Abstract
To determine the effect of continuous positive airway pressure (CPAP), the gold standard treatment for obstructive sleep apnea syndrome (OSAS), on gait control in severe OSAS patients. We conducted a randomized, double-blind, parallel-group, sham-controlled monocentric study in Grenoble Alpes University Hospital, France. Gait parameters were recorded under single and dual-task conditions using a visuo-verbal cognitive task (Stroop test), before and after the 8-week intervention period. Stride-time variability, a marker of gait control, was the primary study endpoint. Changes in the determinants of gait control were the main secondary outcomes. ClinicalTrials.gov Identifier: (NCT02345694). 24 patients [median (Q1; Q3)]: age: 59.5 (46.3; 66.8) years, 87.5% male, body mass index: 28.2 (24.7; 29.8) kg. m−2, apnea–hypopnea index: 51.6 (35.0; 61.4) events/h were randomized to be treated by effective CPAP (n = 12) or by sham-CPAP (n = 12). A complete case analysis was performed, using a mixed linear regression model. CPAP elicited no significant improvement in stride-time variability compared to sham-CPAP. No difference was found regarding the determinants of gait control. This study is the first RCT to investigate the effects of CPAP on gait control. Eight weeks of CPAP treatment did not improve gait control in severe non-obese OSAS patients. These results substantiate the complex OSAS-neurocognitive function relationship.
Similar content being viewed by others
Introduction
Obstructive sleep apnea syndrome (OSAS) is one of the most prevalent chronic diseases affecting nearly 1 billion adults aged 30–69 years worldwide1,2. Chronic intermittent hypoxia and sleep fragmentation, resulting in systemic low-grade inflammation and oxidative stress, in time produce neural damage and cerebral homeostatic and neurovascular changes underlying the detrimental consequences of severe OSAS on cerebral structure and function3,4. OSAS-related neurocognitive impairment extensively affects attention and vigilance domains as well as executive functioning, which in turn impact everyday functioning, work performance and productivity5,6,7. Recently, gait abnormalities have been highlighted in severe OSAS8,9,10,11, with a dose–response relationship between OSAS severity and the magnitude of gait impairment8. Rather than a completely automatic task, gait has to be considered as a cognitively-demanding task, requiring attention and executive functions integrity12. Defects in these neurocognitive domains can be revealed and/or documented by gait abnormalities13. While the beneficial effects of continuous positive airway pressure (CPAP, the gold standard treatment for OSAS) on excessive daytime sleepiness and everyday functioning is well established14, its ability to reverse neurocognitive impairment remains debated, highlighting a complex OSAS-neurocognitive relationship15,16,17. To date, one open-labelled9 and one non-randomized controlled study10 have shown positive effects of CPAP treatment on gait control in OSAS patients. To validate these previously described effects a randomized, controlled (effective vs. sham-CPAP, the acknowledged CPAP placebo), double-blind trial was lacking. Furthermore, neurophysiological measurements are required to determine the cerebral correlates of the potential CPAP-induced changes in gait control. The main objective of the present parallel randomized controlled trial (RCT) was to investigate the impact of an 8-week CPAP treatment on gait control evaluated by stride time variability (STV) in severe OSAS patients, compared to sham-CPAP. We hypothesized that: (1) gait control in severe non-obese OSAS patients would be improved by CPAP treatment and (2) those improvements might be paralleled by changes in the determinants of gait control, i.e. postural control, cognitive performance and cerebral oxygenation assessed by functional near-infrared spectroscopy (fNIRS) while walking.
Results
Patients characteristics
Twenty-four severe OSAS patients were included and randomized to be treated by effective CPAP (n = 12) or by sham-CPAP (n = 12). Twenty-one patients completed all the evaluations, as three patients in the effective CPAP group withdrew, due to personal constraints (Fig. 1). Patient characteristics are shown in Table 1. There was no significant difference regarding all baseline anthropometric and sleep apnea characteristics. Three out of nine patients (33.3%) in the CPAP group and eight out of 12 patients (66.7%) in the sham-CPAP group showed low compliance (< 4 h of use per night) and the percentage of nights with CPAP usage > 4 h/night was significantly lower in the sham-CPAP group (p = 0.02). Patients in the CPAP group were effectively treated, as shown by them achieving normal values for residual AHI (Table 1).
Primary outcome: stride time variability
There was no significant difference between the CPAP and sham-CPAP groups for the primary outcome, STV, both under ST [Group effect β (Standard Error (SE)) = 0.46 (0.31), p = 0.14; Period effect β (SE) = −0.09 (0.13), p = 0.50] and DT [Group effect β (SE) = 0.45 (0.38), p = 0.25; Period effect β (SE) = −0.26 (0.09) p < 0.01] (Fig. 2 a. and Supplementary Table S1).
Effect of continuous positive airway pressure (CPAP) on gait control (a) and Stroop test performance (b) assessed in single (gait or Stroop test only) and dual task (gait and Stroop test performed simultaneously) (linear mixed effect model analysis). CPAP continuous positive airway pressure, CRR correct response rate, DT dual task, ST single task, STV stride time variability.
Overground gait parameters and dual-task gait performance
Gait parameters did not differ between the two groups at baseline and CPAP elicited no significant improvement in gait parameters under either ST or DT conditions (Supplementary Table S1). Effective CPAP produced no significant change in gait speed (Fig. 2a). Cognitive performance in the Stroop Test, evaluated by the CRR, was not improved by CPAP treatment in both ST and DT (Fig. 2b).
Cognitive performance
Results of the neurocognitive evaluations are presented in Table 2. There was no significant difference between the two groups at baseline. The only significant improvement observed in the effective CPAP group after the 8 weeks of intervention was for the Stroop Interference test (p = 0.02).
Standing balance
There was no significant difference in postural control between the two groups at baseline (Supplementary Table S2). CPAP elicited no significant change in postural control evaluated by the CoP Area both in ST [Group effect β (SE) = −15.48 (27.88), p = 0.58; Period effect β (SE) = 0.37 (17.36) p = 0.98] and DT [Group effect β (SE) = −16.85 (22.60), p = 0.46; Period effect β (SE) = 26.22 (15.59) p = 0.11]. There was no significant improvement for all the other postural parameters following CPAP treatment (Supplementary Table S2). Cognitive performance at the Stroop Test, evaluated by the CRR, was not improved by CPAP treatment both in ST [Group effect β (SE) = 1.23 (9.13), p = 0.89; Period effect β (SE) = 10.31 (3.64) p = 0.01] and in DT [Group effect β (SE) = 2.62 (4.32), p = 0.55; Period effect β (SE) = 6.42 (1.71) p < 0.01].
Treadmill dual-task gait and cerebral oxygenation
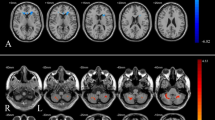
Preferred walking speed at baseline did not differ between the two groups [median (Q1; Q3) gait speed = 3.00 (2.58; 3.30) in the sham-CPAP group vs. 2.85 (2.35; 3.38) in CPAP group, p = 0.56]. There was no significant difference between the two groups at baseline. After eight weeks of intervention, CPAP elicited no significant change in cognitive performance in either ST or DT (Supplementary Table S3). Regarding pre-frontal cortex hemodynamics assessed by fNIRS, 11 out of 12 patients (91.7%) in the sham-CPAP group and 6 out of 9 (66.7%) in the CPAP group had valid pre- and post-intervention recordings (p = 0.27). There was no significant difference regarding [HbO2], [HHb], [HbTot] and TSI between the two groups, pre and post intervention, despite higher baseline values in the sham-CPAP group (Fig. 3).
Evolution of prefrontal cortices Oxy[HbO2]- and Total[HbTot]-hemoglobin concentration assessed by functional Near Infrared Spectroscopy during the treadmill test. (a) Evolution of [HbO2] and [HbTot] (in mmoles−1) pre (continuous line) and post (dotted line) intervention for sham-CPAP (n = 11, represented in black) and CPAP (n = 6, represented in red) groups. Data are the measured mean values of [HbO2] and [HbTot] over the first 30-s of each task, averaged for left and right prefrontal cortices. To assess the evoked hemodynamic response, five activation indices were calculated as the difference in [HbO2] and [HbTot] between task and rest (in ST) or task and walk (in DT): ① S-7 ST; ② Stroop test ST; ③ Walk ST; ④ S-7 DT; ⑤ Stroop test DT. (b) Comparisons of delta pre-post intervention of [HbO2] and [HbTot] between sham-CPAP (n = 11) and CPAP (n = 6) groups. Data are mean ± 1 standard deviation. Analysis of data by Wilcoxon-Mann–Whitney test for continuous data, provided with Cohen’s D for effect size estimation. CPAP continuous positive airway pressure, DT dual task, HbO2 Oxy-hemoglobin concentration, HbTot total hemoglobin concentration, S-7 Serial S-7 tasks, ST single task.
Discussion
In this randomized, sham-controlled trial (RCT), eight weeks of CPAP treatment did not improved gait control of severe OSAS patients.
We found no improvement in STV, our primary outcome, following eight weeks of CPAP treatment. The STV values obtained at baseline were comparable to those of the severe OSAS patients reported by Celle et al.8 and in our previous study10 showing impaired gait control in OSAS patients. This negative result contrasts with that of two recent uncontrolled interventional studies. Allali et al.9 showed improvements in gait speed and temporal gait parameters specifically in dual tasks. Their study included mostly obese patients, lacked an appropriate control group and the changes in gait performance they reported were subtle with limited clinical relevance. In our previous prospective, non-randomized controlled study10, we showed that at baseline OSAS patients had significantly larger STV compared to matched healthy controls. After eight weeks of CPAP treatment, STV was significantly improved and no longer different from controls. The lack of an OSAS group treated by sham-CPAP and the absence of a retest of the controls eight weeks after baseline assessment limited the conclusions of this previous study. The present study, using a stronger methodological design, suggests that eight weeks of CPAP may be insufficient to reverse gait control impairments in severe OSAS patients. This result was supported by the lack of changes after CPAP treatment in the main determinants of gait control (i.e. brain oxygenation and cognitive performance). A key outcome was DT gait assessment using a Stroop test. We found no difference between the two groups but a mutual facilitation18 reflecting an improvement in DT performance both in gait (i.e. a reduction in STV) and cognition (i.e. an increased Stroop CRR) compared to ST performance. This might be explained by an exercise-induced arousal effect19, the positive incidence of acute physical activity on cognitive performance. However, such an effect is expected to occur only in patients with morphologically and functionally unaltered prefrontal cortices suggesting only slight neurological consequences of OSAS prior to treatment in the included patients18.
All patients underwent a comprehensive neurocognitive evaluation, covering the domains supposed to be most impaired in OSAS5,20. CPAP treatment elicited no significant change in cognitive performance, except an improvement in the Stroop Interference test. We had included patients without significant cognitive deficiency at baseline and most of them were highly educated, with at least half having more than 11 years of formal education. Thus, included patients were normal or supra normal, free of comorbidities, and with probably a high cognitive reserve6, representing a preserved adaptability of cognitive processes explaining their reduced susceptibility to cognitive deterioration with aging or pathologies21.
During cognitive or motor tasks, cerebral hemodynamic regulation is essential so as to deliver the adequate amount of oxygen and substrates required for brain metabolism22. Severe OSAS might have impaired cerebral hemodynamic regulation23,24. CPAP treatment, through suppression of nocturnal intermittent hypoxia and fragmented sleep, is thought to limit OSAS-related neuroinflammation and improve cerebrovascular hemodynamics3. CPAP has a clear acute impact in suppressing acute cerebral hemodynamic abnormalities occurring concurrently with sleep obstructive events25. Some studies have suggested a partial improvement in cerebral blood flow (CBF) in the frontal areas during wakefulness in OSAS patients with a dose–response effect-size related to CPAP adherence26,27. However, in our well controlled RCT no chronic impact of CPAP treatment on prefrontal cortices hemodynamics was found, as assessed by fNIRS during daytime walking. This result is consistent with those of a previous study from our group28, showing no improvement in prefrontal cortex hemodynamics while performing an incremental cycling test following eight weeks of CPAP treatment.
The major strength of our study is its randomized controlled design which is unique in the field of gait control and OSAS. We acknowledge that the duration of the CPAP intervention might not have been long enough for CPAP to have any effect. Although we note that most of the existing studies on neurocognitive function or brain imaging in OSAS patients have used similar timeframes. Moreover, one third of the patients of the CPAP group showed low CPAP compliance (< 4 h of use per night). Altogether, this may have dampened the expected positive effect of CPAP on gait control.
We included only non-obese OSAS patients. This choice was driven by the fact that obesity is a cofounding factor when assessing gait control and postural control, as overweight and even more obesity directly affect gait control and gait kinetics29,30. Another limitation could be that the gait and postural tasks might have been too easy for participants, with parameters already satisfactory at baseline, which reduced the chances to conclude. Lastly, the small sample size of highly selected patients, together with three loss of follow-up patients in the CPAP group may have impacted our results. As we included only severe OSAS patients, further generalization of the study findings should be restricted to OSAS patients with an AHI > 30 events. h−1, with a low burden of comorbidities.
Conclusion
This first randomized controlled trial in the field showed that eight weeks of CPAP treatment did not improve gait control in severe non-obese OSAS patients. Long-term real-life observational data on different clusters of phenotypes31 might help to identify specific subgroups for whom CPAP could have a significant impact on gait control, which could then be tested in further RCTs.
Methods
Study design
This randomized, double-blind, parallel-group, sham-controlled study was monocentric, performed at Grenoble Alpes University Hospital, France. The study was approved by an independent Ethics Committee (CPP Sud-Est V, Grenoble, France, 14-CHUG-46, ID RCB: 2014-A01523-44, date of initial approval: November 12th, 2014); conducted in accordance with applicable good clinical practice requirements in Europe, French law and ethical principles of the Declaration of Helsinki; and is registered on the ClinicalTrials.gov site (NCT02345694; date of first registration 26/01/2015; study start date 03/02/2015). Written informed consent was obtained from all patients prior to their participation in the study. Informed consent was obtained to publish the images in an online open access publication.
An external data quality control was performed systematically for the following criteria: informed consent, complications, adverse events, and case report forms.
The primary study endpoint was the change in STV of OSAS patients after eight weeks of CPAP treatment, in comparison with sham-CPAP treatment.
Participants
Patients were recruited from a consecutive sample at the Sleep Laboratory of Grenoble Alpes University Hospital and from the Outpatient Sleep Clinic in a French tertiary-care university hospital (Grenoble, France) between February 2015 and December 2018. Inclusion criteria were: (1) age ≥ 18 years and < 70 years, (2) non-obese patients (body-mass index (BMI) < 30 kg. m−2), to control for obesity-related gait kinetic changes29 (3) severe OSAS (apnea–hypopnea index, AHI > 30. events h−1) on polysomnography or respiratory polygraphy, (4) to be naive of CPAP treatment and (5) to present with a strictly normal neurological examination. Patients were not included if they declined to participate or were unable to give their informed consent. Patients with any of the following criteria were also not included: (1) the presence of any medical condition supposed to interfere with gait, (2) cognitive impairment, defined as a score on the Mini-Mental State Examination (MMSE32) < 24/30, (3) current pregnancy, (4) ongoing hypnotic or central nervous system targeted medication, (5) chronic alcohol consumption and (6) a profession requiring an effective CPAP treatment (e.g. public transport or truck drivers).
Overnight sleep studies
Obstructive sleep apnea syndrome (OSAS) diagnosis was based on an overnight sleep study (respiratory polygraphy or polysomnography), performed before inclusion and treatment by continuous positive airway pressure (CPAP) or sham-CPAP according to standard procedures33. Polysomnographic recordings were undertaken with electroencephalography (EEG) electrode positions C3/A2-C4/A1-Cz/01 of the international 10–20 Electrode Placement System, eye movements, chin electromyogram and ECG with a modified V2 lead. Sleep was scored manually according to standard criteria. For polysomnographic and polygraphic recordings, airflow was measured with nasal pressure prongs, together with the sum of oral and nasal thermistor signals. Respiratory effort was monitored using abdominal and thoracic bands. Oxygen saturation was measured using a pulse oximeter and oxygen desaturation index (ODI), mean nocturnal SpO2, and percentage of recording time spent at a SpO2 < 90% were also calculated. Respiratory events were scored manually by a trained sleep specialist and the apnea–hypopnea index (AHI) was calculated from the number of apneas and hypopneas per hour of sleep according to international guidelines34. An apnea was defined as the complete cessation or a reduction of at least 90% of airflow for at least 10 s and hypopnea as a reduction of at least 30% in the nasal pressure signal associated with either oxygen desaturation of at least 3% or an EEG arousal, both lasting for at least 10 s35. Apnea was classified as obstructive, central or mixed, according to the presence or absence of respiratory efforts. The classification of hypopnea as obstructive or central was based on the thoraco-abdominal band signal and the shape of the respiratory nasal pressure curve (flow limited aspect or not). Severe OSAS was defined as an AHI > 30 events h−1.
Evaluation protocol
At baseline and at the end of the 8-week interventional period the same parameters were recorded following the same protocol for evaluation, performed in the same order at the same hour of the day: (1) baseline clinical assessment, (2) single task (ST) and dual task (DT) overground gait assessments, using a dual-task paradigm similar to our previous work10, (3) comprehensive neuropsychological assessment, (4) postural control assessment during ST and DT, using a dual-task paradigm similar to our previous work10 and (5) treadmill gait assessment in ST and DT, with continuous recording of prefrontal cortex functional activation using functional Near-Infrared Spectroscopy (fNIRS). Following the collection of baseline parameters patients were randomized to be treated by CPAP or sham-CPAP for eight weeks (the duration of interventions previously used in studies investigating the effects of CPAP treatment on cognition15 and gait9,10). Patients assigned to the CPAP group were equipped with an auto-titrating device (Autoset Spirit, ResMed, UK or Remstar Auto, Philips Respironics, Murrysville, PA, USA) provided by a home care provider (Agir à Dom, France). Pressure was homogenously set between 6 and 14 cmH2O in the effective CPAP group. Patients receiving sham-CPAP had a similar device, delivering a pressure that was too low to suppress sleep respiratory events, as previously validated36,37,38. As a double-blind protocol, investigators and the study team as well as patients were blinded to treatment allocation. CPAP compliance and residual AHI39 under CPAP were downloaded from the CPAP device software.
Baseline clinical assessment
Prior to all gait and postural assessments, patients underwent a screening history and physical examination to ensure that they were free of significant orthopedic, neurological and visual disorder which could interfere with the outcomes of the present study. Cardiovascular risk factors were recorded, including past or actual smoking, systemic hypertension, diabetes and dyslipidemia (Table 1). Past cardiovascular events, including non-fatal stroke and non-fatal myocardial infarction, transient ischemic attack, any cardiac event of coronary origin (including vascular recanalization procedures), hospitalization due to cardiac or cerebrovascular causes (acute coronary syndrome, stroke, heart failure, heart rhythm disorder including atrial fibrillation, bleeding, etc.) were retrieved from medical interviews. Patients were then tested for their ability to distinguish the four colors (red, yellow, blue, green) used in our Stroop test appropriately by using the same screen setting as during the dual-task assessments. They completed an Epworth Sleepiness Scale (ESS).
Overground gait assessment
Spatiotemporal gait parameters were recorded using a modular optoelectronic floor-based system (OptoGait, Microgate, Bolzano, Italy), which consists of two parallel 1-m bars (one emitting, containing 96 lights diodes and one receiving bar). The system demonstrated high reliability for the assessment of spatiotemporal gait parameters40. Ten emitting and ten receiving bars were disposed parallel to each other to build a 10-m long, 1.5-m width walkway. Patients walked barefoot, at their self-selected comfortable speed, continuously around an oval circuit10 (Fig. 4a, b). Each participant completed three familiarization loops prior to the 20 evaluation loops, alternating ST (10 evaluation loops, gait only) and DT (10 evaluation loops, gait and Stroop test performed simultaneously). Gait kinetic parameters were recorded at 1 kHz sample frequency and analyzed using the OptoGait software (version 1.10.7.0, Microgate). The average value of all recorded steps was used for data analysis. The following gait kinetic parameters were recorded and calculated: speed (m.s−1), cadence (step.s−1), stride time (s), total double support (percentage of total gait cycle time), step width (cm). The walk ratio (WR, cm/(steps/min))41, a speed-independent index of the overall neuromotor gait control, which reflects balance, between-step variability, and attentional demand was calculated as follows: WR = Step Length (cm)/cadence (steps/min).
Overground gait and stride time variability assessment experimental setting. (a) Picture of the experimental setting. (b) Schematic representation of the oval gait circuit (outward in the 10-m long corridor delineated by the Optogait system, return outside of the corridor). Stroop test was displayed on a black background screen installed at the end of the corridor. (c) Schematic representation of the visuo-verbal Stroop test. Patients were instructed to name the words font color and to inhibit reading the word (Correct answers here: “red” then “yellow” then “blue”). Words were presented one by one, and the evaluator skipped manually to the next one after the subject gave an oral response.
Stride time variability (STV), our primary study endpoint, is a marker of gait control related to cerebral integrity and greater STV values reflect impaired gait control42. STV was calculated as the mean of the coefficient of variation of stride time as follows42:
Dual-task paradigm
The DT paradigm consists in the assessment of the interferences occurring when a motor and a cognitive task are performed simultaneously12,43. This can result in performance decrements in one or both of the tasks, suggesting the simultaneous engagement of the same functional brain subsystems44. We used the same DT paradigm as in our previous work10, combining gait and postural assessments with a Stroop color-word interference test (Fig. 4c), a cognitive task considered to specifically assess executive functions. Stroop test consists in color names (blue, red, green and yellow) written in a conflicting font color. Participants were instructed to name the word font color and to inhibit reading the word (e.g., the word “green” written in red font color). To avoid learning effects, we used 30 different versions of the Stroop test, presented randomly to the participants throughout the different assessments. The Stroop test was displayed on a black background screen installed at the end of the corridor, which height was adjusted for each participant and for each evaluation. Words were presented one by one, and the evaluator skipped manually to the next one after the subject gave an oral response. The number of correct answers and errors were recorded by a trained evaluator. In ST, a red sight was displayed on the black background screen. In DT, participants were asked to perform both the motor and cognitive tasks at the best of their capacity without any task prioritization. The correct response rate for Stroop test performance in ST and DT gait assessments45 was calculated as follows:
To deepen our understanding of the complex interactions between gait, posture and cognition, we calculated the dual task effect (DTE)18 for each gait, postural and cognitive parameter, as follows:
DTE quantifies the dual-task interference, i.e. the relative change in performance associated with dual-tasking. Indeed, as attention or executive functions are limited resources, dividing attention between two concurrent tasks can result in a decrement in performance in one or both of the tasks, relative to when each task is performed alone18. Therefore, the magnitude of DT interference is influenced by the interaction between the two tasks.
Neuropsychological assessment
A comprehensive neuropsychological assessment, covering the most frequently impaired domains in OSAS5,20 (i.e. episodic memory and executive functions) was administered by a certified neuropsychologist, blind for treatment allocation, always at the same time of the day, in the same order:
-
Global cognitive function: MMSE
-
Episodic memory: 16-item free and cued recall test
-
Executive functions: processing speed and working memory (Code and digit span forward and backward, Wechsler Adult Intelligence Scale IV), sustained attention (Paced Auditory Serial Addition test), visuomotor speed (Trail making test A), mental shifting (Trail making test B), interference and inhibition (Stroop test), planification skills (Tower of Hanoï).
Neuropsychological assessment lasted approximatively 1 h, with short breaks between each test to avoid fatigue.
Raw performances at the different evaluations are displayed in Table 2.
Standing posture
Posture and gait are interrelated functions and their control is anatomically and functionally intertwined46. Moreover, postural control is altered in OSAS47. We assessed standing balance using a posturographic platform (Feetest 6, TechnoConcept, Céreste, France), composed of two dynamometric posturographic clogs (with a total of 12 strain gauges). Participants stood upright barefoot on the clogs, in a conventional manner (feet side by side, forming an angle of 30° with both heels separated by 4 cm), with their arms alongside the body. To ensure participants security, the platform was settled in the middle of handlebars and an evaluator stood behind the participants to avoid them falling. The examination took place in a dedicated quiet room with standardized lighting conditions. Assessments were alternatively performed in ST (posture only, 4 trials) and in DT (posture and Stroop test performed simultaneously, 4 trials). Each trial lasted 30 s and a minimal 30 s-period of rest sitting was systematic between trials. In ST, subjects were instructed to maintain their balance while looking straight-ahead at a fixed red sight displayed on the screen installed 1.5 m ahead of each participant. In DT, subjects were asked to maintain the erect posture as still as possible and to perform the Stroop test at the best of their capacity without any task prioritization. Data were recorded with a sampling rate of 40 Hz and calculated using the Posturewin 4 software. As a marker of an efficient standing postural control, the amount of sway was assessed by calculating the center of pressure (CoP) area (90% confidence ellipse, mm2). The smaller the area, the better the postural control48. Beyond center of pressure (CoP) Area, the following postural kinetic parameters were recorded and calculated: mediolateral instability, defined as one standard deviation of the CoP displacement along the mediolateral axis, anteroposterior instability, defined as one standard deviation of the CoP displacement along the anteroposterior axis and mean speed of CoP displacement (mm.s−1).
Treadmill dual-task gait and cerebral oxygenation
To investigate the link between brain oxygenation and cognitive and gait performances, we designed a DT gait test on a treadmill (Gait Trainer 3, Biodex Medical System, NY, USA), with continuous recording of cerebral oxygenation using bilateral fNIRS on the two prefrontal cortices. fNIRS is a non-invasive, optical neuroimaging technique based on neurovascular coupling to infer changes in neuronal activity49. fNIRS provides reproducible measurements for investigating functional activation of the human cerebral cortex by tracking changes in cerebral O2 status while performing cognitive tasks or walking50.
Left and right pre-frontal cortices hemodynamics were assessed respectively between Fp1 and F3 (left prefrontal cortex) and Fp2 and F4 (right prefrontal cortex) locations according to the international 10–20 EEG system with a 3.5-cm inter-optodes distance. The probe holders were secured to the skin with double-sided tape and maintained with Velcro headbands. Oxyhemoglobin ([HbO2]) and deoxyhemoglobin ([HHb]) concentration changes and the tissue saturation index (TSI) were measured throughout the testing sessions using a two-wavelength (780 and 850 nm) multichannel, continuous wave NIRS system (Oxymon MkIII, Artinis Medical Systems, the Netherlands). Total hemoglobin concentration ([HbTot]) was calculated as the sum of [HbO2] and [HHb] and reflects changes in tissue blood volume within the illuminated area. Data were recorded continuously at 10 Hz and filtered with a 1-s width moving Gaussian smoothing algorithm before analysis. Each measurement was visually and manually checked by a trained evaluator and only valid recordings (with at least > 90% of valid signal after having removed artefacts) were kept for final analysis.
Following a 10-min period of habituation to the treadmill51, each participant’s preferred walking speed was determined according to a standardized protocol (updated from52): participants were instructed to walk on the treadmill at an initial speed of 1.5 km.h−1. Speed was progressively increased manually by the investigator in increments of 0.2 .km h−1 every 30 s until subjects reported that they were walking at their preferred walking speed. Then 1 km.h−1 was added to the current speed, followed by a decrease of 0.2 km.h−1 to confirm preferred walking speed. This procedure was repeated until a ± 0.4 km.h−1 agreement was obtained in preferred walking speed, as recommended52. The same speed was retained for the post-intervention evaluation.
The DT gait evaluation protocol was divided in 3 consecutive phases (Supplementary Figure S1): (1) Standing phase composed of 5 min of quiet standing immediately followed by 2 different conditions of cognitive assessment performed in ST (cognition only, baseline cognitive performance) and ending with 3 min of rest. The two different cognitive assessments were: a serial-7 test (S-7 test), consisting in subtracting 7 from a random 3-digit number and a Stroop test (performed according to the methodology previously described10). Each cognitive test consisted in 3 consecutive 1-min blocks interspersed by 1-min rest periods. CRR accounted for cognitive performance in ST and in DT; (2) Walking phase composed of 5 min of walk in ST (gait only, baseline gait performance) immediately followed by the 2 cognitive assessments (S-7 test and Stroop test) performed in DT (gait and cognition) and ending with 3 min of walk in ST. In ST gait, participants were instructed to walk according to their natural pattern, arms moving freely by their sides, while looking straight-ahead at a fixed red sight displayed on the screen. In DT, subjects were asked to walk as naturally as possible and to perform the S-7 test or Stroop test at the best of their capacity without any task prioritization; (3) Recovery phase consisting in 5 min of quiet standing. The DT gait evaluation protocol is further illustrated in Supplementary Figure S1.
Data and statistical analysis
Study design and data are reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) criteria53. Patients were randomized by an independent statistician according to a computer-generated list following a 1:1 ratio. Data were analyzed in complete case analysis. Except where clearly stated, results are presented as Median [Quartile 1 (Q1); Quartile 3 (Q3)]. All data have been tested for normality prior to further analysis. For non-normally distributed data, a logarithmic transformation has been applied. Baseline data were compared (CPAP vs. sham-CPAP) by a non-parametric Mann–Whitney test for continuous variables, and χ2 test or Fisher exact test for categorical variables. For the analysis of data evolution between baseline (pre) and eight weeks (post-intervention), a linear mixed effect model was used with a patient random effect. Group effects (CPAP vs. sham-CPAP) and period effects (pre vs. post CPAP/sham-CPAP) were added as fixed effects. Interaction terms (Group * Period) were tested for all variables, but no significant effect was observed. Therefore, interaction terms were not included in the final model. For complete information of the readers, results of the linear mixed effect model with interaction terms are displayed in Supplementary Tables S4 and S5. Deltas (Pre-Post intervention in Sham-CPAP and CPAP group) for primary outcome were also calculated and between group comparisons using independent sample t-tests were performed. Results are displayed in Supplementary Table S6.
A p value < 0.05 was considered significant. Sample size calculation was based on a previous study from our group10. The sample size needed to observe a significant difference in STV of 0.8, with a standard deviation of 0.6, a power of 80% and an α value of 0.05 was 10 patients per arm. Moreover, we expected that approximatively 20% of OSAS patients would not be compliant with CPAP therapy. Consequently, we estimated that 12 patients in each group would be sufficient to show differences before and after effective or sham-CPAP therapy. Statistical analyses were performed using SAS (V.9.4, SAS Institute, Cary, NC, USA).
Data availability
Study protocol as well as data collected for the study, including deidentified individual participant data will be made available to others following the publication of this article, for academic purposes (e.g. meta-analyzes) on request to the corresponding author.
References
Benjafield, A. V. et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir. Med. 7, 687–698. https://doi.org/10.1016/S2213-2600(19)30198-5 (2019).
Levy, P. et al. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 1, 15015. https://doi.org/10.1038/nrdp.2015.15 (2015).
Rosenzweig, I. et al. Sleep apnoea and the brain: a complex relationship. Lancet Respir. Med. 3, 404–414. https://doi.org/10.1016/S2213-2600(15)00090-9 (2015).
Leng, Y., McEvoy, C. T., Allen, I. E. & Yaffe, K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol. 74, 1237–1245. https://doi.org/10.1001/jamaneurol.2017.2180 (2017).
Bucks, R. S., Olaithe, M. & Eastwood, P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology 18, 61–70. https://doi.org/10.1111/j.1440-1843.2012.02255.x (2013).
Bucks, R. S., Olaithe, M., Rosenzweig, I. & Morrell, M. J. Reviewing the relationship between OSA and cognition: where do we go from here?. Respirology 22, 1253–1261. https://doi.org/10.1111/resp.13140 (2017).
Stepnowsky, C. et al. Comorbidities, health-related quality of life, and work productivity among people with obstructive sleep apnea with excessive sleepiness: findings from the 2016 US National Health and Wellness Survey. J. Clin. Sleep Med. 15, 235–243. https://doi.org/10.5664/jcsm.7624 (2019).
Celle, S. et al. Sleep-related breathing disorders and gait variability: a cross-sectional preliminary study. BMC Pulm. Med. 14, 140. https://doi.org/10.1186/1471-2466-14-140 (2014).
Allali, G. et al. Gait abnormalities in obstructive sleep apnea and impact of continuous positive airway pressure. Respir. Physiol. Neurobiol. 201, 31–33. https://doi.org/10.1016/j.resp.2014.06.012 (2014).
Baillieul, S. et al. Continuous positive airway pressure improves gait control in severe obstructive sleep apnoea: a prospective study. PLoS ONE 13, e0192442. https://doi.org/10.1371/journal.pone.0192442 (2018).
Lee, S. & Shin, C. Interaction of obstructive sleep apnoea and cognitive impairment with slow gait speed in middle-aged and older adults. Age Ageing 46, 653–659. https://doi.org/10.1093/ageing/afw228 (2017).
Yogev-Seligmann, G., Hausdorff, J. M. & Giladi, N. The role of executive function and attention in gait. Mov. Disord. 23, 329–342. https://doi.org/10.1002/mds.21720 (2008) (quiz 472).
Morris, R., Lord, S., Bunce, J., Burn, D. & Rochester, L. Gait and cognition: mapping the global and discrete relationships in ageing and neurodegenerative disease. Neurosci. Biobehav. Rev. 64, 326–345. https://doi.org/10.1016/j.neubiorev.2016.02.012 (2016).
Marshall, N. S. et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax 61, 430–434. https://doi.org/10.1136/thx.2005.050583 (2006).
Kushida, C. A. et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 35, 1593–1602. https://doi.org/10.5665/sleep.2226 (2012).
Joyeux-Faure, M. et al. Continuous positive airway pressure treatment impact on memory processes in obstructive sleep apnea patients: a randomized sham-controlled trial. Sleep Med. 24, 44–50. https://doi.org/10.1016/j.sleep.2016.06.023 (2016).
Dalmases, M. et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized Pilot study. Chest 148, 1214–1223. https://doi.org/10.1378/chest.15-0171 (2015).
Plummer, P. & Eskes, G. Measuring treatment effects on dual-task performance: a framework for research and clinical practice. Front. Hum. Neurosci. 9, 225. https://doi.org/10.3389/fnhum.2015.00225PMID-25972801 (2015).
Schmit, C. & Brisswalter, J. Executive functioning during prolonged exercise: a fatigue-based neurocognitive perspective. Int. Rev. Sport Exerc. Psychol. https://doi.org/10.1080/1750984X.2018.1483527 (2018).
Olaithe, M., Bucks, R. S., Hillman, D. R. & Eastwood, P. R. Cognitive deficits in obstructive sleep apnea: insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med. Rev. 38, 39–49. https://doi.org/10.1016/j.smrv.2017.03.005 (2018).
Stern, Y. et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement https://doi.org/10.1016/j.jalz.2018.07.219 (2018).
Secher, N. H., Seifert, T. & Van Lieshout, J. J. Cerebral blood flow and metabolism during exercise: implications for fatigue. J. Appl. Physiol. (1985) 104, 306–314. https://doi.org/10.1152/japplphysiol.00853.2007 (2008).
Innes, C. R., Kelly, P. T., Hlavac, M., Melzer, T. R. & Jones, R. D. Decreased regional cerebral perfusion in moderate-severe obstructive sleep apnoea during wakefulness. Sleep 38, 699–706. https://doi.org/10.5665/sleep.4658 (2015).
Prilipko, O., Huynh, N., Thomason, M. E., Kushida, C. A. & Guilleminault, C. An fMRI study of cerebrovascular reactivity and perfusion in obstructive sleep apnea patients before and after CPAP treatment. Sleep Med. 15, 892–898. https://doi.org/10.1016/j.sleep.2014.04.004 (2014).
Matsuo, A., Inoue, Y., Namba, K. & Chiba, H. Changes in cerebral hemoglobin indices in obstructive sleep apnea syndrome with nasal continuous positive airway pressure treatment. Sleep Breath 15, 487–492. https://doi.org/10.1007/s11325-010-0367-y (2011).
Shiota, S. et al. Effect of continuous positive airway pressure on regional cerebral blood flow during wakefulness in obstructive sleep apnea. Sleep Breath 18, 289–295. https://doi.org/10.1007/s11325-013-0881-9 (2014).
Kim, J. S. et al. Effect of continuous positive airway pressure on regional cerebral blood flow in patients with severe obstructive sleep apnea syndrome. Sleep Med. 32, 122–128. https://doi.org/10.1016/j.sleep.2016.03.010 (2017).
Marillier, M. et al. Impaired cerebral oxygenation and exercise tolerance in patients with severe obstructive sleep apnea syndrome. Sleep Med. 51, 37–46. https://doi.org/10.1016/j.sleep.2018.06.013 (2018).
Browning, R. C. & Kram, R. Effects of obesity on the biomechanics of walking at different speeds. Med. Sci. Sports Exerc. 39, 1632–1641. https://doi.org/10.1249/mss.0b013e318076b54b (2007).
Naugle, K. M., Higgins, T. J. & Manini, T. M. Obesity and use of compensatory strategies to perform common daily activities in pre-clinically disabled older adults. Arch. Gerontol. Geriatr. 54, e134-138. https://doi.org/10.1016/j.archger.2011.10.017 (2012).
Bailly, S. et al. Obstructive sleep apnea: a cluster analysis at time of diagnosis. PLoS ONE 11, e0157318. https://doi.org/10.1371/journal.pone.0157318 (2016).
Folstein, M. F., Robins, L. N. & Helzer, J. E. The mini-mental state examination. Arch. Gen. Psychiatry 40, 812. https://doi.org/10.1001/archpsyc.1983.01790060110016 (1983).
Kapur, V. K. et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep Apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 13, 479–504. https://doi.org/10.5664/jcsm.6506 (2017).
Berry, R. B. et al. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 13, 665–666. https://doi.org/10.5664/jcsm.6576 (2017).
Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22, 667–689 (1999).
Farre, R. et al. Sham continuous positive airway pressure for placebo-controlled studies in sleep apnoea. Lancet 353, 1154. https://doi.org/10.1016/S0140-6736(99)01056-9 (1999).
Jenkinson, C., Davies, R. J., Mullins, R. & Stradling, J. R. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 353, 2100–2105. https://doi.org/10.1016/S0140-6736(98)10532-9 (1999).
Rodway, G. W. et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep 33, 260–266. https://doi.org/10.1093/sleep/33.2.260 (2010).
Schwab, R. J. et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am. J. Respir. Crit. Care Med. 188, 613–620. https://doi.org/10.1164/rccm.201307-1282ST (2013).
Lienhard, K., Schneider, D. & Maffiuletti, N. A. Validity of the Optogait photoelectric system for the assessment of spatiotemporal gait parameters. Med. Eng. Phys. 35, 500–504. https://doi.org/10.1016/j.medengphy.2012.06.015 (2013).
Rota, V., Perucca, L., Simone, A. & Tesio, L. Walk ratio (step length/cadence) as a summary index of neuromotor control of gait: application to multiple sclerosis. Int. J. Rehabil. Res. 34, 265–269. https://doi.org/10.1097/MRR.0b013e328347be02 (2011).
Hausdorff, J. M. Gait variability: methods, modeling and meaning. J. Neuroeng. Rehabil. 2, 19. https://doi.org/10.1186/1743-0003-2-19 (2005).
Beauchet, O. et al. Gait control: a specific subdomain of executive function?. J. Neuroeng. Rehabil. 9, 12. https://doi.org/10.1186/1743-0003-9-12 (2012).
Woollacott, M. & Shumway-Cook, A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 16, 1–14 (2002).
Hall, C. D., Echt, K. V., Wolf, S. L. & Rogers, W. A. Cognitive and motor mechanisms underlying older adults’ ability to divide attention while walking. Phys. Ther. 91, 1039–1050. https://doi.org/10.2522/ptj.20100114 (2011).
Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 10, 1–17. https://doi.org/10.14802/jmd.16062 (2017).
Degache, F. et al. Sleep-disordered breathing and daytime postural stability. Thorax 71, 543–548. https://doi.org/10.1136/thoraxjnl-2015-207490 (2016).
Caron, O., Gelat, T., Rougier, P. & Blanchi, J. P. A comparative analysis of the center of gravity and center of pressure trajectory path lengths in standing posture: an estimation of active stiffness. J. Appl. Biomech. 16, 234–247. https://doi.org/10.1123/jab.16.3.234 (2000).
Obrig, H. NIRS in clinical neurology—a “promising” tool?. Neuroimage 85(Pt 1), 535–546. https://doi.org/10.1016/j.neuroimage.2013.03.045 (2014).
Metzger, F. G. et al. Functional brain imaging of walking while talking—an fNIRS study. Neuroscience 343, 85–93. https://doi.org/10.1016/j.neuroscience.2016.11.032 (2017).
Van de Putte, M., Hagemeister, N., St-Onge, N., Parent, G. & de Guise, J. A. Habituation to treadmill walking. Biomed. Mater. Eng. 16, 43–52 (2006).
Jordan, K., Challis, J. H. & Newell, K. M. Walking speed influences on gait cycle variability. Gait Posture 26, 128–134. https://doi.org/10.1016/j.gaitpost.2006.08.010 (2007).
Calvert, M. et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 309, 814–822. https://doi.org/10.1001/jama.2013.879 (2013).
Funding
JLP, RT and SB are supported by the French National Research Agency in the framework of the "Investissements d’avenir” program (ANR-15-IDEX-02) and the “e-health and integrated care and trajectories medicine and MIAI artificial intelligence” Chairs of excellence from the Grenoble Alpes University Foundation. This work has been partially supported by MIAI @ Grenoble Alpes (ANR-19-P3IA-0003) SOFMER by Société Française de Médecine Physique et de Réadaptation.
Author information
Authors and Affiliations
Contributions
S. Baillieul designed and conceptualized this study, played a major role in the acquisition of data, analyzed the data and drafted the manuscript for intellectual content. B.W. designed and conceptualized this study, drafted the manuscript for intellectual content. D.P. designed and conceptualized this study, analyzed the data and drafted the manuscript for intellectual content. R.T. interpreted the data and revised the manuscript for intellectual content. S. Bailly analyzed and interpreted the data and drafted the manuscript for intellectual content. B.M. analyzed and interpreted the data. T.L.R. and C.P. played a major role in the acquisition of data. S.V. and J.L.P. designed and conceptualized this study, interpreted the data and revised the manuscript for intellectual content. Pr. J.-L.P. takes full responsibility for the data, the analyses and interpretation, and the conduct of the research. Pr. J.-L.P. certifies that he has full access to all the data and that I have the right to publish any and all data separate and apart from any sponsor. This work is based on original data that has not been previously published and is not being considered for publication in another journal. The authors are grateful to Fond de dotation AGIR pour les maladies chroniques; the homecare company Agir à Dom, France, which provided patient equipment and follow-up. These sponsors had no role in the study design, data analysis, and study conclusions. Other contributions: The authors are also grateful to Sleep Laboratory technicians for their collaboration during the sleep studies; Angélique Grangier for data collection (INSERM U1042, HP2 Laboratory, Grenoble University Hospital, Grenoble, France); Claire Bérenger (PMR Department, Grenoble Alpes University Hospital, Grenoble, France) for the neuropsychological assessments. We thank Alison Foote (Grenoble Alpes University Hospital) for editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baillieul, S., Wuyam, B., Pérennou, D. et al. A randomized sham-controlled trial on the effect of continuous positive airway pressure treatment on gait control in severe obstructive sleep apnea patients. Sci Rep 11, 9329 (2021). https://doi.org/10.1038/s41598-021-88642-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88642-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.