Abstract
It is a great challenge to convert thermochemically stable CO2 into value-added products such as CH4, CH3OH, CO via utilizing solar energy. It is also a difficult task to develop an efficient catalyst for the reduction of CO2. We have designed and synthesized noble metal-free photocatalytic nanostructure Ni2P/CdS and Pt/TiO2 for conversion of CO2 to methanol in the presence of sacrificial donor triethylamine (TEA) and hydrogen peroxide. The synthesised catalysts physicochemical properties were studied by using several spectroscopic techniques like; XRD, UV-DRS, XPS, TEM, SEM and PL. Quantification of methanol by GC–MS showed encouraging results of 1424.8 and 2843 μmol g−1 of catalyst for Pt/TiO2 and 5 wt% Ni2P/CdS composites, respectively. Thus, Ni2P/CdS is a promising catalyst with higher productivity and significant selectivity than in-vogue catalysts.
Similar content being viewed by others
Introduction
From the past few decades, substantial changes have been witnessed in the atmosphere due to the burning of fossil fuels1 leading to an increase in the demand for renewable energy2. Also, the natural resources of fossil fuels are decreasing day by day, thus increasing the demand for an alternate source of energy3,4. At present, much of our energy demands are met by fossil fuel, but this resource is not a renewable source and is associated with contemporary environmental issues. Thus, renewable sources of energy shall play a critical role in fulfilling the requirement of energy demand5. More efforts have been focused on substituting renewable biomass sources for chemicals and fuels, which possess high energy density and environmentally friendly properties6. Mixing alcohol with petroleum products increases the fuel's combustion efficiency due to oxygen and reduces the emission of pollutants into the atmosphere7,8. The regular trend for methanol synthesis uses syngas conversion with high pressure and high thermal energy with heterogeneous catalytic reaction over alumina supported Cu/ZnO catalyst9. In the petrochemical industry, the production of methanol from CO2 reduction an important achievement. In this research area, efforts are continuing to increase the selectivity for methanol by reverse-water–gas-shift (RWGS) reaction in thermal catalytic CO2 hydrogenation via Cu/ZnO-based catalyst. The great challenge in this reaction is to maintain the operating temperature and pressure. Due to the competing RWGS reaction, the exothermic nature of methanol synthesis, equilibrium-limited conversion, and the very high activation energy barrier of the CO2 hydrogenation to methanol reaction, the process must balance operation temperature megapascal pressures to achieve high selectivity and production rate10,11. Fujishima first reported the photocatalysis study in 1972; in recent years, it’s become a common word in chemistry and also various technology products12. The theoretical target is to get chemical energy by using light energy with semiconductor materials. The electron present on the conduction band gets activated and migrates to the valance band of the semiconductor with the holes in the conduction band. Thus, the charge carriers facilitate the reaction wherein the electrons react with dissolved CO2 present in the reactant stream13. The reduction of CO2 is mainly depending on the highly reducing electron, which has a high reduction ability to convert CO2 into useful methanol14. As per Gibb’s free energy values, the CO2 and methanol having − 394.4 and − 166.4 kJ/mol energy indicating that the reduction of CO2 is an exothermic reaction15. Many processes are used for the conversion of CO2 into methanol such as (1) chemical conversion method, (2) electrochemical conversion method, (3) photochemical reduction method and (4) photoelectrochemical reduction method (Eq. 1).
Miguel et al.16 studied the hydrogenation of CO2 to MeOH and CH4 which disclosed that thermodynamically CH4 formation is more favorable than MeOH generation. Fiordaliso and Diez-ramiez17,18 used Pd2Ga and Cu/ZnO catalyst, reported 100% selectivity for MeOH at ambient pressure but these gave a low yield of conversion. TiO2 is widely studied as a photocatalyst with a wide bandgap semiconductor (3.2 eV) which can only be excited by UV light. CdS has a narrow bandgap of 2.4 eV and has received considerable interest in the field of water splitting and environmental remediation19. It can be a suitable candidate for visible light absorption and fits the thermodynamics requirements for H2O/H220,21,22. However, the photocatalytic activity over pure CdS is relatively low due to the instability and recombination of the electron–hole pairs. To overcome the above-mentioned problems, investigators are making a series of approaches that can be applied to improve the photocatalytic activity of CdS and TiO223,24,25. Combining Ni2P with CdS has proved to be an efficient way to enhance photocatalytic performance. With this background, we have synthesized two nano-systems Pt/TiO2 and Ni2P/CdS to convert CO2 into methanol in the presence of sacrificial donor triethylamine. The loading Ni2P on CdS is also studied by increasing concentration of Ni2P from 1 to 5% weight to find the optimum concentration of Ni2P. H2O2 can act as a reducing as well as oxidizing agent depending on pH value. It works as a potent reducing agent in the presence of a basic medium and produces O2 and 2H+ ions. The present manuscript discusses the significant conversion of CO2 to methanol with H2O2, the selectivity of methanol production (no by-products were observed) and the excellent yield of methanol (2843 μmol/g cat using 5 wt%-Ni2P/CdS catalyst). The finding of this has been compared with the conventional catalyst reported in the literature26.
Material and methods
Thiourea (NH2CSNH2), cadmium nitrate tetrahydrate Cd(NO3)2·4H2O, ethylenediamine, Nickel phosphide (Ni2P) and H2O2 34%. These chemicals are of analytical grade and used directly without further purification.
Synthesis of Ni2P/CdS
The solvothermal method was used to prepare cadmium sulphide nanorods. 10 mmol and 30 mmol of cadmium nitrate tetrahydrate and thiourea were dissolved in ethylenediamine (30 ml) respectively. These were transferred into 80 ml of Teflon-lined autoclave (stainless steel), which was then kept for 48 h at 160 °C in a hot air oven. After 48 h, an autoclave is set aside to cool at ambient temperature. The yellow colored product is rinsed with deionized (DI-water) water and ethyl alcohol several times. The yellow-colored cadmium sulphide was dried in a vacuum oven at around 60 °C for 6 h27,28. Preweighed quantity of nickel phosphide and synthesized cadmium sulphide were dissolved in ethylenediamine. The same autoclave is used to heat the mixture at 140 °C for 12 h. The yellow-green colored product obtained was collected from the cooled autoclave, which was rinsed with ethanol and DI-water several times. The final Ni2P/CdS catalyst was dried into a vacuum oven at around 60 °C for 5 h.
Synthesis of Pt/TiO2 nanoparticles
The photo-deposition technique was used to deposit platinum over titanium dioxide using hydro chloroplatinic acid. The experiment was conducted in a 500 ml irradiation type (inner) reactor. A 350 w medium pressure mercury vapor lamp was used as an irradiation light source and cooled to room temperature with cold circulating water. This photochemical reaction was performed in 400 ml of aqueous reactant solution with 150 mg of titanium dioxide and was continuously stirred magnetically for about 30–45 min to accomplish complete dispersion. Before starting the photo-deposition reaction, nitrogen was purged within a glass reactor to withdraw the air for 25 to 30 min (Scheme 1)29.
Characterization
Morphology of the photocatalysts was determined by scanning electron microscope (SEM) (Tescan, HiPace 10) and HRTEM using JEM-2100F JEOL Japan. The XRD patterns were recorded by using a benchtop X-ray Diffractometer (Model: Rigaku Miniflex II at 30 kV) having a scintillation counter detector. X-ray photoelectron spectroscopy (XPS) analysis has been done by using KRATOS AXIS 165 with Mg Kα irradiation. An Agilent Cary 5000 UV/VIS/NIR spectrophotometer was used to determine the UV/VIS absorption at ambient conditions. Photoluminescence was recorded at Hitachi F-7000 spectrofluorometer. Liquid samples were taken at a different time interval with an airtight syringe and separated by offline GC detected by a flame ionized detector (FID) using helium as carrier gas (Perkin Elmer Clarus 680).
Results and discussion
XRD studies
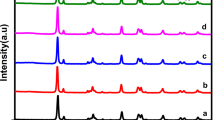
The XRD patterns of the TiO2, Ni2P, CdS, Pt/TiO2, CdS, 1 wt% Ni2P/CdS, 3 wt% Ni2P/CdS, 5 wt% Ni2P/CdS and 6 wt% Ni2P/CdS are shown in Fig. 1. It is observed that synthesized CdS nanorods have prominent diffraction peaks located at 25.04°, 26.66°, 28.39°, and 43.94° corresponding to the (100), (002), (101) and (110) planes of pure CdS with the hexagonal phase structure (JCPDS file no. 65-3414), respectively, suggesting that CdS has good crystallinity. In the case of Ni2P used in the preparation of Ni2P/CdS composite diffraction peaks at 40.58°, 44.39°, 47.18°, and 54.08° are observed which matches well with the hexagonal phase standard card of Ni2P (JCPDS file no. 65-3544) with (111), (201), (210) and (300) planes, respectively30. Besides, the XRD pattern of Ni2P/CdS shows prominent diffraction peaks at 25.11°, 26.77°, 28.46°, 36.84°, 43.95°, 48.14° and 51.89° for CdS and Ni2P, clearly showing that crystallinity of CdS and Ni2P is retained in Ni2P/CdS composite31. The deposition of Ni2P onto CdS shifted the diffraction angle from 43.8° to 43.9°, as indicated in the diffraction patterns of Ni2P doped CdS. The diffraction peak shift towards the higher angle for the doped material induces the expansion in the crystal lattice that increases the interplanar spacing. The XRD pattern of Pt/TiO2 shows diffraction peaks at 25.34°, 38.15°, 48.10° and 55.11° which was assigned to Pt/ TiO2. For bare TiO2, an intense peak at 25.30°, 37.79° and 48.03° corresponds to (101), (004) and (200) of the anatase phase of TiO2, respectively. In Pt/TiO2 a TiO2 has been detected at 47.44°. The absence of diffraction peak of the Pt on the Pt/TiO2 composite indicated that the platinum was well dispersed in TiO2. The high degree of dispersion was also confirmed by the HR-TEM image of the TiO2 and Pt/TiO2 in Fig. 2H, in which a clear lattice image of TiO2 and Pt particles on TiO2 are evident. The amount of doped Pt was less than 2.0% which is very less concentration to be detected by the XRD instrument significantly.
SEM and HRTEM studies
SEM and HRTEM analysis were carried out to investigate the morphology and microstructure of prepared photocatalysts. In Fig. 2a–d shows crystalline CdS nanorods with an average diameter of 100–5 nm while nickle phosphide deposited over CdS via solvothermal process showed similar geometry of nanorods and their diameter has not apparent change. The distance of lattice fringe of 0.33 and 0.22 nm for CdS and Ni2P, respectively, clearly shows that they are in close contact. The images clearly showed that the length of Ni2P/CdS is a little smaller than pure CdS while the structure and the results confirmed that morphology had no effect of the deposition of Ni2P on CdS. In Fig. 2e–h Pt/TiO2 crystal clearly shows platinum deposited on the TiO2 crystals which are seen as black spots at high resolution in TEM images32. In Fig. 3 SEM images of CdS (a–d) and Ni2P/CdS (e–h) from 200 to 1 μm indicates that there is no change after deposition of nickel on CdS nanorods, especially in d and h clearly showing that after and before deposition of Ni2P the nanorods structures are clearly observed in both images. SEM results corroborate the finding of TEM that deposition of co-catalysts like Ni2P and Pt nanostructure on CdS and TiO2 has minimal impact on the structural morphology of the materials.
UV–Vis diffuse reflectance spectroscopic (DRS) studies
The diffuse reflectance UV–Vis absorption spectra of synthesized composites were assembled for an area of 200–700 nm. The strong absorption peak ~ 264 to 296 nm indicates the interband transition between Ni2P–CdS and Pt–TiO2 (Fig. 4A). The absorption at a wavelength shorter than 390 and 580 nm in Ni2P/CdS and Pt/TiO2 can be ascribed as intrinsic bandgap absorption. These catalysts show the broad absorption in a visible range due to Ni2P and Pt deposition on CdS and TiO233. The bandgap Eg, of both the samples estimated using the Tauc’s relation given below in Eq. (2) is as follows;
where A is a constant, Eg the semiconductor bandgap and n is a number equal to 1/2 for the direct gap. The (αhυ)2 versus energy graphs were plotted. In Fig. 4, a straight line was fitted for the straight segment, this straight line was extrapolated to the X-axis to get the band gap values. The catalysts Ni2P, TiO2 and Ni2P/CdS have shown a bandgap of 1.05 eV, 3.3 eV and ~ 2.33 eV, respectively (Fig. 4B). The bandgap of CdS is shifted to left after loading of Ni2P and maximum shift has been observed in 5 wt% Ni2P/CdS indicating deposition of Ni2P on CdS and improved photocatalytic efficiency for CO2 reduction.
A series of experiments were conducted to understand the catalyst better. UV-DRS and photoluminescence (PL), was performed to investigate the photocatalytic mechanism for Ni2P/CdS composite. In the Fig. 4A, it is seen that CdS shows an absorption edge of approximately 490 nm34. The UV and visible light absorption intensity increased compared to pure CdS and 1–6 wt% Ni2P/CdS samples, which was attributed to the reduction of reflectivity35. The photoabsorption property has been increased by increasing the amount of Ni2P because of its dark colour. A slight redshift is seen in the samples of Ni2P/CdS and thus, the Ni2P is loaded onto the surface rather than the crystal lattice. In the present work, the optimal value was attained in 5 wt% Ni2P/CdS sample. In addition, as the diameter grows, it takes longer for the electrons to pass nanoparticles into the surface of Ni2P and less electrons to produce methanol. The maximum yield of methanol was obtained (2843 μmol/g cat) by using 5 wt% Ni2P/CdS catalyst under a visible light source of 300 W Xe lamp with 420 nm cutoff filter and 300 W tungsten light for 1 h. The Hg lamp with 300 W was the light source for Pt/TiO2 catalyst. As expected, increased Ni2P loading resulted in increased photoactivity. Ni2P served as an active centre for the production of methanol by electron trapping to decrease the recombination rate of electron–hole pairs during photocatalyst excitation. The adsorption of reactant CO2 can be supported by surface hydroxyl (OH) of CdS to improve photoreaction36. The amount of hydroxyl on the surface of CdS increased with increasing Ni2P loading, and the total CO2 photoreduction increased significantly. However, excess of Ni2P on the CdS surface (6 wt%-Ni2P/CdS), resulted in less catalyst light exposure. As a result, electron and hole pair photoexcitation was decreased because less photo-energy was absorbed. Therefore, an optimal Ni2P loading on the catalyst is a 5 wt% Ni2P and the highest methanol yields were found at this percentage under experimental conditions.
The photoluminescence (PL) is obtained from the radiative recombination of the photo-generated electron–hole pair. The (PL) emission experiment was carried out at an excitation wavelength of 350 nm. A series of studies includes loading of Ni2P on CdS (1 wt% Ni2P/CdS, 3 wt% Ni2P/CdS, 5 wt% Ni2P/CdS and 6 wt% Ni2P/CdS) as shown in Fig. 4C were restricted to the recombination of photoexcited electron–hole pairs. It appears that all samples exhibit a high emission at 535 nm because free electrons are radially recombined with free holes at the valence bands. Due to the increase in Ni2P concentration on CdS the PL strength compared to bare CdS was noticeably reduced. The lowest intensity was attained with a 5 wt% Ni2P/CdS composite which suggested that co-catalysts Ni2P was able to substantially reduce the population of single-excitons with the presence of an intimate interface of Ni2P and CdS composites.
XPS spectra
The valence state of the elements and the chemical composition of Ni2P/CdS photocatalyst were studied through XPS measurements. Figure 5 represents the binding energy (BE) curve for the core level spectra of Cd 3d, S 2p, Ni 2p, and P 2p constituent elements in the photocatalyst. Figure 5a displays the core level spectrum of Cd 3d area where 403.04 eV and 408.80 eV peaks correspond to Cd 3d5/2 and Cd 3d3/2, respectively. These values are close to earlier reported values37. In Fig. 5b, S2p has two peaks positioned at 160.54 eV and 159.38 eV are corresponding to S 2p1/2 and S 2p3/2 orbitals of divalent sulfide ions (S2−), respectively, which are in line with the formation of CdS38. The characteristic binding energies of peaks located at 854.35 eV, 871.72 eV, 130.82 eV and 131.15 eV correspond to Ni 2p3/2, Ni 2p5/2, P 2p3/2 and P 2p1/2 respectively, shown in Fig. 5c,d39,40. These results clearly demonstrate the existence and strong interaction between Ni2P and CdS, which is in good agreement with the TEM analysis.
Photocatalytic reduction of CO2 to methanol
A known amount of CO2 is purged into DMF after removal of moisture from the solvent with nitrogen purging at least for 30 min. 20 mg of the prepared photocatalyst was well dispersed in the mixture of DMF with TEA as a sacrificial electron donor. Carbon dioxide is added to the reaction mixture, followed by hydrogen peroxide and irradiated with light for the different time intervals. Liquid samples were filtered with a microsyringe filter and injected in offline GC to detect products (Scheme 2).
The photocatalytic reduction of CO2 to methanol was performed for both prepared catalysts. The catalysis test results showed that Ni2P/CdS is highly active than Pt/TiO2 under the influence of light (Fig. 6). An increment in the concentration of H2O2 shows a positive effect on methanol generation until the concentration researches 1.5 ml. There is no detection of methanol over oxidation products like formaldehyde and formic acid etc., in the GC and GC–MS spectrum indicting high selectivity towards methanol. There is less variation in methanol formation between 1 and 1.5 ml of H2O2, indicating that the reaction is completed at this point. However, the additional increment in H2O2 concentration of more than 1.5 ml shows the reduction in the formation of methanol. This may be attributed to a decrease in the availability of CO2 in the reaction mixture.
Therefore the ideal concentration of H2O2 for the reduction of CO2 is 1.5 ml/20 mg of catalyst. The effect of light over a different time is also done using the same catalyst. It is concluded that as the time duration increases the concentration of methanol increases. After an hour, no significant impact on the generation of methanol suggesting the catalyst can get exhausted (Fig. 7A). Nanocomposites typically have specific features that support the catalyst and are reusable. To illustrate this characteristic, we have studied the performance of selected catalyst 5 wt%-Ni2P/CdS to reduce CO2 to CH3OH. The obtained results were strongly supporting to the production of methanol after 3-cycle at 1 h time. After completion of 1 h reaction, the catalyst was removed from the reaction mix and dried and the next reaction was conducted. Based on the findings, the formation of CH3OH after each run does not effect a significant loss in efficiency of catalyst (Fig. 7B).
Quantum efficiency for CO2 reduction
Photochemical efficiency describes the percent of absorbed photons that reduce CO2 to products. CO2 adsorption on photocatalysts affects the efficacy of photochemical efficiency. The quantum outcome of the reaction is widely called photochemical efficiency. The photo-reduction of CO2 to methanol requires two electrons, the photochemical efficiency of the reaction is obtained by the equation given below41.
Here we calculate the quantum efficiency at λ = 420 ± 15 nm. The catalyst mixture was irradiated by a 300 W Xe lamp for 1 h. The average incident irradiation was determined to be 2.75 W/cm2 and the area was 17.59 cm2. The amount of methanol produced in 1 h was 2843 μmol. All the calculations are given below.
The number of incident photons (N) in 1 h over 17.59 cm2 area:
Photochemical efficiency depends on the intensity and wavelength of radiation. According to experiment results, a maximum quantum yield of 27.91% was obtained by 5 wt% Ni2P/CdS (Table 1).
Mechanism
Based on the experimental results a plausible schematic mechanism basic the robust methanol production over 5 wt%-Ni2P/CdS composite was proposed (Fig. 8). It is well known that the CB edge of CdS is more negative than that of Ni2P. Thus on irradiation with visible light the photogenerated electrons can efficiently transfer from the conduction band (CB) of CdS to Ni2P. The electrons accumulating on the Ni2P particles can reduce CO2 into CO2− while the holes on CdS can oxidize H2O2 to produce O2 and H+. Then CO2− reacts with H+ to produce CH3OH in presence of sacrificial electron donor TEA (Eq. 2). The effective separation of the photo-generated electrons and holes in CdS further improves photocatalytic activity.
Probable mechanism of charge transfer and CO2 reduction by 5 wt%-Ni2P/CdS composite and chemical equations. Comparision of Ni2P/CdS and Pt/TiO2 for photoreduction of CO2 to methanol is provided in Table 2. The synthesised 5% Ni2P/CdS photocatalyst in this work appears to outperforming the other reported photocatalysts.
Conclusion
The reduction of CO2 via visible light to methanol is achieved successfully. Here we are reporting methanol generation over noble metal-free hybrid semiconductor photocatalyst (Ni2P/CdS) under visible-light-driven CO2 reduction. The photocatalytic activity of the catalyst was enhanced by the homogenous dispersion of the co-catalyst. The synthesized Pt/TiO2 photocatalyst was adopted as the reference for comparison of the photocatalytic activity of the Ni2P/CdS nanocomposites under similar experimental conditions with an increase in Ni2P loading, the photocatalysts showed increased crystallinity. The CdS loaded with Ni2P showed greater efficiency than CdS for the formation of methanol from CO2. The 5 wt% loading of Ni2P on CdS was found to be optimal among the different compositions and afforded the highest product yield (2843 μmol/g cat). The reaction was found to be completely photocatalytic, as in the absence of visible light, no conversion was observed. The synthesized photocatalyst was heterogeneous and showed clear 3 recycle runs with no change in catalytic efficiency and also no significant leaching and change in morphologywas observed. Also, the reduction rate of CO2 is much higher than the previously reported catalysts. These results may provide a new avenue for various photocatalytic applications.
References
Perera, F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: Solutions exist. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph15010016 (2018).
Owusu, P. A. & Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 3(1), 1167990. https://doi.org/10.1080/23311916.2016.1167990 (2016).
He, X. & Liu, H. Efficient synthesis of 1,1-diethoxyethane via sequential ethanol reactions on silica-supported copper and H-Y zeolite catalysts. Catal. Today 233, 133–139. https://doi.org/10.1016/j.cattod.2014.01.023 (2014).
Zhang, H., Yupeng, Wu., Li, Li. & Zhu, Z. Photocatalytic direct conversion of ethanol to 1,1-diethoxyethane over noble-metal-loaded TiO2 nanotubes and nanorods. Chemsuschem 8, 1226–1231. https://doi.org/10.1002/cssc.201403305 (2015).
Gielenac, D. et al. The role of renewable energy in the global energy transformation. Energy Strat. Rev. 24, 38–50. https://doi.org/10.1016/j.esr.2019.01.006 (2019).
Sharma, A., Erdenedelger, G., Jeong, H. M., Jeong, H. M. & Lee, B. K. Controlled oxygen functional groups on reduced graphene using rate of temperature for advanced sorption process. J. Environ. Chem. Eng. 8(10374), 9. https://doi.org/10.1016/j.jece.2020.103749 (2020).
Surisetty, V. R., Dalai, A. K. & Kozinskib, J. Alcohols as alternative fuels: An overview. Appl. Catal. A Gener. 404, 1–11. https://doi.org/10.1016/j.apcata.2011.07.021 (2011).
Nguyen, T. D. et al. Tuyen Van Nguyen6 BiVO4 photocatalysis design and applications to oxygen production and degradation of organic compounds: A review. Environ. Chem. Lett. https://doi.org/10.1007/s10311-020-01039-0.3 (2020).
Martinez-Suarez, L., Siemer, N., Frenzel, J. & Marx, D. Reaction network of methanol synthesis over Cu/ZnO nanocatalysts. ACS Catal. 5, 4201–4218. https://doi.org/10.1021/acscatal.5b00442 (2015).
Wang, L. et al. Photocatalytic hydrogenation of carbon dioxide with high selectivity to methanol at atmospheric pressure. Joule 2, 1369–1381. https://doi.org/10.1016/j.joule.2018.03.007 (2018).
Dazaa, Y. A. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 6, 49675–49691. https://doi.org/10.1039/C6RA05414E (2016).
Hashimoto, K., Irie, H. & Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. Soc. Appl. Phys. 44, 8269–8285. https://doi.org/10.1143/JJAP.44.8269 (2005).
Taraka, T. P. Y., Gautam, A., Jain, S. L., Bojja, S. & Pal, U. Controlled addition of Cu/Zn in hierarchical CuO/ZnO p–n heterojunction photocatalyst for high photoreduction of CO2 to MeOH. J. CO2 Util. 31, 207–214. https://doi.org/10.1016/j.jcou.2019.03.012 (2019).
Fresno, F. et al. Selectivity in UV photocatalytic CO2 conversion over bare and silver-decorated niobium-tantalum perovskites. Catal. Today https://doi.org/10.1016/j.cattod.2020.01.013 (2020) ((In Press)).
Meng, X. & Zhang, Z. Photo-catalytic conversion of CO2 to hydrocarbons: Introduction, challenges and possible approaches. Prog. Petrochem. Sci. 1, 93–96. https://doi.org/10.31031/PPS.2018.01.000520 (2018).
Fiordaliso, E. M. et al. Intermetallic GaPd2 nanoparticles on SiO2 for low-pressure CO2 hydrogenation to methanol: Catalytic performance and in situ characterization. ACS Catal. 5, 5827–5836. https://doi.org/10.1021/acscatal.5b01271 (2015).
Diez-Ramirez, J., Dorado, F., de la Osa, A. R., Valverde, J. L. & Sánchez, P. Hydrogenation of CO2 to methanol at atmospheric pressure over Cu/ZnO catalysts: Influence of the calcination, reduction, and metal loading. Eng. Chem. Res. 56, 1979–1987. https://doi.org/10.1021/acs.iecr.6b04662 (2017).
Miguel, C. V., Soria, M. A., Mendes, A. & Madeira, L. M. Direct CO2 hydrogenation to methane or methanol from post-combustion exhaust streams—a thermodynamic study. J. Nat. Gas Sci. Eng. 22, 1–8. https://doi.org/10.1016/j.jngse.2014.11.010 (2015).
Peng, Q. X., Xue, D., Zhan, S. Z. & Jiang, X. F. A heterogeneous photocatalytic system based on a nickel complex over a CdS nanorod photosensitizer for H2 generation from water under visible light. Catal. Commun. 103, 15–18 (2018).
Chai, N. N., Wang, H. X., Hu, C. X., Wang, Q. & Zhang, H. L. Well-controlled layerby- layer assembly of carbon dots/CdS heterojunction for efficient visible-light-driven photocatalysis. J. Mater. Chem. A 3, 16613–16620 (2015).
Jiang, W. J. et al. Photocatalytic hydrogen generation on bifunctional ternary heterostructured In2S3/MoS2/CdS composite with high activity and stability under visible light irradiation. J. Mater. Chem. A 3, 18406–18412 (2015).
Reddy, D. A. et al. Heterostructured WS2-MoS2 ultrathin nanosheets integrated on CdS nanorods to promote charge separation and migration and improve solar-driven photocatalytic hydrogen evolution. Chemsuschem 10, 1563–1570 (2017).
Wu, X. Q. et al. Carbon dots as solid-state electron mediator for BiVO4/CDs/CdS Z-scheme photocatalyst working under visible light. Appl. Catal. B 206, 501–509 (2017).
Han, B., Liu, S., Zhang, N., Xu, Y. J. & Tang, Z. R. One-dimensional CdS@MoS2 core-shell nanowires for boosted photocatalytic hydrogen evolution under visible light. Appl. Catal. B 202, 298–304 (2017).
Hong, S., Kumar, D. P., Reddy, D. A., Choi, J. & Kim, T. K. Excellent photocatalytic hydrogen production over CdS nanorods via using noble metal-free copper molybdenum sulfide (Cu2MoS4) nanosheets as co-catalysts. Appl. Surf. Sci. 396, 421–429 (2016).
Chao, Y. et al. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 6, 1890–1895. https://doi.org/10.1039/C3NR05380F (2014).
Chao, Y. et al. Highly efficient visible light-driven hydrogen production of precious metal-free hybrid photocatalyst: CdS@NiMoS core–shell nanorods. Catal. Sci. Technol. https://doi.org/10.1039/C7CY00964J (2017).
Anushree, A., Priti Mangrulkara, A. C., Moinuddin, A. A., Nagababu, P. & Rayalu, S. S. In-situ Cl− ions formation during photocatalytic reaction of platinized nanocomposite for hydrogen generation. Sol. Energy 174, 1019–1025. https://doi.org/10.1016/j.solener.2018.09.047 (2018).
Pal, K., Maiti, U. N., Majumder, T. P. & Debnath, S. C. A facile strategy for the fabrication of uniform CdS nanowires with high yield and its controlled morphological growth with the assistance of PEG in hydrothermal route. Appl. Surf. Sci. 258, 163–168. https://doi.org/10.1016/j.apsusc.2011.08.024 (2011).
Wang, Q. et al. Highly efficient photocatalytic hydrogen production of flower-like cadmium sulfide decorated by histidine. Sci. Rep. 4, 1–9. https://doi.org/10.1038/srep13593 (2015).
Lakshminarasimhan, N., Bokare, A. D. & Choi, W. Effect of agglomerated state in mesoporous TiO2 on the morphology of photodeposited Pt and photocatalytic activity. J. Phys. Chem. C 116(33), 17531–17539 (2012).
Song, R., Luo, B., Geng, J., Song, D. & Jing, D. Photothermocatalytic hydrogen evolution over Ni2P/TiO2 for full-spectrum solar energy conversion. Ind. Eng. Chem. Res. 57(23), 7846–7854 (2018).
Zhao, D. et al. Promoting visible light-driven hydrogen evolution over CdS nanorods using earth-abundant CoP as a cocatalyst. RSC Adv. 6, 33120–33125 (2016).
Wu, T. F. et al. Noble-metal-free nickel phosphide modified CdS/C3N4 nanorods for dramatically enhanced photocatalytic hydrogen evolution under visible light irradiation. Dalton Trans. 46, 13793–13801 (2017).
Kong, T., Jiang, Y. & Xiong, Y. Photocatalytic CO2 conversion: What can we learn from conventional COx hydrogenation?. Chem. Soc. Rev. 49, 6579–6591 (2020).
Tang, Y. et al. Snowflake-like Cu2S/Zn0.5Cd0.5S p–n heterojunction photocatalyst for enhanced visible light photocatalytic H2 evolution activity. J. Taiwan Inst. Chem. Eng. 96, 487–495. https://doi.org/10.1016/j.jtice.2018.12.021 (2019).
Han, A., Chen, H., Zhang, H., Sun, Z. & Du, P. Ternary metal phosphide nanosheets as a highly efficient electrocatalyst for water reduction to hydrogen over a wide pH range from 0 to 14. J. Mater. Chem. A 4, 10195–10202. https://doi.org/10.1039/C6TA02297A (2016).
Zhu, D. et al. Two-dimensional metal-organic frameworks with high oxidation states for efficient electrocatalytic urea oxidation. Chem. Commun. 53, 10906–10909. https://doi.org/10.1039/C7CC06378D (2017).
Yin, X. et al. A novel structure of Ni-(MoS2/GO) composite coatings deposited on Ni foam under supergravity field as efficient hydrogen evolution reaction catalysts in alkaline solution. Electrochim. Acta 249, 52–63. https://doi.org/10.1016/j.electacta.2017.08.010 (2017).
Kang, X. et al. Titanium dioxide: From engineering to applications. Catalysts 9, 1–32. https://doi.org/10.3390/catal9020191 (2019).
Raziq, F. et al. Enhanced cocatalyst-free visible-light activities for photocatalytic fuel production of g-C3N4 by trapping holes and transferring electrons. J. Phys. Chem. C 120(1), 98–107. https://doi.org/10.1021/acs.jpcc.5b10313 (2016).
Abdullah, H., Ismail, N. A., Yaakob, Z., Khan, M. R. & Rahim, S. A. Ceo2-Tio2 for photoreduction of CO2 to methanol under visible light: Effect of ceria loading. Malays. J. Anal. Sci. 21, 166–172. https://doi.org/10.17576/mjas-2017-2101-19 (2017).
Li, H., Li, C., Han, L., Li, C. & Zhang, S. Photocatalytic reduction of CO2 with H2O on CuO/TiO2 catalysts. Util. Environ. Eff. 38, 420–426. https://doi.org/10.1080/15567036.2011.598910 (2016).
Xie, Y. P., Yang, Y., Wang, G. & Liu, G. Oxygen vacancies promoted interfacial charge carrier transfer of CdS/ZnO heterostructure for photocatalytic hydrogen generation. J. Colloid Interfernce Sci. 503, 198–204. https://doi.org/10.1016/j.jcis.2017.05.006 (2017).
Liu, S., Zhao, Z. & Wang, Z. Photocatalytic reduction of carbon dioxide using sol–gel derived titania-supported CoPc catalysts. Photochem. Photobiol. Sci. 6, 695–700. https://doi.org/10.1039/B613098D (2007).
Gusain, R., Kumar, P., Sharma, O. P., Jain, S. L. & Khatri, O. P. Reduced graphene oxide–CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B 181, 352–362. https://doi.org/10.1016/j.apcatb.2015.08.012 (2016).
Wesselbaum, S., vom Stein, T., Klankermayer, J. & Leitner, W. Hydrogenation of carbon dioxide to methanol by using a homogeneous ruthenium–phosphine catalyst. Angew. Chem. Int. Ed. 51, 7499–7502. https://doi.org/10.1002/anie.201202320 (2012).
Kumar, P., Chauhan, R. K., Sain, B. & Jain, S. L. Photo-induced reduction of CO2 using a magnetically separable Ru-CoPc@TiO2@SiO2@Fe3O4 catalyst under visible light irradiation. Dalton Trans. 44, 4546–4553. https://doi.org/10.1039/C4DT02461C (2015).
Lu, X. L. et al. Facile one step method realizing scalable production of g-C3N4 nanosheets and study of their photocatalytic H2 evolution activity. J. Mater. Chem. A 2, 18924–18928. https://doi.org/10.1039/C4TA04487H (2014).
Kumar, P. et al. Core–shell structured reduced graphene oxide wrapped magnetically separable rGO@CuZnO@Fe3O4 microspheres as superior photocatalyst for CO2 reduction under visible light. Appl. Catal. B 205, 654–665. https://doi.org/10.1016/j.apcatb.2016.11.060 (2017).
Acknowledgements
This project is supported by CSIR-New Delhi under the category of NCP/E3OW-Theme project (no. CSIR-NEERI/PMPD/FBR/NCP Project-2018-19-6) and Demonstration and validation of hydrogen ecosystem for stationary power backup application for telecommunication towers - Hybrid Broad Band Absorption PV Cell based Water Electrolysis for Solar Hydrogen technology (GAP-2536). KRC no.: CSIR-NEERI/KRC/2020/MARCH/EMD/2.
Author information
Authors and Affiliations
Contributions
N.B. conceived the idea and conceptualized the research work. N.B. also prepared the catalyst, few experiments, and prepared the original draft. S.A.M.A., Y.T.P., S.B. and A.K conducted experiments related to catalytic conversion of CO2 to methanol and GC and GC-MS studies and manuscript writing SR.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagababu, P., Ahmed, S.A.M., Prabhu, Y.T. et al. Synthesis of Ni2P/CdS and Pt/TiO2 nanocomposite for photoreduction of CO2 into methanol. Sci Rep 11, 8084 (2021). https://doi.org/10.1038/s41598-021-87625-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87625-w
This article is cited by
-
Investigations on effect of Mn doping on the photocatalytic properties CdS nanoparticles for effective degradation of organic pollutants and enhanced production of solar fuels
Journal of Materials Science: Materials in Electronics (2024)
-
Synthesis of single-walled carbon nanotubes functionalized with platinum nanoparticles to sense breast cancer cells in 4T1 model to X-ray radiation
Microchimica Acta (2023)
-
Synthesis and characterization of InP quantum dots for photovoltaics applications
Journal of Materials Science: Materials in Electronics (2023)
-
Enhanced visible light photocatalytic CO2 reduction over direct Z-scheme heterojunction Cu/P co-doped g-C3N4@TiO2 photocatalyst
Chemical Papers (2022)
-
An Efficient Photocatalyst with Pt/TiO2@CdS/Co3O4 Hollow Core–Shell Nanostructure for Overall Water Splitting
JOM (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.