Abstract
We evaluate the risks of various urological disorders that require treatments according to obesity and metabolic health status using a nationwide dataset of the Korean population. 3,969,788 patients who had undergone health examinations were enrolled. Participants were classified as “obese” (O) or “non-obese” (NO) using a BMI cut-off of 25 kg/m2. People who developed ≥ 1 metabolic disease component in the index year were considered “metabolically unhealthy” (MU), while those with none were considered “metabolically healthy” (MH). There were classified into the MHNO, MUNO, MHO, and MUO group. In BPH, chronic renal disease, neurogenic bladder, any medication related to voiding dysfunction, alpha-blocker, and antidiuretics, age and gender-adjusted hazard ratio (HR) was highest in MUO, but higher in MUNO than in MHO. In stress incontinence, prostate surgery, and 5alpha-reductase, HR increased in the order of MUNO, MHO, and MUO. In prostatitis, anti-incontinence surgery, and cystocele repair, HR was higher in MHO than MUNO and MUO. In cystitis, cystostomy, and anticholinergics, HR was higher in MUNO and MUO than MHO. In conclusion, obesity and metabolic health were individually or collaboratively involved in urological disorders related to voiding dysfunction. Metabolic healthy obesity needs to be distinguished in the diagnosis and treatment of urological disorders.
Similar content being viewed by others
Introduction
The reported global prevalence of metabolic syndrome is < 10% to 84%, according to how the syndrome is defined and the population that is studied (race/ethnicity, sex, age, location, and environmental factors)1,2. Impaired glucose tolerance, dyslipidemia, hypertension, and abdominal obesity are shared pathophysiological contributors to metabolic syndrome and lower urinary tract symptoms (LUTS), which are both multifactorial conditions with shared risk factors1,2,3,4. The components of metabolic syndrome in men over 60 years are closely associated with LUTS, which frequently develop as a result of increased tone of smooth muscle in the prostate and bladder and enlarged prostate volume5. In men, weight gain, a large waist circumference, and a high body mass index (BMI) increase the risk of LUTS and exacerbate their progression6. Women with high BMI and diabetes are also at an increased risk for urinary incontinence and overactive bladder7,8. Previous studies showed that people in the metabolic healthy obese (MHO) or metabolic unhealthy non-obese (MUNO) group have different risks in terms of the incidence of type 2 diabetes, cardiovascular diseases, and mortality9,10,11. Thus, clinicians should assess the risk of MHO and MUNO individuals for various urological disorders related to voiding dysfunction that require proper treatment. However, no large-scale study has yet investigated this issue. Therefore, using a nationwide dataset of the Korean population, we classified individuals into 4 groups—metabolic healthy non-obese (MHNO), MUNO, MHO, and metabolic unhealthy obese (MUO)—and aimed to evaluate the associations of obesity and metabolic health status with the risk of various urological disorders related to voiding dysfunction requiring treatment.
Results
Baseline characteristics according to BMI and metabolic health status
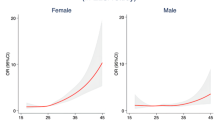
Of the 3,879,449 participants, 2,227,856 (57.4%), 375,836 (9.7%), 693,078 (17.9%) and 582,679 (15.0%) subjects were defined as having the MHNO, MUNO, MHO, and MUO phenotypes, respectively (Table 1) (Fig. 1). The mean BMI was 21.8 ± 2 kg/m2, 22.9 ± 1.6 kg/m2, 26.8 ± 1.9 kg/m2, and 27.9 ± 2.4 kg/m2 in participants with the MHNO, MUNO, MHO, and MUO phenotypes, respectively (Table 1). As expected, the values for metabolic disease components (total cholesterol levels, fasting glucose, and systolic and diastolic BP) were higher in the MUNO and MUO groups than in the metabolically healthy groups (P < 0.001) (Table 1). Significant differences were also found among the groups in age, sex, family history of relevant conditions, smoking/alcohol consumption, income, and exercise (P < 0.001) (Table 1).
Diagnosis related to urological disorders according to BMI and metabolic health status
After adjusting for age and sex, the hazard ratio (HR) (95% CI) of a diagnosis of BPH was 1.185 (1.176, 1.195) for the MUNO group, 1.132 (1.123, 1.141) for the MHO group, and 1.293 (1.284, 1.303) for the MUO group, compared to those in the MHNO group. For prostatitis, the age- and sex- adjusted HRs (95% CI) were 1.013 (0.999, 1.026) for the MUNO group, 1.025 (1.015, 1.035) for the MHO group, and 1.016 (1.006, 1.027) for the MUO group, compared to the MHNO group. For cystitis, the age- and sex-adjusted HRs (95% CI) were 1.085 (1.077, 1.094) for the MUNO group, 0.953 (0.947, 0.96) for the MHO group, and 1.102 (1.094, 1.109) for the MUO group, compared to the MHNO group. For stress incontinence, the age- and sex-adjusted HRs (95% CI) were 1.015 (0.995, 1.037) for the MUNO group, 1.126 (1.105, 1.148) for the MHO group, and 1.153 (1.132, 1.174) for the MUO group, compared to those in the MHNO group. For chronic renal disease, the age- and sex- adjusted HRs (95% CI) were 2.055 (2.017, 2.094) for the MUNO group, 1.114 (1.089, 1.139) for the MHO group, and 2.084 (2.048, 2.12) for the MUO group, compared to those in the MHNO group. For neurogenic bladder, the age-and sex- adjusted HRs (95% CI) were 1.098 (1.088, 1.108) for the MUNO group, 1.025 (1.017, 1.033) for the MHO group, and 1.133 (1.124, 1.142) for the MUO group, compared to the MHNO group (Table 2).
In BPH, chronic renal disease, neurogenic bladder, age and gender-adjusted HR was highest in MUO, but higher in MUNO than in MHO. In stress incontinence, HR increased in the order of MUNO, MHO, and MUO. In prostatitis, HR was higher in MHO than MUNO and MUO. In cystitis, HR was higher in MUNO and MUO than MHO. Rather, the HR of MHO was 0.953, which is lower than that of the control MHNO.
Surgery related to urological disorders according to BMI and metabolic health status
After adjusting the age and sex, the HR (95% CI) for prostate surgery was 1.014 (0.958, 1.073) for the MUNO group, 1.118 (1.054, 1.187) for the MHO group, and 1.22 (1.159, 1.284) for the MUO group, compared to the MHNO group. For prostate hyperthermia, the age- and sex-adjusted HRs (95% CI) were 1.002 (0.847, 1.187) for the MUNO group, 1.089 (0.949, 1.249) for the MHO group, and 1.046 (0.909, 1.204) for the MUO group, compared to the MHNO group. For anti-incontinence surgery, the age- and sex-adjusted HRs (95% CI) were 0.916 (0.869, 0.966) in the MUNO group, 1.493 (1.438, 1.549) in the MHO group, and 1.111 (1.063, 1.162) in the MUO group, compared to the MHNO group. For cystocele repair, the age- and sex-adjusted HRs (95% CI) were 1.224 (1.023, 1.464) in the MUNO group, 1.317 (1.114, 1.557) in the MHO group, and 1.232 (1.043, 1.456) in the MUO group, compared to those in the MHNO group. For cystostomy, the age- and sex-adjusted HRs (95% CI) were 1.503 (1.138, 1.984) in the MUNO group, 0.988 (0.721, 1.353) in the MHO group, and 1.894 (1.494, 2.401) in the MUO group, compared to the MHNO group (Table 3).
In prostate surgery, HR increased in the order of MUNO, MHO, and MUO. In anti-incontinence surgery, and cystocele repair, HR was higher in MHO than MUNO and MUO. In anti-incontinence surgery, the HR of MUNO is 0.916, which is lower than the control MHNO. In cystostomy, HR was higher in MUNO and MUO than MHNO.
Medication related to voiding dysfunction according to BMI and metabolic health status
For any medication related to voiding dysfunction, the age- and sex-adjusted HRs (95% CI) were 1.087 (1.079, 1.094) in the MUNO group, 1.029 (1.022, 1.035) in the MHO group, and 1.139 (1.132, 1.145) in the MUO group, compared to the MHNO group. For alpha-blockers, the age- and sex-adjusted HRs (95% CI) were 1.147 (1.138, 1.156) for the MUNO group, 1.092 (1.084, 1.1) for the MHO group, and 1.219 (1.21, 1.227) for the MUO group, compared to the MHNO group. For anticholinergics, the age- and sex-adjusted HRs (95% CI) were 1.052 (1.042, 1.062) for the MUNO group, 0.973 (0.964, 0.982) for the MHO group, and 1.093 (1.083, 1.102) for the MUO group, compared to the MHNO group. For 5-alpha-reductase, the age- and sex-adjusted HRs (95% CI) were 1.16 (1.145, 1.174) for the MUNO group, 1.162 (1.148, 1.177) for the MHO group, and 1.304 (1.289, 1.318) for the MUO group, compared to the MHNO group. For antidiuretics, the age- and sex-adjusted HRs (95% CI) were 1.184 (1.153, 1.216) for the MUNO group, 1.108 (1.077, 1.14) for the MHO group, and 1.295 (1.264, 1.327) for the MUO group, compared to the MHNO group (Table 4).
In any medication related to voiding dysfunction, alpha-blocker, and antidiuretics, HR was highest in MUO, but higher in MUNO than in MHO. In 5alpha-reductase, HR increased in the order of MUNO, MHO, and MUO. In anticholinergics, HR was higher in MUNO and MUO than MHNO, the HR of MHO is 0.973, which is lower than the control MHNO.
Discussion
Metabolic syndrome is a complex pathologic condition leading to an increased risk of diabetes and death from cardiovascular and non-cardiovascular causes12. Obesity is a common comorbidity associated with LUTS (e.g., frequency, incomplete emptying, intermittency, hesitancy, straining, weak stream, nocturia, and urgency), which are highly prevalent in older men. Abdominal obesity, which tends to co-occur with limited physical activity, may have an association with higher levels of sympathetic activity and is related to the severity of LUTS and the development and progression of BPH to benign prostatic obstruction2,13. Diminished physical activity also has a causal relationship with hyperinsulinemia and abnormal blood glucose levels. Of particular concern, extended durations of high serum glucose levels selectively exert neurotoxic effects on parasympathetic neurons, with implications for the tone of smooth muscle of the bladder2,13. In a cross-sectional study of National Health and Nutrition Examination Survey data, older adults (> 60 years of age) with a BMI exceeding 25 kg/m2 showed more severe LUTS14. Evidence suggests an association between metabolic syndrome and LUTS due to BPH15,16. It has been found that insulin resistance and secondary hyperinsulinemia augment the risk of BPH17, and case series have confirmed that metabolic syndrome is associated with the growth rate of prostate volume18,19. Bunn et al., in a systematic review, concluded that women with metabolic syndrome may be at an elevated risk for overactive bladder20.
In the present study, the authors analyzed the pattern of urological disorders according to obesity and metabolic health status in three areas: diagnostic disease codes, surgical procedures, and medications related to voiding dysfunction. For BPH, chronic renal disease, and neurogenic bladder, the age and sex-adjusted HR was highest in the MUO group, but higher in the MUNO group than in the MHO group. The same pattern was seen for medications related to voiding dysfunction, alpha-blockers, and antidiuretics. This suggests that metabolic health may have a greater effect on these disease and medication than obesity itself. Multiple studies have demonstrated a link between the component of metabolic syndrome and BPH/LUTS complex2,3,4. Factors including autonomic hyperactivity, hyperinsulinemia, inflammation, and obesity may play a role in the causes of metabolic symdrome and BPH/LUTS1,21. Chen et al.22 showed that patients with metabolic syndrome have a 2.5-fold higher risk of developing chronic renal disease. Studies suggest that the renal fibrosis seen in metabolic syndrome might be caused by a constellation of insulin resistance, hypertension, dyslipidemias and inflammation, and result in a heightened expression of adipocytokines, angiotensin and inflammatory cytokines such as interleukin-6 and tumor necrosis factor-alpha22. In this study, all types of neurogenic bladder were included in the analysis, but if we only look at the neurogenic bladder by representative SCI (spinal cord injury) patient, the inactivity imposed by SCI leads to an increase in BMI for many people and consequently an increased incidence of central adiposity and insulin resistance, components of the metabolic syndrome. This in turn leads to an increased risk of diabetes, an independent risk factor for mortality in people with SCI23. Obesity is also known to be associated with an increased risk of urinary tract infection (UTI) in men and women24,25. The obese were up to 2.5 times more likely to be diagnosed with a UTI than were the nonobese24.
For stress incontinence, prostatic surgery, and 5-alpha-reductase, the lowest age and sex-adjusted HR was found for the MUNO group, followed in increasing order by the MHO and MUO groups, indicating the cumulative effects of obesity and metabolic health. For prostatitis, anti-incontinence surgery, and cystocele repair, the age- and sex-adjusted HR was higher in the MHO group than in the MUNO and MUO groups. Increased intra-abdominal pressure owing to obesity appears to be the common pathophysiologic factor in stress urinary incontinence and pelvic organ prolapse for women and midurethral sling surgery is large effective in the obese population26,27. The authors believe that obesity itself is more influential regarding the choice of surgical treatment methods for stress urinary incontinence and cystocele than metabolic health, and that medical control is preferred in the MUO group over surgical treatment. Zhang et al.28 reported that major lifestyle factors such as obesity, smoking and hypertension were not associated with chronic prostatitis/chronic pelvic pain syndrome risk in the large cohort study. The etiology of chronic prostatitis/chronic pelvic pain syndrome remains unknown. For cystitis, cystostomy, and anticholinergics, the age and sex-adjusted HR was higher in the MUNO and MUO groups than in the MHO group, implying that for cystitis and anticholinergics, metabolic health seems to have a greater effect than obesity itself. In particular, in cystitis and anticholinergics, MHO showed lower HR than the control MHNO, which can be seen as strong evidence that obesity and metabolic health status should be separated and evaluated individually.
Several limitations of our study should be noted. First, there might be errors in the classification of metabolic health status according to disease code. The disease code cannot completely represent the patient's current disease status, and there may be an error as to whether each drug prescription accordingly occurs for each disease code. Second, because, our customized NIHS database had built randomly sampled 50% of the examinees who have undergone medical examinations, there may be errors in representing general populations during the study period. Also, the possibility of bias of healthy users should be considered due to the characteristics of the examinees who have undergone general medical examination.
In conclusion, obesity and metabolic health were individually or collaboratively involved in urological disorders related to voiding dysfunction. Metabolic healthy obesity needs to be distinguished in the diagnosis and treatment of urological disorders.
Methods
Study population and data source
The National Health Insurance System (NHIS) maintains a comprehensive set of databases with health information on 50 million Koreans, including an eligibility database (with demographic factors including socioeconomic variables [including income], sex, age, and eligibility type), a health examination database (with data from general health examinations and lifestyle/behavior-related questionnaires), and a medical treatment database (containing information on claims made by medical service providers for medical expenses)29,30,31. The Health Insurance Review and Assessment (HIRA) service provides this information from their database, which includes information on insurance claims for approximately 97% of the Korean population. In the present study, we used a customized NHIS database including approximately 10% of the Korean population. The representativeness of the database was ensured by stratified random sampling. The index year was defined as the year of subjects’ first participation in the health examination. Of the 5,300,646 persons who received health examinations between 2009 and 2016, those who were < 20 years of age (n = 7,717) or had previously been diagnosed with urologic diseases involving voiding dysfunction (n = 1,323,141) were excluded. Within 1 year after the health examination, 3,969,788 patients were diagnosed with urological diseases related to voiding dysfunction and were treated medically or surgically. The final study population consisted of 3,879,449 subjects, with the further exclusion of 90,339 patients with missing values (Fig. 2). This study was developed in accordance with good clinical practice guidelines and the Declaration of Helsinki and was approved by the Hallym University Kangnam Sacred Heart Hospital Institution Review Board (HKS 2017-04-004) and exempted it from the requirement to obtain informed consent due to the use of anonymized and de-identified data.
Flow diagram of study subjects. Of the 5,300,646 persons who received health examinations between 2009 and 2016, those aged < 20 years (n = 7,717) or those who were diagnosed with urologic diseases involving voiding dysfunction before the health examination (n = 1,323,141) were excluded. Within 1 year after the health examination, 3,969,788 patients were diagnosed with diseases related to voiding dysfunction and were treated medically or surgically. The final study population consisted of 3,879,449 subjects, except for 90,339 patients with missing values.
Measurements
As appropriate for Asian populations, obesity was defined using the BMI cutoff of 25 kg/m2. Samples were monitored according to the questionnaire before health examination of Koreans, family histories of cardiovascular disease, diabetes, and cancer were obtained using the questionnaire. Subjects were recorded as smoking status, and as drinking alcohol status based on the information obtained using the questionnaire. Regular exercise was defined as vigorous physical activity performed for at least 20 min, and subjects were classified based on the number of exercises per week.
Metabolic health status and urological disorders related to voiding dysfunction
We classified to subjects by referring to previous study that defined metabolic health status10. Metabolic health status was defined on the basis of three disease components: diabetes, hypertension, and dyslipidemia. Diabetes was defined as (1) at least one claim annually for the prescription of an antidiabetic medication under International Classification of Disease, 10th Revision (ICD–10) codes E10–14, or (2) a fasting glucose measurement of ≥ 7 mmol/L. Hypertension was defined as the presence of one or more claim per year for the prescription of an antihypertensive agent (ICD-10 codes I10–I15) or a systolic/diastolic BP reading ≥ 140/90 mmHg. Dyslipidemia was defined based on the presence of one or more claim per year for the prescription of an antihyperlipidemic agent (ICD-10 code E78) or a total cholesterol measurement of ≥ 6.21 mmol/L. Participants with a BMI < 25 kg/m2 who developed at least one component of metabolic disease in the index year were defined as having the MUNO phenotype, and those with no components of metabolic disease were defined as having the MHNO phenotype. Analogously, participants with a BMI ≥ 25 kg/m2 were defined as having the MUO and MHO phenotypes, respectively, depending on the presence or absence of incident components of metabolic disease in the index yea. We analyzed data on the diagnostic codes according to ICD-10 classification such as benign prostatic hyperplasia (N400, N401, N402, N403, N408), chronic prostatitis (N411, N418), cystitis (N300, N301, N302, N303, N308, N309), neurogenic bladder (N31, N310, N311, N312, N318, N319), urinary incontinence (N393, N394), and cystocele (N811), and data for prescriptions of alpha-adrenergic antagonists, 5-alpha-reductase inhibitors, anticholinergic agents, and antidiuretic agents entered at all medical institutions during the observational period. We also analyzed data on the number of surgical procedures related to voiding dysfunction performed at all medical institutions during the observational period. Prostate operations included prostatectomy, transurethral resection, photoselective vaporization, and holmium laser enucleation. The anti-incontinence surgical procedures of interest included slings, suspensions, injectables, prosthetics, and reconstructions.
Statistical analyses
Data are expressed as mean values (standard deviation), geometric mean values (95% confidence interval [CIs]), or percentages. Comparisons were made among the four groups according to BMI and metabolic health status using one-way analysis of variance or the chi-square tests. The hazard ratios (HRs) and 95% CIs were analyzed using Cox proportional hazards models, with the MHNO group as a reference. The proportional-hazard assumption was assessed using the logarithm of the cumulative hazards function based on Kaplan–Meier estimates for each group. Multivariable-adjusted proportional hazards models were constructed. A P value < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
References
Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 943162. https://doi.org/10.1155/2014/943162 (2014).
Kupelian, V. Nocturia, sleep disturbance and the metabolic syndrome. J. Urol. 188, 2041–2042. https://doi.org/10.1016/j.juro.2012.09.064 (2012).
Araujo, A. B. et al. Sleep related problems and urological symptoms: testing the hypothesis of bidirectionality in a longitudinal, population based study. J. Urol. 191, 100–106. https://doi.org/10.1016/j.juro.2013.07.011 (2014).
Denys, M. A., Anding, R., Tubaro, A., Abrams, P. & Everaert, K. Lower urinary tract symptoms and metabolic disorders: ICI-RS 2014. Neurourol. Urodyn. 35, 278–282. https://doi.org/10.1002/nau.22765 (2016).
Rohrmann, S., Smit, E., Giovannucci, E. & Platz, E. A. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int. J. Obes. (Lond) 29, 310–316. https://doi.org/10.1038/sj.ijo.0802881 (2005).
Mondul, A. M., Giovannucci, E. & Platz, E. A. A prospective study of obesity, and the incidence and progression of lower urinary tract symptoms. J. Urol. 191, 715–721. https://doi.org/10.1016/j.juro.2013.08.110 (2014).
Lee, W. C. et al. Effects of diabetes on female voiding behavior. J. Urol. 172, 989–992. https://doi.org/10.1097/01.ju.0000136255.83054.0c (2004).
Mommsen, S. & Foldspang, A. Body mass index and adult female urinary incontinence. World J. Urol. 12, 319–322. https://doi.org/10.1007/bf00184112 (1994).
Pajunen, P. et al. Metabolically healthy and unhealthy obesity phenotypes in the general population: the FIN-D2D Survey. BMC Public Health 11, 754. https://doi.org/10.1186/1471-2458-11-754 (2011).
Yang, H. K. et al. Obesity, metabolic health, and mortality in adults: A nationwide population-based study in Korea. Sci. Rep. 6, 30329. https://doi.org/10.1038/srep30329 (2016).
Lee, K. Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: Characteristics and health behaviors. Asia Pac. J. Clin. Nutr. 18, 280–284 (2009).
Ford, E. S. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care 28, 1769–1778. https://doi.org/10.2337/diacare.28.7.1769 (2005).
Keto, C. J., Masko, E. M. & Freedland, S. J. Physical activity, obesity, and lower urinary tract symptoms. Eur. Urol. 60, 1181–1183. https://doi.org/10.1016/j.eururo.2011.08.012 (2011) (discussion 1183).
Rohrmann, S., Smit, E., Giovannucci, E. & Platz, E. A. Associations of obesity with lower urinary tract symptoms and noncancer prostate surgery in the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 159, 390–397. https://doi.org/10.1093/aje/kwh060 (2004).
Parsons, J. K., Sarma, A. V., McVary, K. & Wei, J. T. Obesity and benign prostatic hyperplasia: Clinical connections, emerging etiological paradigms and future directions. J. Urol. 182, S27-31. https://doi.org/10.1016/j.juro.2009.07.086 (2009).
Gacci, M. et al. Metabolic syndrome and benign prostatic enlargement: A systematic review and meta-analysis. BJU Int. 115, 24–31. https://doi.org/10.1111/bju.12728 (2015).
Dahle, S. E. et al. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J. Urol. 168, 599–604 (2002).
Ozden, C. et al. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol 51, 199–203. https://doi.org/10.1016/j.eururo.2006.05.040 (2007) (discussion 204-196).
Hammarsten, J., Hogstedt, B., Holthuis, N. & Mellstrom, D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1, 157–162. https://doi.org/10.1038/sj.pcan.4500221 (1998).
Bunn, F. et al. Is there a link between overactive bladder and the metabolic syndrome in women? A systematic review of observational studies. Int. J. Clin. Pract. 69, 199–217. https://doi.org/10.1111/ijcp.12518 (2015).
Moul, S. & McVary, K. T. Lower urinary tract symptoms, obesity and the metabolic syndrome. Curr. Opin. Urol. 20, 7–12. https://doi.org/10.1097/MOU.0b013e3283336f3f (2010).
Chen, J. et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann. Intern. Med. 140, 167–174. https://doi.org/10.7326/0003-4819-140-3-200402030-00007 (2004).
Garshick, E. et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 43, 408–416. https://doi.org/10.1038/sj.sc.3101729 (2005).
Semins, M. J., Shore, A. D., Makary, M. A., Weiner, J. & Matlaga, B. R. The impact of obesity on urinary tract infection risk. Urology 79, 266–269. https://doi.org/10.1016/j.urology.2011.09.040 (2012).
Saliba, W., Barnett-Griness, O. & Rennert, G. The association between obesity and urinary tract infection. Eur. J. Intern. Med. 24, 127–131. https://doi.org/10.1016/j.ejim.2012.11.006 (2013).
Fuselier, A., Hanberry, J., Margaret Lovin, J. & Gomelsky, A. Obesity and stress urinary incontinence: Impact on pathophysiology and treatment. Curr. Urol. Rep. 19, 10. https://doi.org/10.1007/s11934-018-0762-7 (2018).
Lee, U. J., Kerkhof, M. H., van Leijsen, S. A. & Heesakkers, J. P. Obesity and pelvic organ prolapse. Curr. Opin. Urol. 27, 428–434. https://doi.org/10.1097/MOU.0000000000000428 (2017).
Zhang, R. et al. Lifestyle and risk of chronic prostatitis/chronic pelvic pain syndrome in a cohort of United States male health professionals. J. Urol. 194, 1295–1300. https://doi.org/10.1016/j.juro.2015.05.100 (2015).
Song, S. O. et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab. J. 38, 395–403. https://doi.org/10.4093/dmj.2014.38.5.395 (2014).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 46, e15. https://doi.org/10.1093/ije/dyv319 (2017).
Lee, Y. H. et al. Data analytic process of a nationwide population-based study using national health information database established by National Health Insurance Service. Diabetes Metab. J. 40, 79–82. https://doi.org/10.4093/dmj.2016.40.1.79 (2016).
Author information
Authors and Affiliations
Contributions
Research conception & design was suggested by J.H.H. and Y.G.L. J.K.K. and K.H. conducted to Acquisition of data, Data analysis and interpretation. J.H.H. and J.K.K. wrote the main manuscript with figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J.K., Lee, Y.G., Han, K. et al. Obesity, metabolic health, and urological disorders in adults: a nationwide population-based study. Sci Rep 11, 8687 (2021). https://doi.org/10.1038/s41598-021-88165-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88165-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.