Abstract
The invasive Asian date mussel (Arcuatula senhousia) inhabits diverse global coastal environments, in some circumstances posing significant ecological and economic risks. Recently recorded in the Greater North Sea ecoregion, an established population has not previously been confirmed. Combining historical and field data, we provided baseline information from the UK and recorded colonisation in a variety of habitats. Gonadal development was assessed using the gonadosomatic index (GSI) to determine if an intertidal soft-sediment population is self-sustaining. Arcuatula senhousia records from subtidal muddy/mixed-sediment within a major estuarine system from 2007 to 2016 were also analysed. First detected in 2011, spatial distribution was variable across the years within the subtidal, with individuals found at 4–9 out of 25 sites, and densities per site varying from 10 to 290 individuals per m2. The intertidal population was, in part, associated with seagrass (Zostera spp.) and attached to bivalves. In marinas, individuals were attached to concrete tiles, associated with live Mytilus edulis, and to dead Ostrea edulis. Mean GSI from the intertidal population differed across months, peaking in July before declining in September/October, but with high inter-individual variability. Arcuatula senhousia is reproducing and maintaining viable populations. Using a natural capital approach, we identify the potential impacts on Europe’s functionally important habitats, fisheries and aquaculture if its spread continues.
Similar content being viewed by others
Introduction
Arcuatula senhousia (Benson, 1842), formerly known as Musculista senhousia, and commonly known as the Asian date mussel, is a fast-growing, relatively small (< 40 mm in length), mytilid mussel which can be found in intertidal and subtidal habitats1,2. Its vast native range stretching from Singapore to Siberia3,4 is testament to its environmental adaptability which has led to its extensive distribution as a non-native species5. As a non-native, it was first detected on the Pacific coast of North America in the 1920s6, but has also been reported from Australia and New Zealand7,8; the Mediterranean and Adriatic Seas9,10,11,12; the Azov-Black Sea Basin13 and West Africa14. Finally, it has been reported from the Suez Canal, Red Sea, Aden, Zanzibar, Madagascar, Mauritius, India, Indo-China and New Caledonia15,16.
Successful A. senshousia introductions have been attributed to traits typical of invasive species: high fecundity; high dispersal capability; fast growth rate; phenotypic plasticity and tolerance to a wide range of environmental conditions1,3,17,18. Although small in size, a female can release > 100,000 eggs19, preceding an extended larval planktonic stage lasting two to eight weeks facilitating dispersal16,20,21. Once settled, individuals can mature within nine months22 adapting their reproductive cycle to new conditions1,19,23.
In Europe, A. senhousia was reported from Arcachon Bay, on the Atlantic coast of France in 200224. There were no further reports north of this location until 2017, when A. senhousia was detected in the Solent region of the south coast of England using eDNA metabarcoding and five specimens were found on intertidal sediment within the same region25,26. However, the distribution and abundance of the species in the Solent are not known. The Solent is a 32 km long strait that separates the Isle of Wight from mainland England that sits within the ecoregion of the Greater North Sea (this ecoregion encompasses the coastlines of the UK, France, Belgium, Netherlands, Germany, Denmark, Sweden and Norway27). The Solent hosts a diverse range of temperate coastal habitats, with annual sea surface temperatures typically varying from 9 °C (February) to 17 °C (September)28. It is protected under a variety of local, national and international conservation designations due to important habitats and the biodiversity they support29. The Solent is also subject to great anthropogenic pressure, for example hosting international commercial ports and high levels of recreational boating activity.
Invasive species alter the value of ecosystems in terms of the benefits that people obtain from them (ecosystem goods and services)30. Ecosystem engineers, such as A. senhousia, at high population densities, can be particularly influential due to their ability to modify, create or destroy habitat31. In doing so they impact natural capital, here defined as “…the stock of forests, rivers, land, minerals and oceans, as well as the natural processes and functions that underpin their operation”32. Impacts of non-native species on commercially and ecologically important species as well as native habitats are poorly understood33 and this is also the case for A. senhousia. In the North Pacific there is evidence for A. senhousia inhibiting rhizome growth of seagrass (Zostera marina) where Z. marina is patchy and sparse34, however interactions between the two species are complex; for instance A. senhousia fertilise Z. marina beds35,36. A. senhousia can also attach to hard surfaces and so has the potential to foul and outcompete cultured bivalves37. As an autogenic ecosystem engineer (conspecifics can bind together using byssal threads to form dense mats in its native and non-native ranges22,38,39) high numbers not only alter the space and type of available substrate but also sediment conditions40,41. Intertidal soft sediments, seagrasses (Zostera spp.) and the ecologically and commercially important European flat oyster (Ostrea edulis) may all be affected. An established Solent population could also affect commercially important shellfish, and bait fisheries that extract significant biomass from soft-sediment benthic habitats42,43.
Evaluation of risk associated with a non-native species is based on many factors including introduction, establishment and spread potential as well as impacts. This study provides a first step towards the assessment of risk associated with A. senhousia in European waters. Our aims are to: (i) Assess spatial distribution and temporal trends in a subtidal population from Southampton Water, using historic data from routine coastal surveys performed by the Environment Agency (EA); (ii) Provide the first baseline information on the species’ presence within the Solent and confirm its ability to colonise diverse habitats within the Greater North Sea, using a combination of the historic data and our own field data (2019); (iii) Investigate if an intertidal sediment population of the Solent has the potential to deliver larvae across this region by assessing gonadal development and gametogenic processes; (iv) Identify potential effects (positive and negative) of A. senhousia for species, habitats, fisheries and aquaculture (i.e. goods and services) of Europe if it spreads beyond the Solent region, with reference to current literature and data from this study.
Methods
Assessment of spatial distribution and temporal trends
Subtidal surveys in Southampton Water were undertaken from 2007 to 2016 when the EA carried out routine benthic surveys as part of the monitoring programme for the UK government’s Water Framework Directive (WFD)44. Forty-five sites (2007) and 25 sites (2011, 2013 and 2016) within Southampton Water and its estuaries (Rivers Test, Itchen and Hamble) were semi-randomly selected for sampling each year by considering sediment type, accessibility and potential hazards. A site was approximately defined as a 50 m radius surrounding a target coordinate. One grab sample, using a 0.1 m2 Day grab, was taken to assess macrofauna at each site. Macrofauna processing and identification were undertaken by a contractor using standard operating and quality control procedures used by the industry (e.g. NMBAQCS: North East Atlantic Marine Biological Analytical Quality Control Scheme) with macrofauna extracted using a 0.5 mm sieve. No specific size measurements of A. senhousia were recorded.
Assessment of spatial distribution, gonad staging, habitat preference
The intertidal shore at Brownwich was surveyed in 2019 using six 600 m × 5 m transects parallel to the mean low water springtide line, evenly spaced (by 40 m) from high shore to low shore. The surveyor walked within the transect parameters locating A. senhousia that were immediately apparent on the sediment surface without sediment excavation. Arcuatula senhousia locations were recorded using a GPS device (Garmin eTrex 20x) and shell lengths measured using calipers. Every other measured specimen was transported back to the laboratory and fixed in formalin before the gonadosomatic index (GSI) measurements were obtained. For GSI, gonads and other tissue were dissected and then calculated as follows: ([gonad wet weight (g) / bodyweight without shell (g)] × 100)45.
Surveys not targeted at detecting A. senhousia also provided records for this species from intertidal locations within the Solent region. These surveys were conducted by researchers from the Universities of Portsmouth and Southampton, a volunteer for the Hampshire and Isle of Wight Wildlife Trust and Pisces Conservation Ltd (see Supplementary Table S1). From west to east, surveys included an intertidal macrobenthos survey at Lepe (2019), a fish push-net survey within the River Test (2016) and a seagrass quadrat survey at Portsmouth Harbour (2019). A specimen from the River Itchen (2018) was also found during an intentional search for A. senhousia on mudflats (no methodology recorded). Survey details regarding the specimen found at Chichester Harbour (2019) cannot be provided due to the commercial sensitivity of the location where it was found.
Marina and harbour surveys across the Solent
As part of the Solent Oyster Restoration Project46, O. edulis were purchased in 2016 from the commissioned dredge fishery in Langstone Harbour and were translocated from the seabed into broodstock cages deployed at various locations within the Solent including Saxon Wharf (River Itchen). It should be noted that oysters were not cleaned of epifauna before translocation, in part due to the sheer numbers of oysters being moved. In total, approximately 10,000 O. edulis were purchased from the fishery, with each oyster being at least 3 years in age (> 70 mm). The oysters remained in the cages throughout 2017 and 2018 until the trial concluded in November 2018. At the end of the trial deceased individuals were extracted from the cages and taken to the laboratory where any A. senhousia which had colonised the shells were removed and shell lengths recorded using calipers. As part of the same restoration project, cages containing O. edulis were deployed in 2019 for nine months at Port Hamble in the River Hamble. Arcuatula senhousia individuals were found within the cages during scheduled monitoring of the oysters in April 2019, at which point they were collected, and shell size recorded. Macrobenthos samples were collected from subtidal sites around the Solent in 2019 to investigate possible associations between O. edulis and other macrobenthos. In addition, roof tiles (Burton Roofing Merchants Limited, Redland Plain Tile Antique Red, concrete: 27.0 × 16.5 × 1.0 cm) submerged at a depth of between 0.5–1.0 m for six months on pontoons in Saxon Wharf Marina, originally deployed to assess O. edulis settlement, were removed and placed in flow-through laboratory holding tanks for 21 days prior to the removal of all epifauna.
Statistical analysis
A Kruskal–Wallis test (SPSS v.25) was used to determine whether there was a significant difference between median densities per site of A. senhousia individuals collected from each of the three EA surveys when A. senhousia was detected (2011, 2013 and 2016). This test was chosen because data were not normal and, due to the high number of zeros, could not be transformed. This test was also used to identify significant differences between the median GSI reported for March, May, July and September/October (data were collected during the last week of September and the first three weeks of October and were, therefore, combined). In order to identify which months had a significantly different GSI, pairwise-comparisons were subsequently made using the Wilcoxon rank sum test.
Assessment of potential impacts
A literature review was conducted to gather information on A. senhousia impacts, specifically in relation to natural capital and vulnerable and protected habitats and species. To extract the relevant information, Web of Science and Google Scholar were used to search for common names and synonyms for A. senhousia as listed by CABI5. Other key words searched included “Zostera”, “impact”, “distribution”, “competition”, “clam”, “oyster” and “reproduction”. Impacts were then categorised by the relevant ecosystem services using the commonly used top level categories of Provisioning, Regulating, Cultural and Supporting e.g.47,48. We adapted these definitions to be the following: Provisioning services are products that people obtain from ecosystems (e.g. food and other raw materials); Regulating services are benefits that people obtain from the regulation of ecosystem processes (e.g. climate regulation and water purification); Cultural services are the non-material benefits that people obtain from ecosystems (e.g. recreation and health); Supporting services are those that are necessary for the production of all other ecosystem services (e.g. habitat provision and genetic diversity).
Results
Spatial distribution, temporal trends, habitat preference
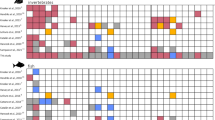
The first scientifically reported sighting of A. senhousia in the UK prior to this study was from 201726, however our study confirms the presence of this species in the UK since 2011 (mean A. senhousia densities for each survey can be found in Table 1). Routine surveys undertaken by the EA throughout Southampton Water and its three estuaries recorded the presence of A. senhousia from 2011–2016. In 2007, no A. senhousia individuals were found at any of the 45 sites (Fig. 1; sites 1–45, Supplementary Table S2). In 2011, five out of the 25 sites sampled contained A. senhousia, concentrated towards the upper reaches of the estuarine system (Fig. 1), and densities varied from 0 to 70 individuals per m2 (m−2) (mean = 7.2 + /− 18.6 SD) (sites 46–70, Supplementary Table S2). In 2013, samples from four out of the 25 sites contained A. senhousia (Fig. 1) with densities ranging from 0 to 70 m−2 (mean = 4.0 + /− 14.1 SD) (sites 71–95 in Supplementary Table S2). In 2016, A. senhousia was found at more sites (nine out of 25) across a greater geographic area (Fig. 1). For example, it was detected for the first time in the River Hamble and near the mouth of Southampton Water. The highest density was recorded in 2016 when there was a range of 0–290 m−2 (mean = 20.4 + /−58.8 SD) (sites 96–120 in Supplementary Table S2). Nevertheless, there is no significant difference in A. senhousia median density per site between 2011, 2013 and 2016 (Kruskal–Wallis test, X2(2) = 3.1, p = 0.215).
(a) Map of the UK denoting the Solent survey region. (b) Locations across the Solent region where A. senhousia has been detected. (c) Distribution of A. senhousia in the Solent focusing on Southampton Water and its tributaries as determined by EA benthic surveys. Dashed rectangle in (b) denotes area (c). Black fill indicates presence of A. senhousia, white fill indicates absence. Overlapping symbols are layered in order of year (most recent at the top). Symbols without numbers are Environment Agency (EA) survey sites (site numbers excluded to maximise clarity of map); EA site locations and associated A. senhousia densities can be found in Supplementary Table S2. Numbers 121–130 refer to surveys by other organisations (see Table 1). Mean densities for all surveys can be seen in Table 1. Site 128 is not a specific location but represents one individual found in Chichester harbour. Map created using ArcGIS Pro 2.6 https://pro.arcgis.com/. The intertidal shore at Brownwich (Fig. 1; site 122) was comprehensively surveyed in 2019. Compared to the subtidal sites in Southampton Water, the population density was low, with only 169 individuals recorded equivalent to 0.06 m−2 (Table 1). Single individuals were found mainly on the higher part of the shore partially buried in the sediment. None were attached to seagrass (Zostera spp.), however, when removed from the sediment a number were attached by their byssal threads to dead cockles (Cerastoderma edule) (empty shells) and living individuals. Arcuatula senhousia shell lengths ranged from 9 to 32 mm (mean = 20.1 + /− 3.9 SD).
In addition to the EA’s surveys, there have been further reports of A. senhousia from a variety of intertidal and marina surveys in all three rivers which discharge into Southampton Water (see Table 1 for survey details and site numbers). Two individuals were found near Hythe at the mouth of the River Test (Fig. 1; site 121), one in 2016 (17 mm length) and another in 2019 (18 mm length). In 2018, two individuals were recorded from Weston Shore in the River Itchen (Fig. 1; site 123). Further, A. senhousia were found attached to empty adult shells of O. edulis that had been removed from oyster cages at Saxon Wharf (Fig. 1; site 124), also in the River Itchen. Fourteen A. senhousia individuals ranging from 13–23 mm (mean = 17.6 + /− 3.0 SD) were removed from the oysters. Concrete tiles deployed in Saxon Wharf Marina (Fig. 1; site 124) had three individuals (mean = 8.7 + /− 3.1 SD) attached to the tiles or to Mytilus edulis when recovered in 2019. Two individuals (19 mm and 28 mm in length) were also found at Shamrock Quay (2019) attached to the metal cages housing the oysters (Fig. 1; site 129). An unknown number of A. senhousia individuals were also collected from oyster cages at Port Hamble (River Hamble). They were found attached to cockles and Ulva spp. that had been caught in cages suspended beneath the marina pontoons (Fig. 1; site 125).
Reports from three intertidal surveys and one subtidal survey provide evidence for the conclusion that A. senhousia is distributed across the Solent region. In 2019, one individual was found in Lepe in the west of the Solent (Fig. 1; site 126). To the east, one individual (18 mm length) was recovered from Portsmouth Harbour (Fig. 1; site 127) growing on mixed eelgrass (Zostera marina and Z. noltei) alongside significant quantities of Ruppia spp. Another individual (4 mm in length) was found on muddy sediment in highly sheltered conditions within Chichester Harbour (Fig. 1; site 128, but exact location cannot be disclosed due to commercial sensitivity of the survey) and another was recovered from the Isle of Wight in Newtown (Fig. 1; site 130).
Gonad staging
Ninety-four individuals collected from Brownwich between March and September/October in 2019 were assessed for reproductive state. Gonad tissue lining the shells of A. senhousia collected in March ranged from extremely thin and barely visible (Supplementary Fig. S1a) to thin translucent tissue with white venation (Supplementary Fig. S1b). The translucent tissue corresponds to a high volume of follicle cells with collapsed or empty gametes indicating spent or developing gonads with no clear differences between sexes19. Arcuatula senhousia from May also resembled those collected in March, however, by July gonad tissue had substantially thickened and channels within the tissue could be seen (Supplementary Fig. S1c, d), suggesting that the gonads were ripe or at the spawning stage19. The colour of the gonads, either white (male) (Supplementary Fig. S1c) or orange (female) (Supplementary Fig. S1d) was also discernible confirming a 3F:2M sex ratio for the 15 A. senhousia individuals collected. By September/October there was a high inter-individual variation in reproductive state, with gonad stage appearing to range from spent to ripe/spawning. One out of the 12 individuals collected in September/October was identified as a female, although a sex ratio could not be established due to the thin gonad tissue of many of the mussels.
To support the gross anatomical observations the GSI was calculated for each month and presented in Fig. 2. Mean GSI was low for both March and May (6.0 + /− 7.2 SD, 5.9 + /− 11.0 SD, respectively), but had increased to 23.1 + /− 6.1 SD by July. By September/October the mean GSI had decreased but remained higher than for March and May (16.7 + /− 13.3 SD). A Kruskal–Wallis test confirms that there are significant differences in median GSIs between the months sampled (Kruskal–Wallis, X2(3) = 41.5, p = < 0.001). A pairwise-comparison of the median GSI for each month indicates that all months are significantly different from each other (Wilcoxon rank sum test, p = < 0.05) apart from March and May.
GSI for A. senhousia collected in March (n = 49), May (n = 18), July (n = 15) and September/October (n = 12) in 2019. The mean values are represented by the line in the centre of the box. Upper and lower limits of the box represent one standard deviation (SD). The whiskers represent data outside of one SD from the mean. Individual GSI data points are represented by the black dots.
Discussion
Baseline biological data and spatial distribution
Our data suggests that A. senhousia arrived in the UK, between 2007 and 2011, which was recently confirmed by Worsfold et al.49. The closest (in distance) European record of A. senhousia prior to this was from Arcachon Bay (Bay of Biscay), on the Atlantic coast of France in 200224. The lack of reported sightings between the UK and the Bay of Biscay suggests a direct introduction event in the Solent as opposed to natural dispersal. As stated by Barfield et al.26, if the French population had gradually extended northwards unaided by any direct anthropogenic vector, it is reasonable to assume that its presence would have been recorded elsewhere before it reached the UK. However, spread of A. senhousia towards UK could have gone undetected due to limited monitoring for non-native species in the region. Potential vectors for introduction to the Solent include as a hitch-hiker with aquaculture species/produce50, but introduction by shipping is most likely. This is supported by the species’ ability to foul boat hulls51 and the detection of A. senhousia DNA in ballast water of boats in Dutch harbours52. A phylogenetic analysis is required to fully explore the likely invasion route(s) into the Solent and contextualise the global colonisation process. Attachment to seaweeds such as Ulva spp., as found in this study, could facilitate more local spread of A. senhousia by acting as a raft for hitchhikers (e.g.53).
Individuals collected ranged in size from 4 mm (Chichester Harbour) to 32 mm (Brownwich shore). Whilst Huber2 indicates an upper length of 40 mm for this species, an upper size limit of around 30–35 mm in its non-native range is most common in the literature (e.g.1,8,24,54). Linked to the small size in terms of traits of a successful invader is the short lifespan with most individuals living for only a year. Morton17 concluded that the small fraction of the population that lives up to two years is an adaptation for the continued survival of population in a variable environment. Considering a growth rate of approximately 2 mm a month depending on environmental conditions 1,16,55 it is possible that a few individuals at Brownwich were potentially older than a year. The size ranges recorded here, combined with the fact that individuals have been recorded from three sites on multiple years (Southampton Water: 2011, 2013, 2016; River Test: 2016, 2019; Brownwich: 2017, 2019) strongly suggest multiple generations from established populations.
Any self-sustaining population requires successful reproduction. While this is supported by the size ranges of A. senhousia (which spanned the 14–20 mm length maturity threshold19,23) the strongest evidence comes from the gonad imagery and GSI scores. From March to May gonads from individuals were not developed, but by July the significant increase in GSI combined with the typical gross morphology for bivalves confirms that individuals have maturing/mature gonads. By September/October, observation of the gonad tissue and the decrease in GSI suggest that some may have spawned. However, timings of the reproductive cycle need to be confirmed by histological analyses and plankton trawls.
The timings of these reproductive stages likely coincides with changes in water temperature; a variable which is well-documented for influencing bivalve reproduction and development56,57, especially in temperate regions58. In its native range of the Sea of Okhotsk, Southern Sakhalin (Russia), the spawning period of A. senhousia coincides with temperatures of 15–20°C3. This temperature range matches the inshore summer temperatures of the Solent (Watson, unpublished data) suggesting summer spawning in Europe’s temperate systems, if other requirements, such as oxygen levels and salinity are met. This is likely considering A. senhousia is also tolerant of a wide range of salinity (multiple Solent sites have reduced or fluctuating salinities) and oxygen levels21. Colder months in the Solent, when the average temperature is < 15 °C (e.g. winter and spring)28, probably limit reproduction59. Despite the evidence indicating a summer spawning population in the Solent, there are inconsistencies in the temperature range reportedly required for A. senhousia reproduction to take place. For example, a temperature of 22.5–28 °C is well documented5,19,60. It is possible that this temperature range only applies to A. senhousia individuals originating from the warmer parts of its native region61. A lineage that is predisposed to colder waters and has high levels of polymorphism may be responsible for adaptation to the relatively cold waters of Northern Europe61. Research should, therefore, focus on identifying the lineage present in this area and determining the temperature limits for reproduction. In addition, the possibility of multiple and prolonged spawning events in the UK cannot be excluded since we observed high inter-individual variability of GSI data. This is not an unusual phenomenon, with prolonged spawning (more than two months) reported outside of its native range1,10,24,55,62.
This study highlights that A. senhousia survives in multiple habitat types present in the Solent confirming the species’ capability for colonising diverse intertidal and subtidal habitats10,34,40,51. Due to the opportunistic collection methods for data used in this study, it is not currently possible to determine the geographical extent of the population or the rate of spread within the Solent since its arrival. Indeed, although the largest density (290 m−2) and greatest number of positive sites (35%) were reported from sampling of Southampton Water in 2016, there was no significant difference in median density between years. Currently, distributions in both Southampton Water and Brownwich beach appear patchy and spatially variable with lower densities than other invaded locations10,40. This may be in part due to limited sampling, but the A. senhousia populations in the Solent could be experiencing an extended lag phase which is typical of newly introduced species63. However, this does not necessarily mean densities will inevitably increase in the future. Local factors might prevent mat formation, for example, anoxia associated with warmer months can induce mass mortalities23,64,65. Arcuatula senhousia is also predated upon by shorebirds birds (diving ducks and oyster catchers)8,62,66, boring carnivorous gastropods51,67,68, fish15 and probably crustaceans and echinoderms due to its thin shell. Therefore, intense activity by predators may limit A. senhousia’s mat-forming abilities. In conclusion, further data to describe the distribution of A. senhousia’s in the Solent are required.
Potential effects on European natural capital
Non-native species impact natural capital and thus alter the value of ecosystems in terms of the ecosystem goods and services provided. Tables 2, 3, 4 and 5 provide summaries of potential impacts (both positive and negative) associated with A. senhousia on ecosystem services (addressing the categories of Provisioning, Regulating, Supporting and Cultural) and identifies key knowledge gaps which should be addressed in the short term as a priority.
Provisioning services
Arcuatula senhousia has been reported to reduce the growth rate and survivorship of commercially important clams by competing for space and food69,70,71 and indirectly increasing predation72. In the Solent, oysters (O. edulis); clams; cockles and polychaetes for angling bait are commercially harvested from intertidal and subtidal soft sediment habitats and many are important fisheries across Europe42,73,74. Arcuatula senhousia collected from Brownwich were often attached to dead C. edule shells, although whether this attachment resulted in the death of C. edule cannot be concluded. High A. senhousia densities can alter sediment conditions40,41, which may have significant implications for the macrofaunal community generally and these commercial species. In Sacca di Goro, Italy, shellfish farmers reported reduced numbers of Ruditapes philippinarum under A. senhousia mats23. However, R. philippinarum has escaped cultivation in Europe and formed invasive populations; this context should be considered when undertaking impact assessments75,76.
We only found one individual from Portsmouth Harbour growing within a bed of Zostera spp., although A. senhousia co-occurs with seagrasses in both its native and introduced ranges3,24,34,77,78. Seagrass beds are biodiverse ecosystems providing a variety of ecosystem services across the world, such as carbon capture, coastal defence and the provision of nursery habitat for juvenile fish, including those of significant commercial value in Europe79,80,81,82,83. Since the late 1800s, seagrass beds have suffered from substantial degradation due to a host of biotic and abiotic factors (although some recent recovery has been reported)84,85. These degraded beds, and new beds transplanted for restoration schemes (for example, Project Seagrass86), may be at risk, since A. senhousia mats have been found to inhibit rhizome growth in recovering populations with low plant density (in contrast, impacts of A. senhousia on established beds have been reported as small and non-consistent)34. Solent densities (290 m−2) may be currently too low to impact seagrass, compared to 15,000 m−2 in San Diego Bay – the site of the aforementioned seagrass study34. Whether higher densities form in the future will depend on a complex interplay of environmental conditions and biological factors.
Within this study we found evidence for A. senhousia attachment to empty O. edulis shells, M. edulis shells and concrete tiles in the Hamble estuary. In a different study, A. senhousia was also found attached to cultured Crassostrea hongkongensis in Hong Kong37. The colonisation of locations in both fully saline and brackish European waters by A. senhousia could increase the cost of shellfish aquaculture via biofouling and directly compete with the commercial species for substrate and food. Biofouling has been estimated to be 20–30% of shellfish production costs, though this cost varies depending on the commercial species and the geographic location of the operation87,88. Disease introduction and hybridisation with commercial species are also possible outcomes that could have significant risks for the European aquaculture industry. For example, the cultivation of the non-native Pacific oyster (Magallana gigas) in France since 1966 is likely to have contributed to the arrival and spread of gill disease to Portuguese oysters (Crassostrea angulata)89. Further, expanding populations of Mytilus trossulus in the UK, likely driven by commercial mussel growing activity, have been associated with the appearance of M. trossulus x M. edulis hybrids which are less valuable as a commercial species90. However, at the time of writing, investigations into potential disease spread from A. senhousia to other shellfish, or hybridisation between A. senhousia and other mussels could not be found. Nonetheless, A. senhousia may be a suitable host of a native generalist parasite, the pea crab Pinnotheres pisum, in the UK, considering that Pinnotheres novaezelandiae was found within A. senhousia in New Zealand91. Pinnotheres spp. are known to negatively impact the condition index, oxygen consumption and filtration rate of Mytilus spp.92,93.
Any non-native species is likely to have positive and negative effects on provisioning services and this is the case for A. senhousia. For example, it can be eaten by humans for food22 or could be used to provide products to the pet trade94. Reusch and Williams34 also found it could be beneficial to seagrasses by providing nutrients and could even protect vulnerable habitats from erosion if it forms mats. Increases in habitat diversity through an increase in structural complexity from mats or aggregations of A. senhousia may provide significant benefits for other species and biodiversity more generally. Thus, any risk assessment needs to cover both potential negative and positive impacts so that informed management decisions can be made.
Regulating, supporting and cultural services
The densities currently reported are unlikely to have an influence on key regulating and supporting services at anything, but the very local scale. Nevertheless, the potential for nutrient bioremediation, carbon sequestration, water clarity improvements and habitat provision will grow if densities increase in combination with the spatial extent of the Solent’s populations across the multiple habitats. The effects on cultural services, such as human health and recreation, are some of the most difficult to predict, but could have the most direct and widespread impact on people within the region and as well as the blue economy.
Impact assessments and management plans for newly arrived species must be balanced by considering both negative and positive impacts, such as those in Tables 2, 3, 4 and 5, and accounting for shifting baselines (see discussion by Crooks71). The imperative is to answer the key questions we have posed in Tables 2, 3, 4 and 5 about the effects (both positive and negative) and the subsequent risks to European habitats and coastal economies. This requires investment in monitoring, but also examination of the potential interactions between A. senhousia and key habitats and species. This two-pronged approach is essential for determining whether A. senhousia or other biotic and abiotic factors are responsible for ecosystem change71. Moreover, as previous invasion trajectories of A. senhousia are diverse, predicting the impacts on services (and any restoration efforts to improve colonised but protected habitats) will be challenging without context-relevant experimental data. For example, Mastrototaro et al.10 found that a population in the Mediterranean had increased to densities of up to 3800 m−2 within two years of arriving. In contrast, the density of a population in Auckland, New Zealand declined by 60% in one year, decreasing from 16,000 m−2 to 5,500 m262. Large temporal variation in density is typical of an opportunistic species, with highly erratic population dynamics, increasing the risk of population extinctions as well as expansions1,23,63. The risk of rapid non-native species population expansion emphasises the need for prompt responses to new introductions. The delay between the earliest detection of A. senhousiain the UK (2011) and the first published report of its arrival (2017) suggests the need for improvement of the national invasive species reporting and response systems. Furthermore, there is a need to prioritise the identified impacts of A. senhousia so that management resources can be effectively allocated. This requires identification of the ecosystem service/s at risk (this study), assessment of the magnitude and scale of ecosystem service impacts, and ecosystem service valuation (ESV)114. ESV can be done in a variety of ways including the assignment of an economic monetary value (e.g. 115). For impacted ecosystem services which have a direct value (such as commercial shellfish stocks) ESV is relatively simple, but for others with an indirect value, such as bioremediation, the replacement cost valuation method can be used (e.g. 116,117). ESV methods are still very much open to discussion118.
Conclusion
Our study confirms that A. senhousia has been in the Solent for at least eight years, indicating stable, self-sustaining populations located on the periphery of the Greater North Sea ecoregion (and by extension Europe). We believe that A. senhousia is likely to spread further within this region. In fact, A. senhousia has already been reported from the Netherlands (in 2018), although it is not clear whether the 30 individuals collected represent an established population119. Where A. senhousia populations establish in the future will be dependent on a wide variety of factors, such as its genetic variation and phenotypic plasticity120,121, hydrodynamics122, propagule processes, and environmental conditions123. If the lineage in the Solent is one that is predisposed to colder water adaptation (Asif and Krug61 suggested this as a reason for its ability to exist in more northerly regions within its introduced range), the colonisation of diverse waters of Europe could be eminently achievable.
The presence of established, self-sustaining A. senhousia UK populations that can reproduce and colonise multiple habitat types, and whilst tolerating variable environmental conditions, highlights a potentially significant risk to the blue economy and natural capital within the Greater North Sea. We advocate that increased monitoring of this species is essential, especially in habitats of conservation and commercial importance. We also recommend the completion of a thorough and standardised risk assessment to aid awareness raising, inform policy and facilitate prioritisation of actions. Concurrently, determined efforts should be made to address the fundamental ecological and biological questions we have highlighted to confirm if A. senhousia will, soon be added to Europe’s list of invasive non-native species.
Data Availability
All raw data can be made available upon request to the authors.
Change history
13 August 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-96119-8
References
Crooks, J. A. The population ecology of an exotic mussel, Musculista senhousia, in a Southern California bay. Estuaries 19, 42–50 (1996).
Huber, M. Compendium of Bivalves: A Full-Color Guide to 3,300 of the World’s Marine Bivalves: A Status on Bivalvia After 250 Years of Research. (ConchBooks, 2010).
Kulikova, V. A. Morphology, seasonal population dynamics, and settlement of larvae of the bivalve mollusc Musculista senhousia in Busse Lagoon (South Sakhalin). Sov. J. Mar. Biol. 4, 769–773 (1978).
Chuang, S. H. On Malayan shores: a log cabin book. (Muwu Shosa, 1961).
CABI. Arcuatula senhousia [original text by A. Zenetos]. In: Invasive Species Compendium. CAB International, Wallingford, UK. www.cabi.org/isc (2019).
Kincaid, T. The acclimitization of marine animals in Pacific northwest waters. Min. Conchol Club South. Calif. 72, 1–3 (1947).
Willan, R. C. Successful establishment of the Asian mussel Musculista senhousia (Benson in Cantor, 1842) in New Zealand. Rec. Auckl. Inst. Museum 22, 85–96 (1985).
Willan, R. C. The mussel Musculista senhousia in Australasia; another aggressive alien highlights the need for quarantine at ports. Bull. Mar. Sci. 41, 475–489 (1987).
Hoenselaar, H. J. & Hoenselaar, J. Musculista senhousia (Benson in Cantor, 1842) in the western Mediterranean (Bivalvia, Mytilidae). Basteria 53, 73–76 (1989).
Mastrototaro, F., Matarrese, A. & D’Onghia, G. Occurrence of Musculista senhousia (Mollusca: Bivalvia) in the Taranto seas (eastern-central Mediterranean Sea). J. Mar. Biol. Ass. UK 83, 1279–1280 (2003).
Micu, D. First record of Musculista senhousia (Brenson in Cantor, 1842) from the Black Sea. (Abstracts of the International Symposium of Malacology, 19–22 Aug 2004, Sibiu, Romania. p. 47, 2004).
Ruci, S., Kasemi, D. & Beqiraj, S. Data on macrozoobenthos in rocky areas of the Adriatic Sea of Albania. IMPACT Int. J. Res. Appl. Nat. Soc. Sci. 2, 63–70 (2014).
Kovalev, E. A., Zhivoglyadova, L. A., Revkov, N. K., Frolenko, L. N. & Afanasyev, D. F. First record of the bivalve Arcuatula senhousia (Benson, 1842) in the Russian part of the the Azov-Black Sea basin. Russ. J. Biol. Invasions 8, 316–320 (2017).
Lourenço, P. M., Henriques, M., Catry, I., Pedro, J. & Catry, T. First record of the invasive Asian date mussel Arcuatula senhousia (Benson, 1842) (Mollusca: Bivalvia: Mytilidae) in West Africa. J. Nat. Hist. 52, 2567–2571 (2018).
Barash, A. & Danin, Z. The Indo-Pacific species of Mollusca in the Mediterranean and notes on a collection from the Suez Canal. Isrl. J. Zool. 21, 301–374 (1972).
George, E. L. & Nair, N. B. The growth rates of the estuarine mollusc Musculista arcuatula Yamamoto and Habe (Bivalvia: Mytilidae). Hydrobiologia 45, 239–248 (1974).
Morton, B. Life-history characteristics and sexual strategy of Mytilopsis sallei (Bivalvia: Dreissenacea), introduced into Hong Kong. J. Zool. 219, 469–485 (1989).
Sakai, A. K. et al. The population biology of invasive species. Annu. Rev. Ecol. Evol. Syst. 32, 305–332 (2001).
Sgro, L., Turolla, E., Rossi, R. & Mistri, M. Sexual maturation and larval development of the immigrant Asian date mussel, Musculista senhousia, in a Po River deltaic lagoon. Ital. J. Zool. 69, 223–228 (2002).
CIESM. Musculista senhousia. In: Atlas of Exotic Species in the Mediterranean. The Mediterranean Science Commission (CIESM). https://www.ciesm.org/atlas (2005).
Cohen, A. N. Musculista senhousia. In: The Exotics Guide: Non-native Marine Species of the North American Pacific Coast. Centre for Research on Aquatic Bioinvasions; San Francisco Estuary Institute. www.exoticsguide.org (2011).
Morton, B. Some aspects of the biology, population dynamics, and functional morphology of Musculista senhousia Benson (Bivalvia, Mytilidae). Pac. Sci. 28, 19–33 (1974).
Mistri, M. Ecological characteristics of the invasive Asian date mussel, Musculista senhousia, in the Sacca di Goro (Adriatic Sea, Italy). Estuaries 25, 431–440 (2002).
Bachelet, G. et al. A round-the-world tour almost completed: first records of the invasive mussel Musculista senhousia in the north-east Atlantic (southern Bay of Biscay). Mar. Biodivers. Rec. 2, e119 (2009).
Holman, L. E. et al. Detection of introduced and resident marine species using environmental DNA metabarcoding of sediment and water. Sci. Rep. 9, 1 (2019).
Barfield, P., Holmes, A., Watson, G. & Rowe, G. First evidence of Arcuatula senhousia (Benson, 1842), the Asian date mussel in UK waters. J. Conchol. 43, 217–222 (2018).
ICES. Maps: ICES statistical rectangles. https://www.ices.dk/data/maps/Pages/ICES-statistical-rectangles.aspx (2020).
World Sea Temperature. Southampton Sea Temperature. https://www.seatemperature.org/europe/united-kingdom/southampton.htm (2020).
Natural England. Solent Maritime EMS. Natural England, UK. http://publications.naturalengland.org.uk/publication/3194402 (2001).
Katsanevakis, S., Wallentinus, I., Zenetos, A., Leppäkoski, E. & Çinar, M. E. Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquat. Invas. 9, 391–423 (2014).
Bouma, T. J., Olenin, S. & Reise, K. Ecosystem engineering and biodiversity in coastal sediments: posing hypotheses. Helgol. Mar. Res. 63, 95–106 (2009).
NCC. Towards a Framework for Defining and Measuring Change in Natural Capital. Working Paper 1. (Natural Capital Committee (NCC), 2014).
Jeschke, J. M. et al. Defining the impact of non-native species. Conserv. Biol. 28, 1188–1194 (2014).
Reusch, T. B. H. & Williams, S. L. Variable responses of native eelgrass Zostera marina to a non-indigenous bivalve Musculista senhousia. Oecologia 113, 428–441 (1998).
Albentosa, M. Effect of food concentration inside eelgrass beds on the energy balance of the invasive mussel Musculista senhousia. Mar. Fresh. Behav. Physiol. 35, 247–260 (2002).
Allen, B. J. & Williams, S. L. Native eelgrass Zostera marina controls growth and reproduction of an invasive mussel through food limitation. Mar. Ecol. Prog. Ser. 254, 57–67 (2003).
Lau, S. C. Y., Brettell, D. L. D. F. & Astudillo, J. C. Rapid assessment of the invasive Xenostrobus securis on cultured oysters in Hong Kong. Reg. Stud. Mar. Sci. 17, 11–16 (2018).
Mistri, M., Rossi, R. & Fano, E. A. The spread of the alien bivalve (Musculista senhousia) in the Sacca Di Goro lagoon (Adriatic Sea, Italy). J. Moll. Stud. 70, 257–261 (2004).
Hosozawa, T. et al. Temporal change in the spatial distribution of Asian bag mussel Arcuatula senhousia (Bivalvia, Mytilidae) population in Ohashi-River, Shimane Prefecture. . Japanese J. Benthol. 70, 1–12 (2015).
Crooks, J. A. Habitat alteration and community-level effects of an exotic mussel, Musculista senhousia. Mar. Ecol. Prog. Ser. 162, 137–152 (1998).
Crooks, J. A. & Khim, H. S. Architectural vs. biological effects of a habitat-altering, exotic mussel, Musculista senhousia. J. Exp. Mar. Bio. Ecol. 240, 53–75 (1999).
Watson, G. J., Murray, J. M., Schaefer, M. & Bonner, A. Bait worms: a valuable and important fishery with implications for fisheries and conservation management. Fish Fish. 18, 374–388 (2016).
Clarke, L. J. et al. Using remote sensing to quantify fishing effort and predict shorebird conflicts in an intertidal fishery. Ecol. Inform. 50, 136–148 (2019).
European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. (2000).
Siah, A., Pellerin, J., Amiard, J. C., Pelletier, E. & Viglino, L. Delayed gametogenesis and progesterone levels in soft-shell clams (Mya arenaria) in relation to in situ contamination to organotins and heavy metals in the St. Lawrence River (Canada). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 135, 145–156 (2003).
Harding, S., Nelson, L. & Glover, T. Solent Oyster Restoration Project Management Plan (Blue Marine Foundation (BLUE), 2016).
Hooper, T. et al. Application of the natural capital approach to the marine environment to aid decision-making. Ecosyst. Serv. 38, 100947 (2019).
Thornton, A. et al. Initial natural capital accounts for the UK marine and coastal environment. Final Report. Report prepared for the Department for Environment Food and Rural Affairs. (Joint Nature Conservation Committee (JNCC); Centre for Environment, Fisheries and Aquaculture Science (CEFAS), 2019).
Worsfold, T. M., Pennisi, N. & Ashelby, C. W. Theora lubrica Gould, 1861 (Bivalvia: Semelidae), new to the UK, with notes on associated non-native species, and an earlier date of introduction for Arcuatula senhousia (Bivalvia: Mytilidae) to the UK. J. Conchol. 43, 665–674 (2020).
Wolff, W. J. & Reise, K. Oyster Imports as a Vector for the Introduction of Alien Species into Northern and Western European Coastal Waters. in Invasive Aquatic Species of Europe. Distribution, Impacts and Management (eds. Leppäkoski, E., Gollasch, S. & Olenin, S.) 193–205 (Springer, 2002).
Slack-Smith, S. M. & Brearley, A. Musculista senhousia (Benson, 1842); a mussel recently introduced into the Swan River estuary, Western Australia (Mollusca: Mytilidae). Rec. West. Aust. Museum 13, 225–230 (1987).
Slijkerman, D. M. E. et al. Monitoring Groningen Sea Ports. Non-indigenous species and risks from ballast water in Eemshaven and Delfzijl. Wageningen Marine Research report C045/17 A. (University of Wageningen, 2017).
Kim, H. M. et al. Epibionts associated with floating Sargassum horneri in the Korea strait. Algae 34, 303–313 (2019).
Reusch, T. B. H. & Williams, S. L. Macrophyte canopy structure and the success of an invasive bivalve. Oikos 84, 398–416 (1999).
Mastrototaro, F., Matarrese, A. & D’Onghia, G. Observations on the recruitment of Musculista senhousia (Mollusca, Bivalvia) in the Taranto seas (Eastern-Central Mediterranean Sea). Biogeographia 25, 55–63 (2004).
Verween, A., Vincx, M. & Degraer, S. The effect of temperature and salinity on the survival of Mytilopsis leucophaeata larvae (Mollusca, Bivalvia): The search for environmental limits. J. Exp. Mar. Bio. Ecol. 348, 111–120 (2007).
Pilditch, C. A. & Grant, J. Effect of temperature fluctuations and food supply on the growth and metabolism of juvenile sea scallops (Placopecten magellanicus). Mar. Biol. 134, 235–248 (1999).
Vélez, A. & Epifanio, C. E. Effects of temperature and ration on gametogenesis and growth in the tropical mussel Perna perna (L.). Aquaculture 22, 21–26 (1981).
Liang, Z. L., Kim, Y. H., Zhang, Z. F., Lim, S. M. & Kang, K. H. Water temperature and salinity tolerance of embryos and spat of the mussel, Musculista senhousia. Korean J. Malacol. 25, 179–187 (2009).
Inoue, T. & Yamamuro, M. Respiration and ingestion rates of the filter-feeding bivalve Musculista senhousia: implications for water-quality control. J. Mar. Syst. 26, 183–192 (2000).
Asif, J. H. & Krug, P. J. Lineage distribution and barriers to gene flow among populations of the globally invasive marine mussel Musculista senhousia. Biol. Invas. 14, 1431–1444 (2012).
Creese, R., Hooker, S., de Luca, S. & Wharton, Y. Ecology and environmental impact of Musculista senhousia (Mollusca: Bivalvia: Mytilidae) in Tamaki Estuary, Auckland, New Zealand. New Zeal. J. Mar. Freshw. Res. 31, 225–236 (1997).
Crooks, J. A. & Soulé, M. Lag times in population explosions of invasive species: causes and implications. in Invasive Species and Biodiversity Management (eds. Sandlund, O. T., Schei, P. J. & Viken, A.) 103–125 (Kluwer Academic Publishers, 1999).
Yamamuro, M. & Jun, Æ. What prevents Musculista senhousia from constructing byssal thread mats in estuarine environments? A case study focusing on Lake Shinji and nearby estuarine waters. Lanscape Ecol Eng 6, 23–28 (2010).
Scirocco, T. & Urbano, F. The population of the non-indigenous bivalve Arcuatula senhousia of the Varano Lagoon (Adriatic Sea, Italy). J. Environ. Sci. Eng. 7, 345–353 (2018).
Yamamuro, M., Oka, N. & Hiratsuka, J. Predation by diving ducks on the biofouling mussel Musculista senhousia in a eutrophic estuarine lagoon. Mar. Ecol. Prog. Ser. 174, 101–106 (1998).
Reusch, T. B. H. Native predators contribute to invasion resistance to the non-indigenous bivalve Musculista senhousia in southern California, USA. . Mar. Ecol. Prog. Ser. 170, 159–168 (1998).
Kushner, R. B. & Hovel, K. A. Effects of native predators and eelgrass habitat structure on the introduced Asian mussel Musculista senhousia (Benson in Cantor) in southern California. J. Exp. Mar. Biol. Ecol. 332, 166–177 (2006).
Sugawara, K., Ebihara, T., Ishii, T., Aoki, K. & Uchida, A. Outbreak of a mussel Brachidontes senhousia in Urayasu shellfish rearing ground. Rep. Chiba Prefect. Inshore Fish. Exp. Stn. 3, 83–92 (1961).
Uchida, A. Growth of a mussel Musculista senhousia and the influence of Musculista senhousia on the clam Tapes philippinarum. Rep. Chiba Prefect. Inshore Fish. Exp. Stn. 7, 69–78 (1965).
Crooks, J. A. Assessing invader roles within changing ecosystems: historical and experimental perspectives on an exotic mussel in an urbanized lagoon. Biol. Invasions 3, 23–36 (2001).
Castorani, M. C. N. & Hovel, K. A. Invasive prey indirectly increase predation on their native competitors. Ecology 96, 1911–1922 (2015).
FAO. Fisheries Global Information System (FIGIS). Food and Agriculture Organization (FAO). http://www.fao.org/figis/servlet/TabSelector (2017).
CEFAS. Sanitary survey of the Solent. CEFAS report on behalf of the Food Standards Agency, to demonstrate compliance with the requirements for classification of bivalve mollusc production areas in England and Wales under of EC Regulation No. 854/2004. (Centre for Environment, Fisheries and Aquaculture Science (CEFAS), 2013).
Humphreys, J., Caldow, R. W. G., Mcgrorty, S., West, A. D. & Jensen, A. C. Population dynamics of naturalised manila clams Ruditapes philippinarum in british coastal waters. Mar. Biol. 151, 2255–2270 (2007).
Pranovi, F. et al. An ecological imbalance induced by a non-native species: The Manila clam in the Venice Lagoon. Biol. Invasions 8, 595–609 (2006).
Kikuchi, T. & Peres, J. M. Consumer ecology of seagrass beds. In Seagrass Ecosystems A Scientific Perspective (eds McRoy, C. P. & Helffrich, C.) (Marcel Dekker Inc, 1977).
Kikuchi, T. Ecology and biological production of Lake Naka-umi and adjacent regions. 3. Macro-benthic communities of Lake Shinji-ko and Lake Naka-umi. Spec. Publ. from Seto Mar. Biol. Lab. 2, 21–44 (1964).
Jackson, E. L., Rees, S. E., Wilding, C. & Attrill, M. J. Use of a seagrass residency index to apportion commercial fishery landing values and recreation fisheries expenditure to seagrass habitat service. Conserv. Biol. 29, 899–909 (2015).
Peters, J. R., McCloskey, R. M., Hinder, S. L. & Unsworth, R. K. F. Motile fauna of sub-tidal Zostera marina meadows in England and Wales. Mar. Biodivers. 45, 647–654 (2015).
Unsworth, R. K. F., Nordlund, L. M. & Cullen-Unsworth, L. C. Seagrass meadows support global fisheries production. Conserv. Lett. 12, 1–8 (2019).
Bertelli, C. M. & Unsworth, R. K. F. Protecting the hand that feeds us: Seagrass (Zostera marina) serves as commercial juvenile fish habitat. Mar. Pollut. Bull. 83, 425–429 (2014).
UNEP. Out of Blue: The value of seagrasses to the enviroment and to people. (United Nations Environment Programme (UNEP), 2020).
Jones, B. L. & Unsworth, R. K. F. The perilous state of seagrass in the British Isles. R. Soc. Open Sci. 3, 1–14 (2016).
de los Santos, C. B. et al. Recent trend reversal for declining European seagrass meadows. Nat. Commun. 10, 1–8 (2019).
Project Seagrass. Project Seagrass. https://www.projectseagrass.org/ (2018).
Claereboudt, M. R., Bureau, D., Côté, J. & Himmelman, J. H. Fouling development and its effect on the growth of juvenile giant scallops (Placopecten magellanicus) in suspended culture. Aquaculture 121, 327–342 (1994).
Lacoste, E. & Gaertner-Mazouni, N. Biofouling impact on production and ecosystem functioning: a review for bivalve aquaculture. Rev. Aquac. 7, 187–196 (2015).
Renault, T. Appearance and spread of diseases among bivalve molluscs in the northern hemisphere in relation to international trade. OIE Rev. Sci. Tech. 15, 551–561 (1996).
Beaumont, A. R., Hawkins, M. P., Doig, F. L., Davies, I. M. & Snow, M. Three species of Mytilus and their hybrids identified in a Scottish Loch: natives, relicts and invaders?. J. Exp. Mar. Bio. Ecol. 367, 100–110 (2008).
Miller, A., Inglis, G. J., Poulin, R. & Inglis, G. J. Use of the introduced bivalve, Musculista senhousia, by generalist parasites of native New Zealand bivalves. New Zeal. J. Mar. Freshw. Res. 42, 143–151 (2008).
Bierbaum, R. & Shumway, S. E. Filtration and oxygen consumption in mussels, Mytilus edulis, with and without pea crabs, Pinnotheres maculatus. Estuaries 11, 264–271 (1988).
Sun, W., Sun, S., Yuqi, W., Baowen, Y. & Weibo, S. The prevalence of the pea crab, Pinnotheres sinensis, and its impact on the condition of the cultured mussel, Mytilus galloprovincialis, in Jiaonan waters (Shandong Province, China). Aquaculture 253, 57–63 (2006).
Morris, J. P., Backeljau, T. & Chapelle, G. Shells from aquaculture: a valuable biomaterial, not a nuisance waste product. Rev. Aquac. 11, 42–57 (2019).
Carlton, J. T. History, biogeography, and ecology of the introduced marine and estuarine invertebrates of the Pacific coast of North America. PhD Thesis. (University of California, 1979).
Michalek, K., Ventura, A. & Sanders, T. Mytilus hybridisation and impact on aquaculture: A minireview. Mar. Genomics 27, 3–7 (2016).
Seed, R. The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores. Oecologia 3, 277–316 (1969).
King, P. A., McGrath, D. & Gosling, E. M. Reproduction and settlement of Mytilus edulis on an exposed rocky shore in Galway bay, west coast of Ireland. J. Mar. Biol. Assoc. United Kingdom 69, 355–365 (1989).
van der Schatte Olivier, A. et al. A global review of the ecosystem services provided by bivalve aquaculture. Rev. Aquac. 1, 1–23. https://doi.org/10.1111/raq.12301 (2018).
Yamamuro, M. & Ishitobi, Y. Seasonal change in a filter-feeding bivalve Musculista senhousia population of a eutrophic estuarine lagoon. J. Mar. Syst. 26, 117–126 (2000).
Broszeit, S., Hattam, C. & Beaumont, N. Bioremediation of waste under ocean acidification: Reviewing the role of Mytilus edulis. Mar. Pollut. Bull. 103, 5–14 (2016).
Valipour, R., Boegman, L., Bouffard, D. & Rao, Y. R. Sediment resuspension mechanisms and their contributions to high-turbidity events in a large lake. Limnol. Oceanogr. 62, 1045–1065 (2017).
Mistri, M. & Munari, C. The invasive bag mussel Arcuatula senhousia is a CO2 generator in near-shore coastal ecosystems. J. Exp. Mar. Bio. Ecol. 440, 164–168 (2013).
Filgueira, R. et al. An integrated ecosystem approach for assessing the potential role of cultivated bivalve shells as part of the carbon trading system. Mar. Ecol. Prog. Ser. 518, 281–287 (2015).
Reaugh, K. E., Harris, J. M. & Branch, G. M. Further refutation of the primary-secondary settlement hypothesis for the brown mussel Perna perna. African J. Mar. Sci. 29, 545–549 (2007).
Cohen, A. N. Guide to the Exotic Species of San Francisco Bay. San Francisco Estuary Institute, Oakland, California, USA. http://www.exoticsguide.org (2005).
Como, S. et al. Assessing the impact of the Asian mussel Arcuatula senhousia in the recently invaded Oristano Lagoon-Gulf system (W Sardinia, Italy). Estuar. Coast. Shelf Sci. 201, 123–131 (2018).
Ragnarsson, S. Á. & Raffaelli, D. Effects of the mussel Mytilus edulis L. on the invertebrate fauna of sediments. J. Exp. Mar. Bio. Ecol. 241, 31–43 (1999).
Barash, A. L. & Danin, Z. Mollusca from the stomach of Sparus auratus fished in the lagoon or Bardwall. Argamon 2, 97–104 (1971).
Taylor, D. et al. Facilitation effects of invasive and farmed bivalves on native populations of the sea slug Pleurobranchaea maculata. Mar. Ecol. Prog. Ser. 537, 39–48 (2015).
Herbert, R. J. H., Stillman, R. A., Davies, C. J., Bowgen, K. M. & Hatton, J. The importance of nonnative Pacific oyster reefs as supplementary feeding areas for coastal birds on estuary mudflats. Aquat. Conserv. Mar. Freshw. Ecosyst. 28, 1294–1307 (2018).
Hanna, G. D. Introduced mollusks of western North America. Occ. Pap. Calif. Acad. Sci. 48, 1–108 (1966).
Vilariño, M. L. et al. Assessment of human enteric viruses in cultured and wild bivalve molluscs. Int. Microbiol. 12, 145–151 (2009).
Vilà, M. & Hulme, P. E. Impact of Biological Invasions on Ecosystem Services. (Springer International Publishing Switzerland, 2017). https://doi.org/10.1007/978-3-319-45121-3_5.
Williams, F. et al. The Economic Cost of Invasive Non-Native Species on Great Britain. Cent. Agric. Biosci. Int. CAB/001/09, 1–199 (2010).
Watson, S. C. L., Preston, J., Beaumont, N. J. & Watson, G. J. Assessing the natural capital value of water quality and climate regulation in temperate marine systems using a EUNIS biotope classification approach. Sci. Total Environ. (2020)
Farber, S. C., Costanza, R. & Wilson, M. A. Economic and ecological concepts for valuing ecosystem services. Ecol. Econ. 41, 375–392 (2002).
Melathopoulos, A. P. & Stoner, A. M. Critique and transformation: On the hypothetical nature of ecosystem service value and its neo-Marxist, liberal and pragmatist criticisms. Ecol. Econ. 117, 173–181 (2015).
Faasse, M. A record of the Asian mussel Arcuatula senhousia (Benson in Cantor, 1842) from NW Europe (the Netherlands). Spirula 416, 14–15 (2018).
Tepolt, C. K. & Somero, G. N. Master of all trades: Thermal acclimation and adaptation of cardiac function in a broadly distributed marine invasive species, the European green crab, Carcinus maenas. J. Exp. Biol. 217, 1129–1138 (2014).
Guardiola, M., Frotscher, J. & Uriz, M. J. High genetic diversity, phenotypic plasticity, and invasive potential of a recently introduced calcareous sponge, fast spreading across the Atlanto-Mediterranean basin. Mar. Biol. 163, 1–16 (2016).
Shanks, A. L. Pelagic larval duration and dispersal distance revisited. Biol. Bull. 216, 373–385 (2009).
Tabak, M. A., Webb, C. T. & Miller, R. S. Propagule size and structure, life history, and environmental conditions affect establishment success of an invasive species. Sci. Rep. 8, 1–9 (2018).
Acknowledgements
We thank Dr. Hannah Tidbury (CEFAS) for assisting with pre-submission reviews of this paper, and Dr. Luke Helmer (University of Portsmouth) and Eric Harris-Scott (University of Portsmouth) for providing data relating to the Solent Oyster Restoration Project. We also thank Tom Newton and Tullus Bergmann for supplying data on behalf of the Environment Agency. Further, we thank Luke Holman (University of Southampton), Robin Somes (Pisces Conservation Ltd.) and Samuel Hatherly (University of Portsmouth) for kindly providing records for A. senhousia. The project was supported by a University of Portsmouth Bursary scheme provided to K. G. Dey as well as the TEMITH project, funded by European Space Agency (contract number: 4000125822/18/I-NB)
Author information
Authors and Affiliations
Contributions
G. J. Watson, J. Dyos and P. Barfield conceived and designed experiments. G. J. Watson and J. Dyos conducted field work. G. J. Watson, J. Dyos and K. G. Dey performed the laboratory work. G. J. Watson, J. Dyos and K. G. Dey analysed the data. K. G. Dey, G. J. Watson, J. Dyos, P. Barfield, and P. Stebbing wrote the manuscript. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Supplementary Information file published with this Article contained an error in the Author list, where Hannah Tidbury was incorrectly listed as an author.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watson, G.J., Dyos, J., Barfield, P. et al. Evidence for self-sustaining populations of Arcuatula senhousia in the UK and a review of this species’ potential impacts within Europe. Sci Rep 11, 9678 (2021). https://doi.org/10.1038/s41598-021-86876-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86876-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.