Abstract
Progressive supranuclear palsy (PSP) is a rare and rapidly progressing atypical parkinsonism. Albeit existing clinical criteria for PSP have good specificity and sensitivity, there is a need for biomarkers able to capture early objective disease-specific abnormalities. This study aimed to identify gait patterns specifically associated with early PSP. The study population comprised 104 consecutively enrolled participants (83 PD and 21 PSP patients). Gait was investigated using a gait analysis system during normal gait and a cognitive dual task. Univariate statistical analysis and binary logistic regression were used to compare all PD patients and all PSP patients, as well as newly diagnosed PD and early PSP patients. Gait pattern was poorer in PSP patients than in PD patients, even from early stages. PSP patients exhibited reduced velocity and increased measures of dynamic instability when compared to PD patients. Application of predictive models to gait data revealed that PD gait pattern was typified by increased cadence and longer cycle length, whereas a longer stance phase characterized PSP patients in both mid and early disease stages. The present study demonstrates that quantitative gait evaluation clearly distinguishes PSP patients from PD patients since the earliest stages of disease. First, this might candidate gait analysis as a reliable biomarker in both clinical and research setting. Furthermore, our results may offer speculative clues for conceiving early disease-specific rehabilitation strategies.
Similar content being viewed by others
Introduction
Progressive supranuclear palsy (PSP) is a rare and rapidly progressing neurodegenerative disease classified among atypical Parkinsonisms, with a prevalence of 5–6 cases per 100,0001. PSP Richardson’s syndrome, the most frequent form of the disease, is characterized by vertical supranuclear gaze palsy and postural instability with early falls2. Extant evidence suggests that the clinical spectrum of PSP is larger than originally described. In particular, the second most common form of disease, accounting for a third of cases, is characterized by a parkinsonian syndrome resembling Parkinson’s disease (PD) especially in the earliest stages3.
Recently, several PSP variants were detailed in the International Parkinson and Movement Disorder Society criteria for diagnosis of PSP (MDS-PSP)4 and subsequently characterized in real-life clinical settings5,6.
Although semi-quantitative rating scales such as the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)7 and PSP Rating Scale (PSP-RS)8 provide a clinician-based quantification of disease burden, these scales do not provide objective quantitative measures. Indeed, there is a need to employ quantitative tools for evaluating motor function in parkinsonism9; among those, gait analysis is one of the main instruments used to assess locomotion. Gait analysis is a non-invasive, 3-dimensional computerized examination of gait, commonly used in the literature to investigate and distinguish various diseases10.
Gait analysis has been employed for different objectives in PD patients, including the investigation of pathophysiological mechanisms underpinning the disease, evaluation of treatment outcomes, automatic recognition of PD symptoms, and implementation of algorithms for PD diagnosis and staging11,12,13,14. In addition, gait analysis has been used to explore the association between specific gait patterns and specific symptoms of PD, such as mild cognitive impairment15,16 and freezing of gait17.
Quantitative tools for assessing locomotion have also been applied in PSP patients. Amano et al. 18 examined the biomechanical features of dynamic postural control during gait initiation and ambulation in PSP patients using a gait analysis system. Hatanaka et al. compared the gait features of PSP, PD patients, and controls using a portable triaxial accelerometer rhythmogram19. Other studies have employed sensor-based approaches. In particular, Raccagni et al. investigated the ability of a gait assessment system to detect differences in gait parameters in atypical parkinsonian disorders20, whereas Gaβner et al. assessed whether sensor-based gait parameters could serve as a complementary tool to clinical scores for distinguishing atypical parkinsonism from PD21. More recently, machine-learning approaches have been introduced to assess the gait patterns of PSP patients, although the rarity of this disease poses challenges to obtaining large datasets for analysis. In our previous work, we distinguished de novo PD, stable PD, and PSP patients using machine-learning techniques applied to gait parameters after artificial data augmentation and achieved promising results22. Subsequently, De Vos et al. performed a similar study using wearable technology23.
Albeit the MDS-PSP criteria4 have recently proven to have good specificity and sensitivity24, there is a need for biomarkers able to capture early objective disease-specific abnormalities and to monitor disease progression over time. The main aim of the present study was to identify gait patterns specifically associated with PSP with two-fold impact: (1) providing a proof of concept that quantitative gait evaluation may represent a reliable biomarker since the earliest stages of disease; (2) recognizing early disease-specific gait patterns useful to design tailored rehabilitation programs.
Methods
Study design and population
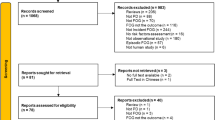
The study population consisted of 104 participants (83 PD and 21 PSP patients) consecutively enrolled between February 2018 and July 2020. Participants were selected from patients referred to the Movement Disorders Unit of the Institute for Diagnosis and Care Hermitage-Capodimonte of Naples and Center for Neurodegenerative Diseases of the University of Salerno. All PD patients fulfilled the Movement Disorder Society (MDS) clinical diagnostic criteria for PD25. Newly diagnosed PD patients presented with symptom onset within 1 year from enrolment and were included after undergoing a [123I]FP-CIT SPECT examination for dopamine transporter assessment which indicated nigro-striatal degeneration. All PSP patients met the clinical diagnostic criteria proposed by MDS4,26 and qualified for diagnosis of probability. Of patients, 11 (52.3%) presented with Richardson’s syndrome. The remaining patients presented with other variant syndromes of PSP (five patients exhibited PSP with predominant parkinsonism and four patients exhibited PSP with predominant gait freezing). Of the PD patients, 56 were stable; i.e., they received stable treatment during the 4 weeks preceding enrolmentt, and 27 were newly diagnosed PD patients who had never received treatment. Of the PSP patients, 12 were early PSP patients (disease duration less than 2 years). The exclusion criteria for all patients were as follows: gait requiring assistance; dementia according to the DSM-V criteria; clinically significant comorbidities, including other neurologic disorders, orthopedic diseases, or cardiovascular/respiratory diseases; anticholinergic or neuroleptic treatment; and/or brain surgery. All participants were evaluated using an assessment including demographic, clinical, and anthropometric data. All participants were evaluated in the self-defined best “on-state” while receiving their typical dopaminergic drugs.
Standard protocol approvals, registrations, and patient consent
This study was performed in accordance with the 1964 Declaration of Helsinki and was approved by Campania Sud, the reference ethics committee of the Center for Neurodegenerative Diseases of the University of Salerno. Written informed consent was obtained from all participants.
Gait analysis
Gait analysis was performed in all subjects using a BTS Bioengineering system. The SMART DX is an optical system equipped with six infrared cameras, two video cameras, two force plates, a set of passive markers, and an elaborator. The Davis protocol was used for all subjects27, comprising the following phases. Anthropometric measurements of the patients (height, weight, leg length, etc.) were obtained. In total, 22 reflective markers were positioned on specific points of the body. The standing phase consisted of assessments of the patient while standing up on a force plate. This was followed by the walking phase on a 10-m path. All patients were evaluated on the straight pathway during two different tasks: (1) GAIT: normal gait, namely the single task; (2) COG: walking while serially subtracting 7 s starting from 100, namely the dual task; each task was performed four times. Prior to commencing the trials, all participants were trained to walk at a normal pace at their usual speed, without any instructions to prioritize walking or calculating. This procedure generated a report from which spatial and temporal parameters were extracted.
Statistical analysis
IBM SPSS v.25 was used to perform all the statistical analyses. For univariate statistical analysis, the Shapiro Wilk and Kolmogorov Smirnov tests were used to assess normality according to the sample size (the former for n < 50, and the latter for n > 50). For normally distributed data, the Levene test was used to assess the homoscedasticity of the variances between the compared groups. A t-test for independent samples was employed when both of the previous assumptions were verified; a Mann Whitney test was otherwise employed. Univariate statistical analysis was performed to quantify the effects of the COG task on the two groups. Binary logistic regression28 was computed to produce models capable of classifying patients into a diagnostic group (PD or PSP) starting from spatial and temporal parameters of gait. The presence of multicollinearity among variables and outliers was verified. The former was assessed by computing the coefficients of correlation, and all variables with a correlation greater than 0.80 were removed; the latter was verified by computing the a-dimensional Cook’s distance and Center Leverage Value. The odds ratios with a confidence interval of 95% and relative p-values were provided for each variable included in the models. The Hosmer Lemeshow goodness-of-fit test was computed to evaluate whether the observed event rates matched expected event rates in subgroups of the model population. Finally, the overall accuracy of the models and capacity to detect each group were determined. Alpha significance level was set to p < 0.05 for all statistical analyses.
Results
Univariate statistical analysis and binary logistic regression were performed twice: first, all PD patients (N = 83) were compared with all PSP patients (N = 21). Subsequently, the analysis was restricted to newly diagnosed PD patients (N = 27) and early PSP patients (N = 12).
PD versus PSP
Univariate statistical analysis
Univariate statistical analysis comparing demographic and clinical features, and spatial and temporal gait parameters for both GAIT and COG tasks between PD and PSP patients are presented in Tables 1 and 2, respectively.
In the GAIT task, PSP patients exhibited poorer gait patterns when compared to PD patients. Namely, relative to PD patients, PSP patients exhibited reduced velocity and cadence, shortened step and cycle lengths, increased cycle duration mainly due to longer double support stance phase duration, and increased swing duration variability (Table 2).
In the COG task, PSP patients exhibited the same gait features as those displayed during the single task, with the exception of two gait variables, namely swing duration and step length variability (Table 2). For both GAIT and COG tasks, the difference in step width between the two groups was not statistically significant. When comparing the effect of the dual task on gait measures in PD and PSP patients, the simultaneous performance of the secondary task significantly worsened most gait measures in both groups with the exception of the step width that was significantly increased in PSP patients but not in PD patients (Table S1).
Binary logistic regression
Binary logistic regression was performed to distinguish PD and PSP patients. The results for GAIT and COG tasks are presented in Table 3.
Two outliers were removed from the GAIT model, and five outliers were removed from the COG model. The graphs depicting Cook’s distance versus center leverage values are presented in the supplemental data (Figs. S1 and S2). The overall accuracies for the GAIT and COG models were 92.3% and 95%, respectively. The capacities to detect PD patients were 96.5% and 96.4%, and the capacities to identify PSP patients were 73.7% and 88.2% for the GAIT and COG models, respectively. The positive predictive value and the negative predictive value were 82.3% and 94.2% for the GAIT task, 83.3% and 97.6% for the COG task. Table 4 shows the confusion matrix of each model.
The odds ratios of the variables included in the GAIT model revealed that longer swing duration and increased cadence and cycle length were associated with a higher probability of being in the PD group. Conversely, longer stance phase was associated with a higher probability of being in the PSP group. The COG model confirmed these results, with the exception of swing duration, which was not entered in this model. The Hosmer Lemeshow goodness-of-fit test demonstrated good overall quality of the models, with p-values of 0.976 and 1.000 for the GAIT model and COG model, respectively.
Newly diagnosed PD versus early PSP
Univariate statistical analysis
Univariate statistical analysis was performed to compare spatial and temporal parameters of gait between newly diagnosed PD and early PSP patients. The results for GAIT and COG tasks are presented in Table 5.
For the single task (GAIT), early phase PSP patients exhibited poorer walking parameters when compared with PD patients. In particular, compared to newly diagnosed PD patients, early PSP patients exhibited reduced velocity and cadence, shortened step and cycle length, and increased cycle duration; these patients tended to rely on a longer double support stance phase (Table 5).
In the dual task (COG), compared to newly diagnosed PD patients, early PSP patients exhibited a gait pattern similar to that during the single task with the exception of two gait variables, namely, swing duration and swing duration variability (Table 5). For both GAIT and COG tasks, the differences in step length variability and step width between the two groups were not statistically significant. When comparing the effects of the dual task on gait measures in newly diagnosed PD patients versus early PSP patients, most gait parameters were similarly altered in the dual task condition in both groups, with the exception of step length variability and step width, which were influenced by the dual task in PSP patients but not in PD patients (Table S2).
Binary logistic regression
The results of the binary logistic regression conducted to differentiate newly diagnosed PD patients and early PSP patients are presented in Table 6 for both GAIT and COG tasks.
Two outliers were removed from each of the two models. The graphs depicting Cook’s distance versus center leverage values are presented in the supplemental data (Figs. S3 and S4). For the GAIT and COG models. The overall accuracies were 89.2% and 91.9% for the GAIT and COG models, respectively. The capacities to detect newly diagnosed PD patients were 92.3% and 96.3%, and the capacities to identify early PSP patients were 81.8% and 80.0% for the GAIT and COG models, respectively. The positive predictive value and the negative predictive value were 81.8% and 92.3% for the GAIT task, 88.9% and 96.2% for the COG task. Table 7 shows the confusion matrix of each model.
The odds ratios of the variables included in the GAIT model revealed that longer cycle length was associated with a higher probability of being in the newly diagnosed PD group. In contrast, longer stance phase was associated with a higher probability of being in the early PSP group. The COG model confirmed the same predictor, i.e. longer stance phase, for PSP diagnosis, whereas it disclosed that increased cadence raises the probability of PD diagnosis. The Hosmer Lemeshow goodness-of-fit test demonstrated good overall quality of the models, with p-values of 0.846 and 0.195 for the GAIT model and COG model, respectively.
Discussion
Here, we demonstrated that PSP patients exhibited disease-specific gait pattern when compared to PD patients, even during the earliest stages of disease. In particular, PSP patients exhibited reduced velocity and increased measures of dynamic instability when compared to PD subjects. Furthermore, application of predictive models to gait data revealed that increased cadence and longer cycle length characterized PD gait pattern, whereas longer stance phase distinguished PSP subjects in both mid and early stages.
Comparison of gait patterns in PD and PSP patients
Itn the single task, PSP subjects exhibited reduced velocity and cadence, shortened step and cycle length, increased cycle duration (mainly due to longer double support stance phase), and increased swing time variability when compared to PD patients. These data indicating that PSP gait pattern is characterized by dynamic instability are consistent with previous findings in smaller samples19,21,29. These results suggest that complex dysfunction of internal motor programming is more prominent in PSP patients than in PD patients, with the former group exhibiting dysfunction in both spatial and temporal domains, and the latter group exhibiting predominantly spatial dysfunction30,31. Notably, because dysfunction of spatial gait variables are generally responsive to dopaminergic treatment in PD patients32, the different gait patterns of the two patient groups could at least partly depend on the effectiveness of medication that is notoriously poor in PSP patients4. Gait patterns of the two groups were similar in both the single and dual tasks, with non-specific detrimental effects of the secondary task on both PSP and PD patients. Importantly, the major effect of the dual task in PSP patients was an increase in step width that could represent an attempt to counteract the lateral instability commonly observed as balance and walking deficits in PSP patients19.
Application of predictive models on the single task data revealed that longer swing duration and increased cadence and cycle length were characteristic gait features of PD patients. In contrast, longer stance phase was a gait feature that typified the gait pattern of PSP patients. Data from the dual task condition confirmed the single task data with the exception of swing duration. These results indicate that relative to PSP patients, PD patients display more stable locomotion, even under the dual task condition, and exhibit superior performance in temporal gait parameters that thus represent the variables better discriminating between PD and PSP30,31.
Comparison of gait patterns in newly diagnosed PD versus early PSP patients
Whole-group differences were recapitulated in the comparison of gait features between newly diagnosed PD and early PSP patients. From early stages, patients with PSP exhibited reduced velocity and cadence, shortened step and cycle length, and increased cycle duration which was predominantly underpinned by a longer double support stance phase. These findings were independent of dopaminergic effects, since newly diagnosed PD patients were drug-naïve. The dual task condition significantly modified most gait measures in both patients groups. Notably, step length variability and step width were influenced by the dual task in PSP patients but not in PD patients. These data highlight two important points. First, early stage PD and PSP patients exhibit distinct gait patterns, which likely reflect different underlying pathophysiological mechanisms. Second, in the early stage, the dual task condition may be more detrimental in PSP patients than in PD patients. Our findings are consistent with the recent observation that gait impairments in PSP are associated with an imbalance in the control of indirect and direct locomotor pathways. In particular, PSP patients display specific dysfunction of the indirect prefrontal–subthalamic–pedunculopontine loop of locomotor control, which drives modulated gait, and increased activity in the direct loop, which regulates stereotyped gait33. Given that the dual task condition mainly involves cognitive-mediated gait34 that is underpinned by the indirect pathway, it is unsurprising that PSP patients exhibit greater effects in the dual task condition, at least in the early stage. With disease progression, this effect tends to be masked by the general deterioration of all gait features, hence the loss of disease specificity of the dual task effect in more advanced patients, as reported above.
Application of predictive models on single task data revealed that increased cycle length was a gait variable characteristic of newly diagnosed PD patients, whereas longer stance phase was a gait feature that typified early PSP gait pattern. In the dual task condition, longer stance phase was confirmed as the best predictor for PSP diagnosis, whereas increased cadence was the strongest predictor for PD diagnosis. These findings indicate that from an early stage, PSP patients exhibit more alterations in temporal gait features when compared to PD patients. Further, they suggest that the dual task condition exerts a major effect on step length35 in PD patients from a very early stage, as reflected by increased cadence.
The present study has some limitations. First, the sample size of PSP patients was relatively small, mainly due to the low prevalence of the disease and the early loss of independent walking. Second, when comparing gait patterns in newly diagnosed PD and early PSP patients, we indeed confronted patients with (PSP) and without (PD) dopaminergic treatment. Nevertheless, given the poor response to dopaminergic treatment in PSP patients4 and the general confirmation of the different gait patterns between the two patients groups in both early and mid-stages of disease, we hypothesize that medication status had a minimal impact on our findings. Finally, we admit that the PSP group included different phenotypes and the “stable” PD group consisted of patients in slightly different Hoehn and Yahr stages; nevertheless, predictive models were able to capture disease-specific gait patterns.
In conclusion, the present study demonstrates that quantitative gait evaluation clearly distinguishes PSP patients from PD patients since the earliest stages of disease. Our findings indicate that gait analysis could be candidate as a reliable biomarker in both clinical and research setting. In addition, our results may offer speculative clues for conceiving early disease-specific rehabilitation strategies.
References
Golbe, L. I. Progressive supranuclear palsy. Semin. Neurol. 34(2), 151–159 (2014).
Steele, J. C., Richardson, J. C. & Olszewski, J. Progressive supranuclear palsy: A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch. Neurol. 10, 333–359 (1964).
Williams, D. R. et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 128, 1247–1258 (2005).
Höglinger, G. U. et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 32(6), 853–864 (2017).
Picillo, M. et al. MDS PSP criteria in real-life clinical setting: Motor and cognitive characterization of subtypes. Mov. Disord. 33(8), 1361–1365 (2018).
Picillo, M. et al. Motor, cognitive and behavioral differences in MDS PSP phenotypes. J. Neurol. 266(7), 1727–1735 (2019).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 23(15), 2129–2170 (2008).
Golbe, L. I. & Ohman-Strickland, P. A. A clinical rating scale for progressive supranuclear palsy. Brain 130(Pt 6), 1552–1565 (2007).
Espay, A. J. et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 31(9), 1272–1282 (2016).
McGinley, J. L., Baker, R., Wolfe, R. & Morris, M. E. The reliability of three-dimensional kinematic gait measurements: a systematic review. Gait Posture 29, 360–369 (2009).
Cascarano, G. D. et al. Biometric handwriting analysis to support Parkinson’s Disease assessment and grading. BMC Med. Inform. Decis. Mak. 19, 252 (2019).
Ricciardi, C. et al. Classifying different stages of Parkinson’s disease through random forests. In Mediterranean Conference on Medical and Biological Engineering and Computing 2019, 1155–1162. (Springer, 2019). https://doi.org/10.1007/978-3-030-31635-8_140.
Mirelman, A. et al. Gait impairments in Parkinson’s disease. Lancet Neurol. 18(7), 697–708 (2019).
di Biase, L. et al. Gait analysis in Parkinson’s disease: An overview of the most accurate markers for diagnosis and symptoms monitoring. Sensors 20(12), 3529 (2020).
Amboni, M. et al. Gait patterns in Parkinsonian patients with or without mild cognitive impairment. Mov Disord. 27(12), 1536–1543 (2012).
Ricciardi, C. et al. Machine learning can detect the presence of Mild cognitive impairment in patients affected by Parkinson’s Disease. In 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), 1–6. https://doi.org/10.1109/MeMeA49120.2020.9137301 (2020).
Ricciardi. C. et al. Classifying patients affected by Parkinson’s disease into freezers or non-freezers through machine learning. In 2020 IEEE International Symposium on Medical Measurements and Applications, 1–6. https://doi.org/10.1109/MeMeA49120.2020.9137317 (2020).
Amano, S. et al. Discriminating features of gait performance in progressive supranuclear palsy. Parkinsonism Relat. Disord. 21(8), 888–893 (2015).
Hatanaka, N. et al. Gait analysis in progressive supranuclear palsy and Parkinson’s disease. Eur. Neurol. 75(5–6), 282–289 (2016).
Raccagni, C. et al. Sensor-based gait analysis in atypical parkinsonian disorders. Brain Behav. 8(6), e00977 (2018).
Gaßner, H., Raccagni, C., Eskofier, B. M., Klucken, J. & Wenning, G. K. The diagnostic scope of sensor-based gait analysis in atypical Parkinsonism: Further observations. Front. Neurol. 22, 10. https://doi.org/10.3389/fneur.2019.00005 (2019).
Ricciardi, C. et al. Using gait analysis’ parameters to classify Parkinsonism: A data mining approach. Comput. Methods Programs Biomed. 180, 105033. https://doi.org/10.1016/j.cmpb.2019.105033 (2019).
De Vos, M., Prince, J., Buchanan, T., FitzGerald, J. J. & Antoniades, C. A. Discriminating progressive supranuclear palsy from Parkinson’s disease using wearable technology and machine learning. Gait Posture. 77, 257–263 (2020).
Ali, F. et al. Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov. Disord. 34(8), 1144–1153 (2019).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30(12), 1591–1601 (2015).
Grim, M. J. et al. How to apply the movement disorder society criteria for diagnosis of progressive supranuclear palsy. Mov. Disord. 34(8), 1228–1232 (2019).
Davis, R. B. III., Ounpuu, S., Tyburski, D. & Gage, J. R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 10(5), 575–587 (1991).
Starkweather, J. & Moske, A. K. Multinomial logistic regression. Consulted page at September 10th. http://www.unt.edu/rss/class/Jon/Benchmarks/MLR_JDS_Aug2011.pdf, 2825–2830 (2011).
Egerton, T., Williams, D. R. & Iansek, R. Comparison of gait in progressive supranuclear palsy, Parkinson’s disease and healthy older adults. BMC Neurol. 12, 116 (2012).
Teasdale, N., Phillips, J. & Stelmach, G. E. Temporal movement control in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 53(10), 862–868 (1990).
Morris, M. E., Iansek, R., Matyas, T. A. & Summers, J. J. Ability to modulate walking cadence remains intact in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 57(12), 1532–1534 (1994).
Curtze, C., Nutt, J. G., Carlson-Kuhta, P., Mancini, M. & Horak, F. B. Levodopa is a double-edged sword for balance and gait in people with Parkinson’s Disease. Mov. Disord. 30(10), 1361–1370 (2015).
Zwergal, A. et al. Functional disturbance of the locomotor network in progressive supranuclear palsy. Neurology 80(7), 634–641 (2013).
Amboni, M., Barone, P. & Hausdorff, J. M. Cognitive contributions to gait and falls: evidence and implications. Mov. Disord. 28(11), 1520–1533 (2013).
Amboni, M. et al. Step length predicts executive dysfunction in Parkinson’s disease: a 3-year prospective study. J. Neurol. 265(10), 2211–2220 (2018).
Acknowledgements
The study was supported by “Fondazione Grigioni per il Morbo di Parkinson”.
Funding
Dr Marianna Amboni is supported by by Fondazione Grigioni per il Morbo di Parkinson; Dr Marina Picillo is supported by the Michael J Fox Foundation for Parkinson’s research; Prof Paolo Barone received consultancies as a member of the advisory board for Zambon, Lundbeck, UCB, Chiesi, Abbvie and Acorda; the other authors report no financial diclosures.
Author information
Authors and Affiliations
Contributions
M.A. and C.R. wrote the main manuscript. C.R. performed the statistical analysis. M.A., M.P., G.C., G.D.A., M.C.C., M.C., P.B. checked the data quality and reviewed the manuscript. C.D.S., G.R., F.A., M.F.T. and G.V. collected the data. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amboni, M., Ricciardi, C., Picillo, M. et al. Gait analysis may distinguish progressive supranuclear palsy and Parkinson disease since the earliest stages. Sci Rep 11, 9297 (2021). https://doi.org/10.1038/s41598-021-88877-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88877-2
This article is cited by
-
Gait analysis of patients with Parkinson-plus syndromes: a research article
Bulletin of the National Research Centre (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.