Abstract
The actin binding protein drebrin plays a key role in dendritic spine formation and synaptic plasticity. Decreased drebrin protein levels have been observed in temporal lobe epilepsy, suggesting the involvement of drebrin in the disease. Here we investigated the effect of drebrin knockout on physiological and pathophysiological neuronal network activities in mice by inducing gamma oscillations, involved in higher cognitive functions, and by analyzing pathophysiological epileptiform activity. We found that loss of drebrin increased the emergence of spontaneous gamma oscillations suggesting an increase in neuronal excitability when drebrin is absent. Further analysis showed that although the kainate-induced hippocampal gamma oscillations were unchanged in drebrin deficient mice, seizure like events measured in the entorhinal cortex appeared earlier and more frequently. The results suggest that while drebrin is not essential for normal physiological network activity, it helps to protect against the formation of seizure like activities during pathological conditions. The data indicate that targeting drebrin function could potentially be a preventive or therapeutic strategy for epilepsy treatment.
Similar content being viewed by others
Introduction
Epilepsy is a disease of high prevalence (~ 1%) with a third of the patients having pharmacoresistant seizures intractable to available treatments1. In addition, people with epilepsy often have long-term cognitive impairments such as memory loss, learning disabilities and behavioral disorders frequently correlating with the frequency and severity of epilepsy2. Current antiepileptic drugs symptomatically suppress the seizures without affecting the underlying mechanisms of epileptogenesis and brain injury. Comorbidities of epilepsy, such as cognitive impairments, are also rarely targeted by antiepileptic strategies, although they can be as disabling as the seizures themselves1.
Dendritic abnormalities have been increasingly observed in both epilepsy patients and animal models3. After seizures, a transient beading of the dendrites occurs followed by a more persistent loss of dendritic spines4,5. This dendritic spine loss is well documented also in neocortex distant from the epileptic focus6 but it is still not clear what is the role of these dendritic abnormalities in promoting epileptogenesis. One possibility is that spine loss is epileptogenetic and enhances the probability of future seizures by disturbing the fine-tuned balance between excitatory and inhibitory circuits, especially when inhibitory inputs are more affected. However, it is also possible that a loss of spines and synapses is a consequence of the seizures with a beneficial role in suppressing seizures, by inhibiting synaptic transmission and the propagation of seizures in the brain.
The cytoskeletal protein actin, existing in depolymerized monomeric (G-actin) and stable filamentous form (F-actin), plays a major role in generating the structural support for dendrites and spines. The organization and turnover of actin filaments within dendritic spines is modulated by actin-binding proteins, such as the developmentally regulated brain protein (drebrin)7. In its function as actin filament modulator, drebrin stabilizes actin filaments and inhibits their depolymerisation in spines7.
Several studies have reported changes in synaptic transmission and plasticity following drebrin down regulation or drebrin loss. Downregulation of drebrin reduces dendritic spine density, alters spine morphogenesis and inhibits both glutamatergic and GABAergic synaptic transmission in cultured hippocampal neurons8,9,10. Drebrin knock out mice as well as depletion of a splice variant of drebrin in mice has been shown to cause LTP impairment combined with decreased spine density11,12. Additionally, depletion of one of the splice variants of drebrin show impaired context dependent fear conditioning12. We recently generated a drebrin deficient mouse line and observed no changes in spine morphogenesis or glutamatergic transmission in young adults, suggesting that loss of drebrin alone is not sufficient to induce glutamatergic synaptic dysfunction13. We surmised that a specific drebrin KO phenotype only becomes evident under certain pathophysiological conditions where the loss of drebrin cannot be compensated.
Decreased levels of drebrin in the brain were shown in states with high epilepsy prevalence14 such as Alzheimer’s disease15 and Down's syndrome16 and a lower drebrin level in the hippocampi of temporal lobe epilepsy patients was associated with more frequent seizures17. Decrease and reactivation of drebrin expression in post-status epilepsy models have been described already18,19,20, but it still remains unclear whether the changes are pro- or antiepileptogenic and if they are the consequence of the seizures or play a role in the epileptogenesis.
Here we measured the effect of drebrin ablation on increased neuronal network activity in physiological and pathological conditions. First, we induced gamma oscillations in hippocampal slices from wild type (WT) and drebrin knockout (KO) mice. Gamma oscillations represent physiological synchronization of neuronal activity at frequencies between 30 and 90 Hz and are associated with higher cognitive tasks such as sensory processing, working memory, attention, learning and memory21,22. Second, we induced epileptiform activity in the entorhinal cortex in vitro23,24 and analyzed whether the loss of drebrin affects the emergence of epileptiform discharges. We found that drebrin ablation was largely compensated in neural networks when activity remained in a physiological frame; however, networks without drebrin developed seizure-like events with shorter onset latency and higher incidence.
Results
Drebrin loss does not alter gamma oscillations in hippocampal slices
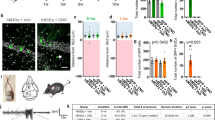
Occurring in different regions of the brain, gamma oscillations are physiological rhythmic fluctuations of field potentials enabling the temporal synchronization of neuronal activity within and across groups of neurons. In the cortex and hippocampus, they are generated by recurrent rhythmic synaptic connections between pyramidal cells and perisomatic parvalbumin-containing basket cells25. Drebrin is expressed in the dendrites of both glutamatergic and GABAergic neurons at the site of excitatory synapses26. Given that drebrin is a regulator of synaptic transmission and that changes in synaptic morphology are associated with memory consolidation, we tested if loss of drebrin influences the development of gamma oscillations in the CA3 region of the hippocampus, where gamma oscillations are primarily generated and have highest amplitudes27. We induced gamma oscillations by bath application of kainate (KA, 500 nM) onto hippocampal slices from WT and KO mice (Fig. 1). The responder rate of the slices was comparable in WT and KO animals (WT: 75.0%; KO: 68.8%; p = 0.782, not shown). The induced oscillations had similar peak power from WT (geometric mean: 1.35 µV2, SD factor: 8.21, n = 24) and KO animals (1.50 µV2, SD factor: 7.96, n = 22) without statistical difference between the two groups (p = 0.76; Fig. 1c). Similarly, there was no difference in peak frequency (Fig. 1d), Q factor (WT: 8.19 ± 1.21; KO 8.57 ± 1.44; p = 0.84; not shown) and half bandwidth of the oscillations (WT: 8.6 ± 2.1 Hz; KO: 6.8 ± 1.0 Hz; p = 0.45; not shown).
Effect of drebrin loss on gamma oscillations in the CA3 area of the hippocampus. (a,b) Original traces (left) and power spectra (right) before and after induction of gamma oscillations by kainate (KA; 500 nM) in slices from wildtype (WT; a) and drebrin knockout (KO; b) mice. Traces were lowpass filtered at 200 Hz. In a fraction of the slices, spontaneous gamma oscillations emerged before the application of KA. (c) Absolute power of induced gamma oscillations in WT (black) and drebrin KO (red) mice. (d) Peak frequency of induced gamma oscillations in WT (black) and drebrin KO (red) mice. WT: 35.1 ± 0.9 Hz, n = 24; KO: 37.3 ± 1.1 Hz, n = 22; p = 0.11. (e) Power of spontaneous gamma oscillations in WT (black) and drebrin KO (red) mice. Geometric mean of power in WT: 0.030 µV2, SD factor: 2.19, n = 21; KO: 0.045 µV2, SD factor: 3.3, n = 12, p = 0.27. (f) Fraction of slices generating spontaneous oscillations in WT and drebrin KO. WT: 3/24 (12.5%), KO: 10/22 (45.5%), p = 0.021. (g) Drebrin expression in hippocampal slices responding or not to KA-induced gamma oscillations. Hippocampal slices, previously stimulated with KA to develop gamma oscillations, were probed for drebrin proteins. We compared responder (gamma; grey) to non-responder (no gamma; black) slices. All samples were run on the same gel and transferred on the same blot. Data shown are representative of 17 slices from 4 experiments. Western blot band densitometry revealed no altered drebrin levels in kainate induced gamma oscillations tissue compared to no gamma oscillations (gamma: drebrin/tubulin: 0.75 ± 0.05, n = 17; no gamma: drebrin/tubulin: 0.84 ± 0.15, n = 6; p = 0.59). Bars show mean ± SEM. * p < 0.05. Full-length blots are presented in Supplementary Fig. 1.
Similar to the induced gamma power, we observed no differences in spontaneous network activity measured prior to the induction of oscillations with KA in WT and KO mice (Fig. 1a,b,e). In a fraction of slices, spontaneous oscillations were recorded before the application of KA. Similar to the induced gamma oscillation, the peak power of these spontaneous oscillations did not differ between WT and KO (geometric mean WT: 0.08 µV2, SD factor: 1.75, n = 3; KO: 0.126 µV2, SD factor: 4.09, n = 10; p = 0.63, not shown). Likewise, we did not find any differences in the peak frequency, half bandwidth and Q factor of the spontaneous oscillations between WT and KO (frequency WT: 29.7 ± 1.3; KO: 33.1 ± 1.4; p = 0.24; half bandwidth WT: 19.2 ± 8.8; KO: 15.3 ± 3.0, p = 0.59; Q factor WT: 3.0 ± 1.8; KO: 3.2 ± 0.8, p = 0.91, not shown). Interestingly, however, the fraction of slices showing spontaneous gamma oscillations in drebrin KO mice were significantly higher suggesting an increase in neuronal excitability when drebrin is absent (Fig. 1f).
In summary, our results indicate that drebrin loss does not influence the amplitude of gamma oscillations in the hippocampus, however, increased the likelihood of spontaneous oscillation generation in brain slices.
Gamma oscillations do not alter drebrin protein levels in hippocampal slices
We previously demonstrated that regulation of drebrin protein stability is linked to increased neuronal activity and may protect from synaptic dysfunction28,29. We therefore investigated whether the emergence of gamma oscillations altered the protein levels of drebrin in hippocampal slices from WT mice. Quantitative assessments by western blotting showed no difference in the amount of drebrin protein following development of gamma oscillations compared to slices where no oscillations were induced. These results indicate that permanent gamma oscillations lasting for more than three hours did not influence drebrin protein expression in the hippocampus (Fig. 1g).
Drebrin loss favors the development of epileptiform activity in the medial entorhinal cortex
While drebrin KO did not affect the physiological gamma oscillations, we observed more frequent development of spontaneous oscillations before the pharmacological induction suggesting an increased excitability in hippocampal slices. In following experiments, therefore, we investigated whether the loss of drebrin affects the development of pathophysiological epileptiform activity in the medial entorhinal cortex. This area is characterized by generation of seizure-like events (SLEs) with the lowest threshold within the temporal lobe30,31,32. We induced seizure-like activity by omitting Mg2+ from the ACSF and observed that SLEs appeared within tens of minutes after induction. SLEs further converted into continuous late recurrent discharges (LRD) with continued omission of Mg2+ from the bath solution (Fig. 2).
Epileptiform activity of WT and drebrin KO mice in medial entorhinal cortex slices induced by omission of Mg2+ from the bath solution. (a,b) Original field potential traces of seizure-like events (SLEs) characterized by negative potential shifts superimposed by high-frequency oscillations developed after the onset latency (first SLEs marked with an arrow) in WT (a) and drebrin KO (b) animals. (c) Ongoing omission of Mg2+ from the bath resulted in conversion of the SLEs into continuous late recurrent discharges (LRDs) after the LRD onset latency (marked with #). (d) Duration of one SLE. (e) Proportion of slices developing seizure-like-events (SLE; left; WT: 87.5% (21 out of 24) of the slices, KO: 100% (27 out of 27) of the slices; p = 0.10 compared to WT) and continuous late recurrent discharges (LRD; right; WT: 70.8% (17 out of 24), KO: 92.6% (25 out of 27); p = 0.07 compared to WT) after removing Mg2+ from the bath solution (f) Drebrin KO shortened the SLE onset latency (WT (black): 32.8 ± 4.4 min, n = 21; KO (red): 20.2 ± 1.5 min, n = 27; p = 0.01). (g) Drebrin KO increased SLE incidence. WT: 0.152 ± 0.019 min−1, n = 21, KO: 0.247 ± 0.035 min−1, n = 26, p = 0.03. (h) Drebrin KO did not change the duration of single SLEs (WT: 69.9 ± 7.3 s, n = 21, KO: 68.6 ± 4.4 s, n = 27; p = 0.88 compared to WT). (i) Drebrin KO did not affect the LRD onset latency (WT: 72.8 ± 7.7 min, n = 17, KO: 80.5 ± 8.0 min, n = 25, p = 0.51 compared to WT). Bar graphs show mean ± SEM. *p < 0.05.
Analyzing the fraction of slices developing SLEs and LRDs in all investigated entorhinal cortex slices revealed that SLEs and LRDs appeared more often in KO compared to WT animals, although the difference was not significant (SLE: p = 0.09, LRD: p = 0.07 compared to WT; Fig. 2e). However, the onset of SLEs was significantly shorter (Fig. 2a,b,f) and SLEs were more frequent in KO slices compared to WT (Fig. 2g). SLE duration did not differ between WT and drebrin KO slices (Fig. 2h). In contrast to the SLEs, development of LRDs (conversion latency of SLE into LRD) was not different between WT and KO animals (Fig. 2i). These results suggest that while physiological network activity was not affected by drebrin loss, seizure-like activity appeared earlier and more often in drebrin KO mice suggesting that the neural networks without drebrin are more susceptible to develop pathophysiological epileptiform activity.
Epileptiform activity does not alter drebrin protein levels in medial entorhinal cortex slices
Previous studies describe decrease in drebrin protein levels in response to KA- or pilocarpin induced seizures18,19,20. We thus set out to investigate whether the emergence of SLEs and LRDs has an impact on drebrin protein levels in the entorhinal cortex of WT mice. SLEs or LRDs did not alter drebrin levels compared to slices without epileptiform activity during the time window of over three hours of recordings (Fig. 3).
Drebrin expression following epileptiform activity in the medial entorhinal cortex. (a) Representative western blot shows levels of drebrin and tubulin in slices that previously developed seizure like events (SLEs) with or without late recurrent discharges (LRDs). All samples were run on the same gel and transferred on the same blot. Separation with a white line indicates cropping of the same blot. (b) Quantification of the drebrin/tubulin levels shows no effect of seizure-like activities on drebrin expression (drebrin/tubulin: no seizure (black): 1.0 ± 0.13, n = 6, SLE + LRD (light grey): 1.09 ± 0.07, n = 16, p = 0.54, only SLE (dark grey): 1.04 ± 0.07, n = 4, p = 0.84). Full-length blots are presented in Supplementary Fig. 2.
Discussion
We have investigated the effects of drebrin loss on physiological and pathophysiological network activities in hippocampal and entorhinal cortex slices, respectively. While drebrin KO did not alter hippocampal gamma oscillations, it enhanced the susceptibility of entorhinal cortex slices to develop SLEs. In drebrin KO mice, SLEs developed faster and with higher incidence compared to WT mice. Although previous studies reported a decrease in drebrin protein and mRNA levels in epilepsy pathologies18,19,20, we did not find alteration of drebrin expression following in vitro physiological or pathophysiological network activities. The results suggest that drebrin could play a role in controlling the excitation in neuronal circuits and the development of pathological network activity. The results support previous studies showing that drebrin deficiency alone is not sufficient in causing synaptic dysfunction in physiological conditions13 and may require additional burdens such as increased excitability to observe a drebrin deficient phenotype.
Our findings demonstrate that gamma oscillations do not differ in drebrin KO animals from those in WT mice. In addition, the induction of gamma oscillations in WT animals did not affect drebrin protein amount in the hippocampal slice, suggesting that drebrin neither modifies gamma oscillations nor gets modified by the evoked oscillatory activity. Although drebrin is expressed in both excitatory and inhibitory neurons26 and drebrin downregulation inhibits both glutamatergic and GABAergic synaptic transmission8,9,10 our data suggest that loss of drebrin does not affect normal physiological neuronal activity such as gamma oscillations. The lack of change observed for gamma oscillations in drebrin deficient mice may be due to the presence of compensatory mechanisms by other actin binding proteins enabling activation of alternative pathways to safeguard actin cytoskeleton dynamics. In this case an abnormal phenotype may only be observed in conditions when drebrin cannot be compensated for such as disease or stress.
In these lines, our results further indicate that drebrin loss augmented the susceptibility of neurons to develop seizure-like activity. The results suggest that neuronal networks with no drebrin expression are more likely to develop SLEs. Pathological network events such as hypersynchronized epileptiform activity also depend on GABAergic interneurons. One function of interneurons under normal conditions is to restrain seizure activity by feed-forward inhibition33 and failure of this restraint can lead to faster spread of seizures in the brain. GABAergic interneurons are very diverse and at present, no data are available on the expression of drebrin in different interneuron cell types in the brain. However, the loss of drebrin may alter the development of neuronal circuits leading to an imbalance between excitation and inhibition, increased excitability and easier seizure evolution. This is corroborated by our finding that drebrin loss increases the probability of the CA3 network to generate gamma oscillations without exogenous induction. Interestingly, in a recent publication, authors detected anti-drebrin autoantibodies in patients with adult onset epilepsy and suspected encephalitis. Exposure of hippocampal neurons to anti-drebrin autoantibodies resulted in aberrant drebrin distribution within neurons and network hyperexcitability34. These results are in accordance with our results and suggest that drebrin dysfunction can lead to impaired synaptic connectivity and increased seizure activity.
There is increasing evidence for dendritic spine abnormalities in the epileptic neocortex and hippocampus, including changes in both structure and number of dendritic spines. Dendritic spine abnormalities were observed in neurodegenerative diseases that have an increased risk of seizures such as Alzheimer’s disease35 and juvenile Huntington’s disease36. Genetic disorders with dendritic spine abnormalities were also documented to have a high epilepsy prevalence14. Among them, Fragile X syndrome, Rett syndrome, tuberous sclerosis and Down syndrome are all characterized by altered spine morphology and, with the exception of Fragile X syndrome, a decreased spine density16,37,38,39. Spine loss and swelling of dendrites have been frequently observed in neocortical and hippocampal pyramidal cells in patients with temporal lobe epilepsy4,6. Morphological and structural changes of spines are tightly coupled to reorganization of the actin cytoskeleton mediated by actin-binding proteins40,41,42. In accordance with this, epilepsy is associated with detectable changes in the expression of different actin-binding or their upstream proteins. KA-induced seizures have been shown to activate the actin-depolymerizing protein cofilin and cause a loss of stable actin filaments43. Human temporal lobe epilepsy is also associated with decreased expression of reelin, a cofilin phosphorylating and inactivating protein in the hippocampus44. Profilin, a protein essential for actin polymerization was also found to be reduced in the hippocampus of temporal lobe epilepsy patients45. These findings suggest that epilepsy patients have less stable actin filaments within their spines. In line with this, a recent study found that lower drebrin levels in the hippocampi of temporal lobe epilepsy patients were associated with higher seizure frequency and less neuron survival17. These dendritic changes may represent a trait in the pathophysiology of seizure development or a consequence of them or even a compensatory response as a form of homeostatic plasticity to dampen excessive neuronal excitability14. Our data on drebrin KO mice suggest that less stable actin filaments in the spines increase the excitability and the probability of seizure-like activity. On the contrary, we did not observe alterations in drebrin expression in the entorhinal cortex after three hours of seizure-like activity, however, we investigated drebrin levels in the whole slice and cannot exclude local or subcellular alterations of drebrin levels after seizures in the hippocampal formation. Previous studies on animal models reported decreased drebrin expression in the hippocampus two hours after systemic KA- or pilocarpin induced seizures18,19,20, possibly by ERK-mediated phosphorylation and activation of the calcium-dependent phosphatase calpain-220. The initial reduction was followed by the recovery of drebrin levels in the chronic phase of pilocarpine-induced seizures suggesting the crucial role of drebrin in reactive synaptic plasticity18,19. Another recent study, showed a negative correlation between drebrin expression and seizure frequency in particular in the dentate gyrus17. Taken together it is difficult to conclude on a linear correlation between drebrin expression and seizure susceptibility. Another possibility is that drebrin may be spatially and temporally regulated in response to seizure activity. In these lines, several studies showed changes in drebrin distribution following high-frequency stimulation in vivo, activation of NMDA receptor or induction of LTP with glutamate uncaging40,46,47. Interestingly, induction of LTP using glutamate uncaging, led to an initial decrease in drebrin concentration in the dendritic spine when the spine head volume increases, then later drebrin reenters the spine during the phase of actin stabilization40. This highly dynamic distribution of drebrin during synaptic activity suggests that drebrin may also change its localization to regulate actin dynamics in the dendritic spine in response to seizure activity to help protect the synapse from dysfunction.
In conclusion, our data demonstrate, in line with previous results on the same drebrin KO mice13, that loss of the dendritic spine enriched actin-binding protein drebrin does not alter physiological oscillatory activity. On the contrary, we found that drebrin loss increases the susceptibility of the neuronal network to develop epileptiform discharges. The results confirm that stable actin filaments might have a protecting effect against seizures.
Experimental procedures
Animals and slice preparation
DBN KO mice were generated as previously described13. Ten Drebrin-KO and 10 wildtype B6/N mice of both sexes were used at an age of 8–12 weeks in the study. Animal procedures were conducted in accordance with the guidelines of the European Communities Council approved by the Berlin Animal Ethics Committee (Landesamt für Gesundheit und Soziales Berlin, T0347/11). The mice were anesthetized with isoflurane and then decapitated. Their brains were removed from the skull and immersed in ice-cold carbogenated (95% O2/5% CO2) sucrose-based solution with an osmolarity of ~ 330 mosmol/kg containing (in mM): NaCl, 80; NaHCO3, 25; NaH2PO4, 1.25; KCl, 2.5; glucose, 25; sucrose, 85; CaCl2, 0.5; MgCl, 3. The brain was cut into 400 μm thick horizontal slices containing both the hippocampus and the entorhinal cortex at an angle of about 13° in the fronto-occipital direction with a vibratome (DSK microslicer DTK-1000, Dosaka, Japan). Slices were immediately transferred to an interface-type recording chamber perfused with carbogenated, warm (36 ± 0.2 °C) artificial cerebrospinal fluid (ACSF; flow rate of 1.7 ± 0.1 ml/min; osmolarity of ~ 300 mosmol/kg) of the following composition (in mM): NaCl, 129; NaH2PO4, 1.25; NaHCO3, 21; glucose, 10; MgSO4, 1.8; CaCl2, 1.6; KCl, 3. Slices were incubated for at least one hour before starting recordings.
Electrophysiology
Local field potentials (LFPs) were recorded with glass pipettes filled with ACSF in the stratum pyramidale in CA3b of the hippocampus for recordings of gamma oscillations and in layers IV-V of the medial entorhinal cortex for seizure investigation. Recordings were amplified by an EXT-02 B amplifier (npi, Tamm, Germany), low-pass filtered at 1 kHz and sampled by a CED 1401 interface (Cambridge Electronic Design, Cambridge, UK) at 5 kHz. Gamma oscillations were recorded in AC whereas seizure investigation was done in DC recording mode. For the induction of gamma oscillations in hippocampal slices, kainate (KA, 500 nM, Tocris Bioscience, Bristol, UK) was administered by bath application for 200 min48. To induce epileptiform activity in medial entorhinal cortex slices, Mg2+ was omitted from the bath solution until the end of the recording (190–200 min)23,24.
Western blot
Following the electrophysiological recordings, slices were harvested, snap frozen and stored at -80° until needed. Each slice was lysed in 100 µl Ripa Buffer (50 mM Tris–HCL pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP40, 0.1% SDS) supplemented with protease inhibitors (Calbiochem set III) and phosphatase inhibitors (1 mM Na2MO4,1 mM NaF, 20 mM β-glycerophosphate, 1 mM Na3V04, 500 nM Cantharidin) using a tissue homogenizer (Minilys, Bertin instruments). Homogenates were centrifuged at 20,000 G and supernatant was collected for further protein quantification analysis using BCA Thermo Scientific Pierce Protein Assay. 30 µg of protein was loaded on an SDS-PAGE gel and western blot analysis was performed as previously described49. Anti-Drebrin (M2F6, Enzo) was used at a concentration of 1:1000 and anti-α-Tubulin (Sigma) was used in 1:8000. Quantification of band densities was performed using FIJI. The area of the band and the mean grey value were measured to obtain a relative density. For relative quantifications, measurements were normalized to tubulin loading control.
Data evaluation and statistics
This study was conducted in accordance with the ARRIVE guidelines50. Gamma oscillations were analyzed by calculating power spectra with a 120-s window every 2 min during the whole recording. Spontaneous activity was computed as the peak power between 20 and 50 Hz during a period of 10 min before induction of the oscillations with KA. In some recordings, spontaneous oscillations were observed during this pre-induction period. Network activity was considered an oscillation when the power spectrum had a peak between 30 and 80 Hz and the Q (quality) factor (frequency/half bandwidth) of the oscillation was higher than the subcritical 0.551. The Q factor measures the periodicity of oscillations independently of the peak frequency (where a high Q factor indicates a sharply distributed oscillation in the power spectrum around the peak frequency and a more periodic, predictable and less dampened oscillation)52. KA-induced gamma oscillations were analyzed 20–30 min after induction. Peak power, peak frequency, half bandwidth (at 50% of peak power), and Q factor of the oscillations were extracted by using a custom-made script for the Spike2 software (Cambridge Electronic Design, Cambridge, UK)51,53. D'Agostino-Pearson normality test was used to test the Gaussian distribution of the data. Peak power was found to be distributed lognormally, therefore is represented as geometric mean and geometric SD factor54. All other normally distributed parameters are presented as (arithmetic) mean ± SEM. The calculated parameters in the WT and KO group were compared with the Student’s t-test. The lognormal distributed power values were first transformed to the logarithms and the logs were analyzed statistically.
Epileptiform activity was analyzed by calculating the following parameters. Seizure onset latency was calculated as the time until the first seizure-like event (SLE) after omitting Mg2+ from the extracellular solution55. The time until the first appearance of late recurrent discharges (LRD) after zero Mg2+ application was used as onset latency of LRD. After appearance, SLEs were analyzed for their incidence (events/min) and duration56,57. Statistical comparison of epileptiform activity parameters was done by Student’s t-test. Fisher's exact test was used to compare the fractions of slices developing gamma oscillations or seizure-like activities. Significance level was set at p < 0.05.
Data availability
The data that support the findings of this study are contained within the article. Not shown data are available on request from the corresponding authors ZG and PK.
References
Devinsky, O. et al. Epilepsy. Nat. Rev. Dis. Primers. 4, 1–24. https://doi.org/10.1038/nrdp.2018.24 (2018).
Elger, C. E., Helmstaedter, C. & Kurthen, M. Chronic epilepsy and cognition. Lancet Neurol. 3, 663–672. https://doi.org/10.1016/S1474-4422(04)00906-8 (2004).
Wong, M. Stabilizing dendritic structure as a novel therapeutic approach for epilepsy. Expert Rev. Neurother. 8, 907–915. https://doi.org/10.1586/14737175.8.6.907 (2008).
Belichenko, P. V. & Dahlström, A. Studies on the 3-dimensional architecture of dendritic spines and varicosities in human cortex by confocal laser scanning microscopy and Lucifer Yellow microinjections. J. Neurosci. Methods 57, 55–61. https://doi.org/10.1016/0165-0270(94)00125-Z (1995).
Jiang, M., Lee, C. L., Smith, K. L. & Swann, J. W. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset epilepsy. J. Neurosci. 18, 8356–8368. https://doi.org/10.1523/JNEUROSCI.18-20-08356.1998 (1998).
Multani, P., Myers, R. H., Blume, H. W., Schomer, D. L. & Sotrel, A. Neocortical dendritic pathology in human partial epilepsy: A quantitative golgi study. Epilepsia 35, 728–736. https://doi.org/10.1111/j.1528-1157.1994.tb02503.x (1994).
Mikati, M. A., Grintsevich, E. E. & Reisler, E. Drebrin-induced stabilization of actin filaments. J. Biol. Chem. 288, 19926–19938. https://doi.org/10.1074/jbc.M113.472647 (2013).
Takahashi, H. et al. Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J. Neurosci. 23, 6586–6595. https://doi.org/10.1523/JNEUROSCI.23-16-06586.2003 (2003).
Takahashi, H., Mizui, T. & Shirao, T. Down-regulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurones. J. Neurochem. 97, 110–115. https://doi.org/10.1111/j.1471-4159.2005.03536.x (2006).
Ivanov, A., Esclapez, M., Pellegrino, C., Shirao, T. & Ferhat, L. Drebrin A regulates dendritic spine plasticity and synaptic function in mature cultured hippocampal neurons. J. Cell Sci. 122, 524–534. https://doi.org/10.1242/jcs.033464 (2009).
Jung, G. et al. Drebrin depletion alters neurotransmitter receptor levels in protein complexes, dendritic spine morphogenesis and memory-related synaptic plasticity in the mouse hippocampus. J. Neurochem. 134, 327–339. https://doi.org/10.1111/jnc.13119 (2015).
Kojima, N. et al. Genetic disruption of the alternative splicing of drebrin gene impairs context-dependent fear learning in adulthood. Neuroscience 165, 138–150. https://doi.org/10.1016/j.neuroscience.2009.10.016 (2010).
Willmes, C. G. et al. Investigation of hippocampal synaptic transmission and plasticity in mice deficient in the actin-binding protein Drebrin. Sci. Rep. 7, 1–11. https://doi.org/10.1038/srep42652 (2017).
Wong, M. & Guo, D. Dendritic spine pathology in epilepsy: Cause or consequence?. Neuroscience 251, 141–150. https://doi.org/10.1016/j.neuroscience.2012.03.048 (2013).
Harigaya, Y., Shoji, M., Shirao, T. & Hirai, S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer’s disease. J. Neurosci. Res. 43, 87–92. https://doi.org/10.1002/jnr.490430111 (1996).
Suetsugu, M. & Mehraein, P. Spine distribution along the apical dendrites of the pyramidal neurons in Down’s syndrome—A quantitative golgi study. Acta Neuropathol. 50, 207–210. https://doi.org/10.1007/BF00688755 (1980).
Dombroski, T. C. et al. Drebrin expression patterns in patients with refractory temporal lobe epilepsy and hippocampal sclerosis. Epilepsia 00, 1–14. https://doi.org/10.1111/epi.16595 (2020).
Sbai, O., Khrestchatisky, M., Esclapez, M. & Ferhat, L. Drebrin A expression is altered after pilocarpine-induced seizures: Time course of changes is consistent for a role in the integrity and stability of dendritic spines of hippocampal granule cells. Hippocampus 22, 477–493. https://doi.org/10.1002/hipo.20914 (2012).
Ferhat, L. Potential role of drebrin A, an F-actin binding protein, in reactive synaptic plasticity after pilocarpine-induced seizures: Functional implications in epilepsy. Int. J. Cell Biol. 1–12, 2012. https://doi.org/10.1155/2012/474351 (2012).
Seinfeld, J., Baudry, N., Xu, X., Bi, X. & Baudry, M. Differential activation of calpain-1 and calpain-2 following kainate-induced seizure activity in rats and mice. eNeuro 3, 1–12. https://doi.org/10.1523/ENEURO.0088-15.2016 (2016).
Buzsáki, G. & Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 35, 203–225. https://doi.org/10.1146/annurev-neuro-062111-150444 (2012).
Bosman, C. A., Lansink, C. S. & Pennartz, C. M. A. Functions of gamma-band synchronization in cognition: From single circuits to functional diversity across cortical and subcortical systems. Eur. J. Neurosci. 39, 1982–1999. https://doi.org/10.1111/ejn.12606 (2014).
Mody, I., Lambert, J. D. & Heinemann, U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J. Neurophysiol. 57, 869–888. https://doi.org/10.1152/jn.1987.57.3.869 (1987).
Dreier, J. P. & Heinemann, U. Regional and time dependent variations of low Mg 2+ induced epileptiform activity in rat temporal cortex slices. Exp. Brain Res. 87, 581–596. https://doi.org/10.1007/BF00227083 (1991).
Fries, P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci. 32, 209–224. https://doi.org/10.1146/annurev.neuro.051508.135603 (2009).
Hanamura, K. et al. Low accumulation of drebrin at glutamatergic postsynaptic sites on GABAergic neurons. Neuroscience 169, 1489–1500. https://doi.org/10.1016/j.neuroscience.2010.06.043 (2010).
Wójtowicz, A. M. et al. Monoamines block kainate- and carbachol-induced γ-oscillations but augment stimulus-induced γ-oscillations in rat hippocampus in vitro. Hippocampus 19, 273–288. https://doi.org/10.1002/hipo.20508 (2009).
Kreis, P. et al. Phosphorylation of the actin binding protein drebrin at S647 is regulated by neuronal activity and PTEN. PLoS ONE 8, 1–12. https://doi.org/10.1371/journal.pone.0071957 (2013).
Kreis, P. et al. ATM phosphorylation of the actin-binding protein drebrin controls oxidation stress-resistance in mammalian neurons and C. elegans. Nat. Commun. 10, 1–13. https://doi.org/10.1038/s41467-019-08420-w (2019).
Bear, J. & Lothman, E. W. An in vitro study of focal epileptogenesis in combined hippocampal-parahippocampal slices. Epilepsy Res. 14, 183–193. https://doi.org/10.1016/0920-1211(93)90043-7 (1993).
Gloveli, T., Schmitz, D. & Heinemann, U. Interaction between superficial layers of the entorhinal cortex and the hippocampus in normal and epileptic temporal lobe. Epilepsy Res. 32, 183–193. https://doi.org/10.1016/S0920-1211(98)00050-3(Elsevier (1998).
Vismer, M. S., Forcelli, P. A., Skopin, M. D., Gale, K. & Koubeissi, M. Z. The piriform, perirhinal, and entorhinal cortex in seizure generation. Front. Neural Circ. 9, 27. https://doi.org/10.3389/fncir.2015.00027 (2015).
Schevon, C. A. et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat. Commun. https://doi.org/10.1038/ncomms2056 (2012).
Pitsch, J. et al. Drebrin autoantibodies in patients with seizures and suspected encephalitis. Ann. Neurol. 87, 869–884. https://doi.org/10.1002/ana.25720 (2020).
Amatniek, J. C. et al. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia 47, 867–872. https://doi.org/10.1111/j.1528-1167.2006.00554.x (2006).
Cloud, L. J. et al. Seizures in juvenile Huntington’s disease: Frequency and characterization in a multicenter cohort. Mov. Disord. 27, 1797–1800. https://doi.org/10.1002/mds.25237 (2012).
Belichenko, P. V., Oldfors, A., Hagberg, B. & Dahlström, A. Rett syndrome: 3-D confocal microscopy of cortical pyramidal dendrites and afferents. NeuroReport 5, 1509–1513. https://doi.org/10.1097/00001756-199407000-00025 (1994).
Machado-Salas, J. P. Abnormal dendritic patterns and aberrant spine development in Bourneville’s disease–a Golgi survey. Clin. Neuropathol. 3, 52–58 (1984).
Hinton, V. J., Brown, W. T., Wisniewski, K. & Rudelli, R. D. Analysis of neocortex in three males with the fragile X syndrome. Am. J. Med. Genet. 41, 289–294. https://doi.org/10.1002/ajmg.1320410306 (1991).
Bosch, M. et al. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82, 444–459. https://doi.org/10.1016/j.neuron.2014.03.021 (2014).
Rácz, B. & Weinberg, R. J. Microdomains in forebrain spines: An ultrastructural perspective. Mol. Neurobiol. 47, 77–89. https://doi.org/10.1007/s12035-012-8345-y (2013).
Takahashi, H. & Naito, Y. Drebrin and spine formation. Adv. Exp. Med. Biol. 1006, 157–181. https://doi.org/10.1007/978-4-431-56550-5_10 (2017).
Zeng, L.-H. et al. Kainate seizures cause acute dendritic injury and actin depolymerization in vivo. J. Neurosci. 27, 11604–11613. https://doi.org/10.1523/JNEUROSCI.0983-07.2007 (2007).
Haas, C. A. et al. Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J. Neurosci. 22, 5797–5802. https://doi.org/10.1523/JNEUROSCI.22-14-05797.2002 (2002).
Yang, J. W., Czech, T., Felizardo, M., Baumgartner, C. & Lubec, G. Aberrant expression of cytoskeleton proteins in hippocampus from patients with mesial temporal lobe epilepsy. Amino Acids 30, 477–493. https://doi.org/10.1007/s00726-005-0281-y (2006).
Fukazawa, Y. et al. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 38, 447–460. https://doi.org/10.1016/S0896-6273(03)00206-X (2003).
Sekino, Y. et al. Activation of N-methyl-d-aspartate receptor induces a shift of drebrin distribution: Disappearance from dendritic spines and appearance in dendritic shafts. Mol. Cell. Neurosci. 31, 493–504. https://doi.org/10.1016/j.mcn.2005.11.003 (2006).
Schulz, S. B., Klaft, Z. J., Rösler, A. R., Heinemann, U. & Gerevich, Z. Purinergic P2X, P2Y and adenosine receptors differentially modulate hippocampal gamma oscillations. Neuropharmacology 62, 914–924. https://doi.org/10.1016/j.neuropharm.2011.09.024 (2012).
Schrötter, S., Leondaritis, G. & Eickholt, B. J. Capillary isoelectric focusing of akt isoforms identifies highly dynamic phosphorylation in neuronal cells and brain tissue. J. Biol. Chem. 291, 10239–10251. https://doi.org/10.1074/jbc.M115.700138 (2016).
du Sert, N. P. et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biol. 18, 1–65. https://doi.org/10.1371/journal.pbio.3000411 (2020).
Lemercier, C. E., Holman, C. & Gerevich, Z. Aberrant alpha and gamma oscillations ex vivo after single application of the NMDA receptor antagonist MK-801. Schizophr. Res. 188, 118–124. https://doi.org/10.1016/j.schres.2017.01.017 (2017).
Meier, M. A. et al. The novel antipsychotic cariprazine stabilizes gamma oscillations in rat hippocampal slices. Br. J. Pharmacol. 177, 1622–1634. https://doi.org/10.1111/bph.14923 (2020).
Schulz, S. B. et al. First and second generation antipsychotics influence hippocampal gamma oscillations by interactions with 5-HT3 and D3 receptors. Br. J. Pharmacol. 167, 1480–1491. https://doi.org/10.1111/j.1476-5381.2012.02107.x (2012).
Kirkwood, T. B. L. Geometric means and measures of dispersion. Biometrics 35, 908–909 (1979).
Kovács, R., Kardos, J., Heinemann, U. & Kann, O. Mitochondrial calcium ion and membrane potential transients follow the pattern of epileptiform discharges in hippocampal slice cultures. J. Neurosci. 25, 4260–4269. https://doi.org/10.1523/JNEUROSCI.4000-04.2005 (2005).
Klaft, Z. J. et al. Extracellular ATP differentially affects epileptiform activity via purinergic P2X7 and adenosine A1 receptors in naive and chronic epileptic rats. Epilepsia 53, 1978–1986. https://doi.org/10.1111/j.1528-1167.2012.03724.x (2012).
Klaft, Z. J. et al. Adenosine A1receptor-mediated suppression of carbamazepine-resistant seizure-like events in human neocortical slices. Epilepsia 57, 746–756. https://doi.org/10.1111/epi.13360 (2016).
Acknowledgements
We thank Andrea Wilke and Luisa A. Hasam-Henderson for technical assistance and Zin-Juan Klaft and Richard Kovács for their comments on the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported by the DFG Grant 285933818.
Author information
Authors and Affiliations
Contributions
A.K. and P.K. collected and analyzed data, prepared figures and wrote parts of the manuscript. Z.G. and B.J.E. designed the study, helped with data acquisition and analysis and wrote parts of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klemz, A., Kreis, P., Eickholt, B.J. et al. The actin binding protein drebrin helps to protect against the development of seizure-like events in the entorhinal cortex. Sci Rep 11, 8662 (2021). https://doi.org/10.1038/s41598-021-87967-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87967-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.