Abstract
A mandibular advancement device (MAD) is recommended as an alternative therapy for obstructive sleep apnea (OSA), which effectively reduces the collapsibility of the upper airway during sleep by advancing the mandible. However, the effects of MAD therapy on cardiac autonomic modulation remain unclear. We evaluated the effects of MAD on nocturnal heart rate variability (HRV) in OSA. Anthropometric data, questionnaire results, and HRV parameters (evaluated using time domain and frequency domain methods) of 58 adult patients with OSA treated with MAD therapy were retrospectively reviewed. All patients underwent polysomnography at baseline and 3-month follow-up. The average normal-to-normal (NN) interval, standard deviation of the NN interval, low-frequency power in normalized units (LFnu), and high-frequency power in normalized units (HFnu) showed significant changes with MAD therapy. Based on the criteria for success (decrease in the apnea-hypopnea index by >50% and value <20/h), 34 and 24 patients were classified into the response and nonresponse groups, respectively. No differences in baseline characteristics were detected between groups, except for higher body mass index and lower minimal oxygen saturation in the nonresponse group. A subgroup analysis indicated that the average NN interval and HFnu significantly increased, and that Total power (TP), very low frequency, low frequency(LF), low frequency/high frequency and LFnu significantly decreased compared to baseline in the response group; however, no HRV changes were found in the nonresponse group. After adjusting for age, sex, and body mass index, the response group showed significant changes from baseline in TP and LF compared to the nonresponse group. Therefore, HRV may be useful for determining the efficacy of MAD therapy in OSA.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway obstruction during sleep associated with arterial blood desaturation, sympathetic nervous system activation, and cardiovascular impairment1. Untreated patients with OSA have increased mortality rates compared to the general population2,3. Laboratory polysomnography (PSG) is accepted as a standard method of diagnosing OSA4. However, PSG can be cumbersome because of its time, space, equipment, and tester requirements.
Heart rate variability (HRV) measures the variation in the time interval between heartbeats and reflects cardiac sympathetic and parasympathetic modulation. It can be measured using the single-lead electrocardiography (ECG) signal and is also called cycle length variability, RR variability, or heart period variability5. Reduced HRV was found to be related to increased mortality after myocardial infarction, congestive heart failure, diabetic neuropathy, depression, and poor survival in premature infants5,6,7,8,9. Because patients with OSA show abnormal cardiac autonomic modulation, the association between OSA and changes in HRV has been reported by several studies10,11,12,13.
A mandibular advancement device (MAD) is an oral device that has been recommended as an alternative therapy for OSA14. It can effectively reduce the collapsibility of the upper airway during sleep by advancing the mandible15. MAD was found to have positive effects on cardiac autonomic modulation measured by HRV in OSA that are similar to those of other treatments such as continuous positive airway pressure (CPAP) therapy16,17,18. However, there is still insufficient evidence for HRV changes occurring with successful MAD therapy for OSA. Furthermore, the positive effects of MAD on cardiac autonomic modulation are less clear than those of CPAP. Therefore, studies of the effects of MAD on cardiac morbidities are warranted. We attempted to identify whether MAD can affect cardiac autonomic modulation during an entire night. Our hypothesis was that a positive treatment response to MAD therapy may be associated with positive changes in HRV. This study evaluated the effects of MAD on HRV changes according to the response of treatment in patients with OSA.
Results
Changes in polysomnography statistics
PSG parameters related to the respiratory index were significantly improved by MAD when compared to baseline. MAD treatment significantly decreased the apnea-hypopnea index (AHI), apnea index (AI), hypopnea index (HI), and oxygen desaturation index (ODI). The minimal and average oxygen saturations significantly increased; however, the snoring rates were not changed with MAD. The incidence of sleep stage N3 and REM sleep significantly increased from 4.8 ± 5.5% to 7.2 ± 6.8% (P < 0.001) and from 15.6 ± 5.8% to 18.5 ± 6.2% (P < 0.001), respectively (Table 1).
Changes in nocturnal heart rate variability
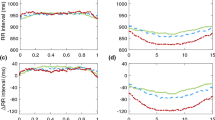
MAD treatment resulted in changes in HRV. Among the time domain measures, the average normal-to-normal (NN) interval significantly increased from 949.3 ± 134.1 ms to 988.4 ± 127.0 ms (P = 0.001), and the standard deviation of the NN interval (SDNN) significantly decreased from 96.8 ± 32.6 ms to 87.4 ± 26.8 ms (P = 0.042). Regarding the frequency domain values, the low-frequency (LF) power in normalized units (LFnu) and high-frequency (HF) power in normalized units (HFnu) showed statistically significant changes. LFnu decreased from 70.5 ± 11.9 to 67.4 ± 13.8 (P = 0.022) and HFnu increased from 29.5 ± 11.9 to 32.6 ± 13.8 (P = 0.022) with the use of MAD (Table 2).
Subgroup analyses of response and nonresponse groups
According to the response criteria, 34 subjects were classified into the response group and 24 were classified into the nonresponse group. No significant differences in baseline characteristics were found between the two subgroups except for higher body mass index (P = 0.004) and lower minimal oxygen saturation (P = 0.048) in the nonresponse group (Table 3). However, significant HRV differences were detected between the two groups. Regarding the time domain measures, the average NN interval significantly increased in the response group (from 947.7 ± 152.0 ms to 998.9 ± 140.0 ms; P = 0.003); however, no significant difference was found in the nonresponse group (from 951.8 ± 106.6 ms to 973.6 ± 107.2 ms; P = 0.111). Other time domain variables did not significantly change in both groups. Among the frequency domain values, total power (TP, P = 0.007), very low frequency (VLF, P = 0.010), LF (P = 0.004), LF/HF (P = 0.031), and LFnu (P = 0.015) significantly decreased in the response group, but not in the nonresponse group. There was also a significant change in HFnu in the response group (from 28.8 ± 10.1 to 33.4 ± 12.6; P = 0.015), but not in the nonresponse group (from 30.6 ± 14.3 to 31.3 ± 14.3; P = 0.686). The remaining frequency domain variable (HF) did not change with MAD in either group (Table 4). According to the success criteria of AHI that decreased by more than 50% and had a value less than 10/h, 24 subjects were classified to success group and 34 subjects were classified to failure group. In analyzing the change in HRV of each group, the average NN interval, TP, VLF, LF, LFnu, and HFnu significantly changed in the success group, while average NN interval and SDNN significantly change in the failure group (Table 5). Finally, the response group showed significant changes from baseline in the TP [−11004.00 (95% CI, −21448.00 to −559.85) in the response group and 5162.16 (95% CI, −7804.99 to 18129.00) in the nonresponse group; P = 0.02] and LF [−3483.21 (95% CI, −6690.23 to −276.19) and 2057.00 (95% CI, −1924.82 to 6038.81); P = 0.012] compared to the nonresponse group after adjusting for age, sex, and body mass index. However, changes in the sleep stage did not differ between the response and nonresponse groups (Table 6).
Discussion
This study revealed that MAD treatment significantly changed cardiac autonomic modulation represented by nocturnal HRV and that the changes were significant only in the treatment response group. MAD reduces AHI less effectively than CPAP, but it has been associated with higher compliance19; therefore, it has been widely used as an alternative treatment for patients with OSA. However, few studies have assessed the effects of MAD on cardiac autonomic modulation. A previous study that included 10 patients with OSA treated with MAD showed significant changes in the NN interval, HF, and the LF/HF ratio16. However, our findings showed significant changes in the NN interval, SDNN, LFnu, and HFnu. This discrepancy could be related to the difference in the number of subjects (10 vs. 58), HRV measurement time (day vs. night), and the treatment success rate (100% success group vs. 62.1% response group). Two previous studies that compared CPAP and MAD also described changes in HRV after MAD treatment. One study showed that MAD significantly decreased TP compared to baseline, and that the decrease was greater than that resulting from the use of a placebo oral appliance but lower than that resulting from CPAP17. Another study found no difference between MAD and CPAP regarding cardiac autonomic function changes during the day, although CPAP was more effective than MAD for eliminating respiratory events18. Although these previous comparative studies showed that MAD treatment induced significant HRV changes, their results were limited by the relatively small numbers of subjects (29 and 40) and restricted numbers of parameters that were used to assess HRV. Because we included 58 subjects and evaluated most HRV parameters, our results are likely to provide more concrete evidence regarding the effects of MAD on HRV. However, the difference in HRV changes was weakened between success and failure groups. HRV significantly changed on average NN interval, TP, VLF, LF, LFnu, and HFnu in the success group, while changed on average NN interval and SDNN in the failure group. Considering the average AHI (41.0/h) of our study subjects, the success criteria of AHI (decreased by more than 50% and had a value less than 10/h) was too strict. Accordingly, some subjects who had a good therapeutic effect on MAD were classified to failure group could be a reason for weakened difference between success and failure groups.
HRV varies considerably between individuals and is affected by age and physical condition20. Therefore, there is no uniform definition of the normal HRV range, and it is more difficult to determine a cutoff value for diagnosing OSA. Previous studies have reported the results of various HRV parameters for the diagnosis of OSA through various research methods. One previous study presented the LF/HF ratio as a useful parameter for diagnosing OSA in correlation with AHI13. Another study that examined the difference between HRV during the day and that during the night found that day and night SDNN were able to screen for OSA with high sensitivity and specificity21. Another study suggested that the NN interval was the best index among the time and frequency domain parameters because only the mean NN interval was shorter in the OSA group than in the control group22. As previously mentioned, studies that used HRV to diagnose OSA showed generally inconsistent results. These different reasons were due to the lack of uniform study methods and HRV characteristics that varied with age and physical condition. Therefore, to clarify the relationship between OSA and HRV, a well-designed research method is needed.
It seems that HRV is more useful for determining treatment results than for diagnosing OSA. A few studies have reported changes in HRV after OSA treatment. One study on changes in HRV after CPAP therapy reported that the SDNN of non-REM sleep decreased after CPAP23. We also found that the SDNN was decreased regardless of the sleep stage after MAD treatment. However, it was impossible to determine the response or nonresponse to treatment using the SDNN. A decrease in the LF/HF ratio was reported for 18 children after adenotonsillectomy24. We also found a change in the LF/HF ratio with response group of MAD therapy. Despite the differences in the age of the subjects (children vs. adults) and treatment modality (adenotonsillectomy vs. MAD) between studies, the change in the LF/HF ratio was similar. Choi et al.25 concluded that HRV showed significant changes in the success group of OSA patients who underwent upper airway surgery, but not in the failure group of OSA patients. They reported that the changes in VLF, LF, LFnu, and HFnu were meaningful, and these findings were in partial agreement with our results. We found that TP, VLF, LF, LFnu, and HFnu showed significant changes among the frequency domain parameters in the response group.
HRV is easier and less expensive to evaluate than PSG. The final model of our study showed that the TP and LF significantly changed in the response group compared to those in the nonresponse group after adjusting for age, sex, and body mass index. Taken together, changes in HRV, including decreased TP and decreased LF, could be used to determine the response of MAD treatment.
Previous studies that have analyzed the changes in HRV and evaluated treatment success have not included time domain paraments25 or only described the results of short-term analyses23. However, after analyzing the time domain parameters during 5 night h according to the standard method, we found that the NN interval had significantly changed in the response group, which was a strength of our study.
This study had several limitations. First, various success criteria were applied in different studies. Among many definitions of OSA treatment success, we used commonly accepted criteria, namely more than a 50% decrease in AHI and a value less than 20/h with MAD treatment. Therefore, the results of this study might differ from those of studies that used other success criteria. Second, the time domain method was applied during 5 h during the night. The HRV Task Force has indicated that it seems appropriate to analyze time domain method results using nominal 24-h long-term recordings5. To overcome this limitation, we used recordings of ECG during the same 5-h periods to analyze the time domain method results according to alternative recommendations. Third, selection bias could not be excluded because patients with better outcomes were more likely to participate in follow-up testing. A prospective study is needed to validate the effects of MAD treatment on cardiac autonomic modulation in OSA. Fourth, the sleep stage may influence the HRV values. However, in this study, we did not perform a detailed analysis of HRV according to sleep stage. Instead, we found that changes in the sleep stage did not differ between the response and nonresponse groups. Therefore, the sleep stage may have had a minor effect on the differences in HRV values between groups. Nevertheless, follow-up studies of HRV according to the sleep stage are needed.
In conclusion, the present study showed that MAD treatment for OSA significantly changed cardiac autonomic modulation as represented by nocturnal HRV. Among the assessed parameters, the average NN interval and HFnu increased, while TP, VLF, LF, LF/HF ratio, and LFnu decreased. However, the changes were observed only in the response group. Moreover, after adjusting for age, sex, and body mass index, the response group showed significant changes in the TP and LF compared to the nonresponse group. Therefore, nocturnal HRV may be a useful screening tool for determining the efficacy of MAD.
Methods
Subjects
We retrospectively reviewed the data of patients who visited a sleep center of a tertiary hospital because of snoring and sleep apnea. We selected subjects according to inclusion and exclusion criteria. The inclusion criteria were as follows: (1) adult patients (age ≥ 18 years); (2) patients who were diagnosed with OSA (AHI > 5/h); (3) patients who were treated with MAD (SomnoDent; SomnoMed Ltd, New South Wales, Australia) (Fig. 1); and (4) patients who underwent follow-up PSG after 3 months of MAD therapy and had the results of two PSG tests (baseline and with MAD) available. The exclusion criteria were the follows: (1) significant arrhythmias; (2) low-quality data (artefact > 20% of total sleep time); (3) total sleep time less than 5 h; (4) awake more than 30 minutes from midnight to 5 am; (5) combined sleep disorders (i.e., insomnia or narcolepsy); (6) habitual use of sedatives and hypnotics; and (7) history of specific pathology related to HRV changes (i.e., myocardial infarction, diabetic neuropathy, cardiac transplantation, myocardial dysfunction, or tetraplegia). Based on the inclusion and exclusion criteria, a total of 58 patients with OSA were included in this study. The anthropometric data of subjects were evaluated. Daytime sleepiness and subjective sleep quality of subjects were evaluated using the Epworth sleepiness scale (ESS) and Pittsburgh sleep quality index (PSQI), respectively. The ethics committee of Seoul National University Bundang Hospital (IRB No. B-1902-522-111) approved the use of these data. The need for written informed consent was waived by the Institutional Review Board.
Analyses of nocturnal heart rate variability
The HRV was measured using exported ECG data and commercially available PSG software (RemLogic 3.0 HRV analyzer; Embla Systems, San Carlos, CA). The HRV analysis was performed without any information regarding PSG results except for the ECG signal. To prevent inappropriate comparisons, we analyzed the ECG signal from midnight to 5 am. In other words, ECG signals exported before midnight and after 5 am were not included in the HRV analysis. The signals were interpolated and resampled at 5.0 Hz; NN intervals more than 2,400 ms and less than 400 ms were omitted.
Calculations of the time domain and frequency domain parameters were performed according to the standard methods for HRV measurements5. The following parameters measured using time domain methods were included: (1) average NN interval; (2) SDNN; (3) standard deviation of the 5-minute averages of NN intervals (SDANN); (4) square root of the mean of the squared differences of adjacent NN intervals (RMSSD); (5) number of pairs of adjacent NN intervals more than 50 ms (NN50 count); (6) the rate of NN50 in the total number of NN intervals (pNN50); and (7) HRV triangular index and geometric measurements. Frequency domain variables were presented as the average of the values calculated every 5 minutes. The parameters of the frequency domain and their brief descriptions were as follows: total power, power of approximately ≤ 0.4 Hz; VLF, power ≤ 0.04 Hz; LF, power of 0.04–0.15 Hz; HF, power of 0.15–0.4 Hz; LF/HF ratio, LF divided by HF; LFnu, LF power of normalized units [LF/(LF + HF) × 100]; and HFnu, HF power of normalized units [HF/(LF + HF) × 100].
Definition of treatment outcomes
Apnea was defined as the complete cessation of airflow for at least 10 s. Hypopnea was defined as a substantial reduction in airflow (≥30%) for at least 10 s associated with electroencephalographic arousal or oxygen desaturation (≥3%). AHI was defined as the total number of apnea incidents and hypopnea incidents per hour of sleep. Snoring was measured with a snore sensor (Piezo; Pro-Tech, Woodinville, Washington, USA). The sensor was attached around the neck of patients at the larynx level and detected whether vibration of the larynx was sufficient. The snoring rate was defined as the ratio of snoring time to total sleep time. The response to treatment was defined as an AHI that decreased by more than 50% and had a value less than 20/h with MAD compared to baseline26. Subjects who did not meet these criteria were included in the nonresponse group. The success to treatment was defined as an AHI that decreased by more than 50% and had a value less than 10/h with MAD compared to baseline. Subjects who did not meet these criteria were included in the failure group.
Statistical analysis
Most values obtained during this study were continuous variables expressed as mean ± standard deviation, except for sex, which was a categorical variable presented as a ratio. The paired t test was used to compare the differences between the baseline values of parameters and those after MAD. The independent t test or chi-squared test was performed for the comparative analysis of baseline characteristics of the response and nonresponse groups. Differences in the change from baseline between the response and nonresponse groups were tested using an analysis of covariance (ANCOVA) with age, sex, and body mass index as covariates. Data analyses were performed using SPSS software (version 18; SPSS Inc., Chicago, IL), and P < 0.05 was considered statistically significant.
Data availability
The data that support the findings of this study are available from Seoul National University Bundang Hospital Sleep Center, but restrictions apply to the availability of these data, which were used under license for the current study; therefore, they are not publicly available. However, data are available from the authors upon reasonable request and with the permission of Seoul National University Bundang Hospital Sleep Center.
References
Phillips, B. Sleep-disordered breathing and cardiovascular disease. Sleep. Med. Rev. 9, 131–140 (2005).
Young, T. et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 31, 1071–1078 (2008).
Marti, S. et al. Mortality in severe sleep apnoea/hypopnoea syndrome patients: impact of treatment. Eur. Respir. J. 20, 1511–1518 (2002).
Kapur, V. K. et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep. Med. 13, 479–504 (2017).
Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 17, 354–381 (1996).
Malik, M. & Camm, A. J. Heart rate variability and clinical cardiology. Br. Heart J. 71, 3–6 (1994).
Malliani, A., Pagani, M., Lombardi, F. & Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 84, 482–492 (1991).
Kamath, M. V. & Fallen, E. L. Power spectral analysis of heart rate variability: a noninvasive signature of cardiac autonomic function. Crit. Rev. Biomed. Eng. 21, 245–311 (1993).
Appel, M. L., Berger, R. D., Saul, J. P., Smith, J. M. & Cohen, R. J. Beat to beat variability in cardiovascular variables: noise or music? J. Am. Coll. Cardiol. 14, 1139–1148 (1989).
Szollosi, I., Krum, H., Kaye, D. & Naughton, M. T. Sleep apnea in heart failure increases heart rate variability and sympathetic dominance. Sleep. 30, 1509–1514 (2007).
Stein, P. K. & Pu, Y. Heart rate variability, sleep and sleep disorders. Sleep. Med. Rev. 16, 47–66 (2012).
Lado, M. J. et al. Detecting sleep apnea by heart rate variability analysis: assessing the validity of databases and algorithms. J. Med. Syst. 35, 473–481 (2011).
Park, D. H. et al. Correlation between the severity of obstructive sleep apnea and heart rate variability indices. J. Korean Med. Sci. 23, 226–231 (2008).
Epstein, L. J. et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep. Med. 5, 263–276 (2009).
Huang, Y., White, D. P. & Malhotra, A. The impact of anatomic manipulations on pharyngeal collapse: results from a computational model of the normal human upper airway. Chest. 128, 1324–1330 (2005).
Coruzzi, P. et al. Autonomic cardiac modulation in obstructive sleep apnea: effect of an oral jaw-positioning appliance. Chest. 130, 1362–1368 (2006).
Dal-Fabbro, C. et al. Mandibular advancement device and CPAP upon cardiovascular parameters in OSA. Sleep. breath. 18, 749–759 (2014).
Glos, M. et al. Comparison of effects of OSA treatment by MAD and by CPAP on cardiac autonomic function during daytime. Sleep. breath. 20, 635–646 (2016).
Tan, Y. K. et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur. J. Orthod. 24, 239–249 (2002).
Thomas, R. J., Mietus, J. E., Peng, C. K. & Goldberger, A. L. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 28, 1151–1161 (2005).
Roche, F. et al. Screening of obstructive sleep apnea syndrome by heart rate variability analysis. Circulation. 100, 1411–1415 (1999).
Zhu, K. et al. Overnight heart rate variability in patients with obstructive sleep apnoea: a time and frequency domain study. Clin. Exp. Pharmacol. Physiol. 39, 901–908 (2012).
Kufoy, E. et al. Changes in the heart rate variability in patients with obstructive sleep apnea and its response to acute CPAP treatment. PLoS One. 7, e33769 (2012).
Muzumdar, H. V. et al. Changes in heart rate variability after adenotonsillectomy in children with obstructive sleep apnea. Chest. 139, 1050–1059 (2011).
Choi, J. H. et al. Effect of upper airway surgery on heart rate variability in patients with obstructive sleep apnoea syndrome. J. Sleep. Res. 21, 316–321 (2012).
Sher, A. E., Schechtman, K. B. & Piccirillo, J. F. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 19, 156–177 (1996).
Acknowledgements
This research was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean Government, Ministry of Science and ICT, Sejong, Republic of Korea (NRF-2015M3A9D7066972 and NRF-2015M3A9D7066973).
Author information
Authors and Affiliations
Contributions
J.W.K. designed the study. W.H.L. wrote the main manuscript text. Both authors collected the data and reviewed the manuscript. S.O.K. analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, JW., Kwon, S.O. & Lee, W.H. Nocturnal heart rate variability may be useful for determining the efficacy of mandibular advancement devices for obstructive sleep apnea. Sci Rep 10, 1030 (2020). https://doi.org/10.1038/s41598-020-57780-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57780-7
This article is cited by
-
Treatment options in obstructive sleep apnea
Internal and Emergency Medicine (2022)
-
A Novel Portable Real-Time Low-Cost Sleep Apnea Monitoring System based on the Global System for Mobile Communications (GSM) Network
Medical & Biological Engineering & Computing (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.