.086. Since a standardized difference < 0.1 has been taken to indicate a negligible difference in the prevalence of a covariate between the 2 groups irrespective of the study sample size36,48, it is appropriate to mention that use of RA would not increase the risk of PMI in complex lesions.
Conclusions
In contemporary elective PCI, use of RA was not associated with PMI after a propensity score-matched analysis, while use of RA was associated with greater risk of slow flow. The fact that RA did not increase the risk of myocardial damage in complex lesions would have an impact on revascularization strategy for severely calcified coronary lesions.
Data availability
All data are available from the corresponding author on reasonable request.
References
Sharma, S. K. et al. North American expert review of rotational atherectomy. Circ. Cardiovasc. Interv. 12, e007448 (2019).
Sakakura, K. et al. Clinical expert consensus document on rotational atherectomy from the Japanese association of cardiovascular intervention and therapeutics. Cardiovasc. Interv. Ther. 36, 1–18 (2021).
Januszek, R., Siudak, Z., Dziewierz, A., Dudek, D. & Bartus, S. Predictors of in-hospital effectiveness and complications of rotational atherectomy (from the ORPKI Polish National Registry 2014–2016). Catheter Cardiovasc. Interv. 92, E278–E287 (2018).
Eftychiou, C. et al. Cardiovascular outcomes following rotational atherectomy: a UK multicentre experience. Catheter Cardiovasc. Interv. 88, 546–553 (2016).
Sakakura, K. et al. Incidence and determinants of complications in rotational atherectomy: insights from the National Clinical Data (J-PCI Registry). Circ. Cardiovasc. Interv. 9, e004278 (2016).
Abbo, K. M. et al. Features and outcome of no-reflow after percutaneous coronary intervention. Am. J. Cardiol. 75, 778–782 (1995).
Sakakura, K. et al. Comparison of frequency of complications with on-label versus off-label use of rotational atherectomy. Am. J. Cardiol. 110, 498–501 (2012).
Ben-Yehuda, O. et al. Impact of large periprocedural myocardial infarction on mortality after percutaneous coronary intervention and coronary artery bypass grafting for left main disease: an analysis from the EXCEL trial. Eur. Heart J. 40, 1930–1941 (2019).
Herrmann, J. Peri-procedural myocardial injury: 2005 update. Eur. Heart J. 26, 2493–2519 (2005).
Zeitouni, M. et al. Periprocedural myocardial infarction and injury in elective coronary stenting. Eur. Heart J. 39, 1100–1109 (2018).
Tricoci, P. et al. Prognostic and practical validation of current definitions of myocardial infarction associated with percutaneous coronary intervention. JACC Cardiovasc. Interv. 11, 856–864 (2018).
Garcia-Garcia, H. M. et al. Impact of periprocedural myocardial biomarker elevation on mortality following elective percutaneous coronary intervention. JACC Cardiovasc. Interv. 12, 1954–1962 (2019).
Yang, X., Tamez, H., Lai, C., Ho, K. & Cutlip, D. Type 4a myocardial infarction: incidence, risk factors, and long-term outcomes. Catheter Cardiovasc. Interv. 89, 849–856 (2017).
Mizuno, Y. et al. Determinants of periprocedural myocardial infarction in current elective percutaneous coronary interventions. Int. Heart J. 61, 1121–1128 (2020).
Abdel-Wahab, M. et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc. Interv. 6, 10–19 (2013).
Abdel-Wahab, M. et al. High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ. Cardiovasc. Interv. 11, e007415 (2018).
Hibi, K. et al. A randomized study of distal filter protection versus conventional treatment during percutaneous coronary intervention in patients with attenuated plaque identified by intravascular ultrasound. JACC Cardiovasc. Interv. 11, 1545–1555 (2018).
Watanabe, H. et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI. JAMA 321, 2414–2427 (2019).
Wolny, R. et al. The obesity paradox revisited: body mass index and -long-term outcomes after PCI from a large pooled patient-level database. EuroInterv. J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 15, 1199–1208 (2020).
Ohashi, J. et al. Determinants of improvement of mid-term ejection fraction in patients with acute myocardial infarction. Int. Heart J. 60, 1245–1252 (2019).
Kasahara, T. et al. Clinical factors associated with in-hospital mortality in patients with acute myocardial infarction who required intra-aortic balloon pumping. Int. Heart J. 61, 209–214 (2020).
Sakakura, K. et al. Comparison of complications with a 1.25-mm versus a 1.5-mm burr for severely calcified lesions that could not be crossed by an intravascular ultrasound catheter. Cardiovasc. Interv. Therap. 35, 227–233 (2019).
Jang, J.-S. et al. Prognostic value of creatine kinase-myocardial band isoenzyme elevation following percutaneous coronary intervention: a meta-analysis. Catheter. Cardiovasc. Interv. 81, 959–967 (2013).
Cavallini, C. et al. Impact of the elevation of biochemical markers of myocardial damage on long-term mortality after percutaneous coronary intervention: results of the CK-MB and PCI study. Eur. Heart J. 26, 1494–1498 (2005).
Tandjung, K. et al. Comparison of frequency of periprocedural myocardial infarction in patients with and without diabetes mellitus to those with previously unknown but elevated glycated hemoglobin levels (from the TWENTE Trial). Am. J. Cardiol. 110, 1561–1567 (2012).
Vranckx, P. et al. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroInterv. J. EuroPCR Collab Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 5, 871–874 (2010).
Moussa, I. D. et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J. Am. Coll. Cardiol. 62, 1563–1570 (2013).
Jeremias, A. et al. Blinded physiological assessment of residual ischemia after successful angiographic percutaneous coronary intervention: the DEFINE PCI study. JACC Cardiovasc. Interv. 12, 1991–2001 (2019).
Choo, E. H. et al. Comparison of successful percutaneous coronary intervention versus optimal medical therapy in patients with coronary chronic total occlusion. J. Cardiol. 73, 156–162 (2019).
Sakakura, K. et al. The incidence of slow flow after rotational atherectomy of calcified coronary arteries: a randomized study of low speed versus high speed. Catheter Cardiovasc. Interv. 89, 832–840 (2017).
Medina A, Suárez de Lezo J and Pan M. A New Classification of Coronary Bifurcation Lesions. Revista Española de Cardiología (English Edition). 59, 183 (2006).
Mintz, G. S. et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation 91, 1959–1965 (1995).
Matsuo, H. et al. Prevention of no-reflow/slow-flow phenomenon during rotational atherectomy—a prospective randomized study comparing intracoronary continuous infusion of verapamil and nicorandil. Am Heart J. 154(994), e1-6 (2007).
Stuart, E. A. Matching methods for causal inference: a review and a look forward. Stat. Sci. 25, 1–21 (2010).
Cavender, M. A. et al. SGLT-2 inhibitors and cardiovascular risk: an analysis of CVD-REAL. J. Am. Coll. Cardiol. 71, 2497–2506 (2018).
Austin, P. C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 46, 399–424 (2011).
Austin, P. C. A tutorial and case study in propensity score analysis: an application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivar. Behav. Res. 46, 119–151 (2011).
Sakakura, K. et al. Comparison of the incidence of slow flow after rotational atherectomy with IVUS-crossable versus IVUS-uncrossable calcified lesions. Sci. Rep. 10, 11362 (2020).
Sakakura, K. et al. Comparison of complications with a 1.25-mm versus a 1.5-mm burr for severely calcified lesions that could not be crossed by an intravascular ultrasound catheter. Cardiovasc. Interv. Ther. 35, 227–233 (2020).
Tsubokawa, A., Ueda, K., Sakamoto, H., Iwase, T. & Tamaki, S. Effect of intracoronary nicorandil administration on preventing no-reflow/slow flow phenomenon during rotational atherectomy. Circ. J. 66, 1119–1123 (2002).
Kini, A., Reich, D., Marmur, J. D., Mitre, C. A. & Sharma, S. K. Reduction in periprocedural enzyme elevation by abciximab after rotational atherectomy of type B2 lesions: results of the Rota ReoPro randomized trial. Am. Heart J. 142, 965–969 (2001).
Chambers, J. W. et al. Outcomes after atherectomy treatment of severely calcified coronary bifurcation lesions: a single center experience. Cardiovasc. Revasc. Med. 20, 569–572 (2019).
Miyagi, M. et al. Impact of renal function on coronary plaque composition. Nephrol. Dial Transpl. 25, 175–181 (2010).
Nakano, T. et al. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am. J. Kidney Dis Offic. J. Natl. Kidney Found. 55, 21–30 (2010).
Kono, K. et al. Composition and plaque patterns of coronary culprit lesions and clinical characteristics of patients with chronic kidney disease. Kidney Int. 82, 344–351 (2012).
Wang, Y. H. et al. Incidence and mechanisms of coronary perforations during rotational atherectomy in modern practice. J. Interv. Cardiol. 2020, 1894389 (2020).
Taniyama, Y. et al. Halfway rotational atherectomy for calcified lesions: comparison with conventional rotational atherectomy in a propensity-score matched analysis. PLoS ONE 14, e0219289 (2019).
Pomponio, M. et al. Impact of 21-gene expression assay on clinical outcomes in node-negative ≤ T1b breast cancer. Ann. Surg. Oncol. 27, 1671–1678 (2020).
Acknowledgements
The authors acknowledge all staff in the catheter laboratory in Saitama Medical Center, Jichi Medical University for their technical support in this study.
Author information
Authors and Affiliations
Contributions
Y.M. and K.S. conceived the idea of the study. Y.M., K.S., H.J., Y.T., T.T., K.Y., M.S., and H.W. collected the data. M. Mizuno performed statistical analysis, and drafted a manuscript. K.S. revised the manuscript. H.F. supervised the project. All authors commented on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
Dr. Sakakura has received speaking honoraria from Abbott Vascular, Boston Scientific, Medtronic Cardiovascular, Terumo, OrbusNeich, Japan Lifeline, and NIPRO; he has served as a proctor for Rotablator for Boston Scientific; and he has served as a consultant for Abbott Vascular and Boston Scientific. Dr. Jinnouchi has received speaking honoraria from Abbott Vascular. Prof. Fujita has served as a consultant for Mehergen Group Holdings, Inc. Other authors have no conflicts of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mizuno, Y., Sakakura, K., Jinnouchi, H. et al. Comparison of the incidence of periprocedural myocardial infarction between percutaneous coronary intervention with versus without rotational atherectomy using propensity score-matching. Sci Rep 11, 11140 (2021). https://doi.org/10.1038/s41598-021-90042-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90042-8
This article is cited by
-
Halftime rotational atherectomy: a unique concept for diffuse long severely calcified lesions
Cardiovascular Intervention and Therapeutics (2023)
-
Clinical expert consensus document on rotational atherectomy from the Japanese association of cardiovascular intervention and therapeutics: update 2023
Cardiovascular Intervention and Therapeutics (2023)
-
Intravascular ultrasound-factors associated with slow flow following rotational atherectomy in heavily calcified coronary artery
Scientific Reports (2022)
-
Factors associated with difficulty in crossing the culprit lesion of acute myocardial infarction
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.