Abstract
Hydroxychloroquine has recently received attention as a treatment for COVID-19. However, it may prolong the QTc interval. Furthermore, when hydroxychloroquine is administered concomitantly with other drugs, it can exacerbate the risk of QT prolongation. Nevertheless, the risk of QT prolongation due to drug-drug interactions (DDIs) between hydroxychloroquine and concomitant medications has not yet been identified. To evaluate the risk of QT prolongation due to DDIs between hydroxychloroquine and 118 concurrent drugs frequently used in real-world practice, we analyzed the electrocardiogram results obtained for 447,632 patients and their relevant electronic health records in a tertiary teaching hospital in Korea from 1996 to 2018. We repeated the case–control analysis for each drug. In each analysis, we performed multiple logistic regression and calculated the odds ratio (OR) for each target drug, hydroxychloroquine, and the interaction terms between those two drugs. The DDIs were observed in 12 drugs (trimebutine, tacrolimus, tramadol, rosuvastatin, cyclosporin, sulfasalazine, rofecoxib, diltiazem, piperacillin/tazobactam, isoniazid, clarithromycin, and furosemide), all with a p value of < 0.05 (OR 1.70–17.85). In conclusion, we found 12 drugs that showed DDIs with hydroxychloroquine in the direction of increasing QT prolongation.
Similar content being viewed by others
Introduction
The COVID-19 pandemic has caused more than 14 million cases of the disease worldwide as of July 22, 20201, and to the best of our knowledge, no drug has been proven to target this virus to date. Meanwhile, one of the many attempts with off-label practice that received attention for treating COVID-19 was the use of hydroxychloroquine, a conventional antimalarial treatment2,3.
However, several studies have reported the cardiotoxic side effects of hydroxychloroquine4. Furthermore, when hydroxychloroquine was administered together with other drugs such as azithromycin, it can exacerbate the risk of QT prolongation due to drug-drug interactions (DDIs)5,6,7,8.
Hydroxychloroquine is also commonly used for some chronic diseases such as rheumatoid arthritis9 or systemic lupus erythematosus10,11. In these chronic diseases, hydroxychloroquine is usually taken over a long term, so it is often used in combination with other drugs. However, the risk of QT prolongation caused by DDIs between hydroxychloroquine and other medications has not yet been assessed comprehensively. The lack of evidence on the level of QT prolongation risk caused by DDIs poses a challenge to both physicians and regulators in treating patients.
To analyze the risk of QT prolongation caused by DDIs retrospectively from the perspective of real-world data, a large amount of electrocardiogram (ECG) results and drug prescription records are needed. Drug prescription data are usually easily accessible in electronic medical records (EMR) but extracting QTc interval information from the ECG results stored in hospital information systems could be a barrier to conducting large-scale studies with ECG data. We have made an effort to collect portable ECG results from inpatients and outpatients for the last few years12. In this database, ECG parameters such as RR, PR, QRS, QT, and QTc intervals were extracted from raw ECG signals. The ECG database enabled us to conduct a study to provide direct evidence of QT prolongation caused by DDIs between hydroxychloroquine and other co-medications.
In this study, we aimed to evaluate the risk of QT prolongation caused by DDIs between hydroxychloroquine and other frequently used co-medications in real-world practice by conducting large-scale retrospective case–control studies.
Methods
This retrospective study was conducted based on EMR data. The Institutional Review Board of Ajou University Hospital approved the study (IRB No. AJIRB-MED-MDB-19-406) and waived the requirement for informed consent because only anonymized data were used retrospectively. All research was performed in accordance with relevant guidelines and regulations.
Data sources
We used the EMR database of Ajou University Hospital, a tertiary teaching hospital in Korea, recorded between January 1996 and May 2018. The database included 177,841,556 prescriptions, 379,994,144 laboratory test results, and 3,024,891 patient demographics.
QTc values from the same observational period were extracted from the local ECG repository in the MUSE system12,13. The ECG report typically contains both alphanumeric values and waveform graphs. The QTc data were extracted by parsing alphanumeric data from the PDF data extracted from the ECG repository using the web-scraping technique in our previous study12. This ECG database contains 1,040,752 ECG results from 447,632 patients as of January 2018 (Fig. 1). Patients whose clinical and ECG data were recorded in both EMR and our ECG repository were finally enrolled in this analysis.
Overview of the study process. Among 1,040,752 ECG results, 992,968 ECGs that have no duplicated or repeated measurements were extracted and selected one ECG for one patient with sex and age information with no age outlier. Total of 444,475 ECG results were enrolled and divided into QT prolongation case (n = 58,258) vs. control (n = 386,317). Multiple logistic regression analysis was repeated iteratively for each target drugs.
Study design and population
This study consisted of a series of retrospective case–control studies. In each study, we assessed the risk of QT prolongation due to DDI between hydroxychloroquine and one of the drugs (target drug) concomitantly used. We iterated this process for selected candidate drugs used in the subject hospital. The differences in drug use between patients with QT prolongation and those without QT prolongation were compared (Fig. 1). QT prolongation was defined as an event where corrected QT (QTc), calculated using the Bazett formula, was greater than 450 ms for males and 460 ms for females.
We excluded the following ambiguous ECG results: (1) automatically duplicated ECG measurements known as systemic errors (n = 84), and (2) sequentially measured ECG performed within 30 min, because these may reflect that the previous measurement could be an error (n = 47,700). We then randomly selected one ECG result per patient (n = 447,632). ECGs without sex or age information in the EMR and ECGs with age outliers (age < 0 and age > 120) were removed (n = 3057).
Selection of candidate drugs for DDI risk analysis
To select candidate drugs, we first extracted all drugs prescribed concomitantly with hydroxychloroquine in the window of interest (from 7 days prior to the date of ECG measurement) from the EMR. Then, drugs used concomitantly more than 10 times were included in the analysis. The list of selected drugs and their frequency of concurrent use are provided in Supplementary Table S1 online.
Definition of covariates
The following variables were included in the analysis to adjust for possible confounding factors on QT prolongation: (1) demographic information including sex and age at the ECG examination date, (2) comorbidities recorded in the EMR within a year before the ECG measurement date based on the International Classification Disease-10 (ICD-10). These comorbidities included myocardial infarction, congestive heart failure, ischemic stroke, hemorrhagic stroke, diabetes mellitus, hypothyroidism, renal disease, AIDS/HIV, alcohol abuse, drug abuse, liver disease, and severe liver disease; (3) the latest serum potassium and calcium levels within a year before the ECG measurement date. In patients without laboratory test results within 1 year before the ECG examination date, we imputed the missing values using the median value from patients of the same age group divided by 10-year intervals, and (4) the use of other drugs known to increase the risk of QT prolongation prescribed within 7 days before ECG measurement. The list of medications and comorbidities applied as parametric covariates is shown in Supplementary Table S2 online.
Subgroup analysis
Because the risk of QT prolongation varies according to age and sex, we conducted a subgroup analysis. For this, we divided the subjects by sex (male and female) and age (age equal to or under 60 and age over 60). In this subgroup analysis, we included all drugs that were included in the main analysis.
Statistical analysis
We compared the demographic characteristics of the subjects, laboratory test results, comorbidities present, medication use, and year of the ECG measurement between the QT prolongation cases and controls using Pearson's chi-square tests (for categorical data) and independent two-sample t tests (for continuous data).
We adopted multiple logistic regression with interaction terms to investigate the DDI. The detailed logit model for the jth drug in the candidate drug list is as follows:
where \(P\) j(Yi = 1) is the ith subject's QT prolongation probability, α is the intercept, \({x}_{i}^{(chl)}\) is the ith study subject's use of hydroxychloroquine, \({x}_{i}^{({drug}_{j})}\) is the ith study subject's use of jth drug. \({x}_{i}^{(chl)}\times {x}_{i}^{({drug}_{j})}\) is the interaction term between \({x}_{i}^{(chl)}\) and \({x}_{i}^{({drug}_{j})}\), and \({x}_{ik}^{(cov)}\) is the kth covariate of the ith subject.
In the regression model, we calculated the coefficient of the interaction term (β3), odds ratio, and p value. When the p value of β3 was less than 0.05, we judged that hydroxychloroquine and the ith candidate drug had a statistically significant DDI.
For all drugs in the candidate drug lists, the multiple logistic regression was repeated iteratively to investigate which drugs showed a statistically significant DDI with hydroxychloroquine.
To visualize the interactions of statistically significant DDI drugs, we plotted the changes in the logit value of QT prolongation (Y-axis) according to the use of the target drug (X-axis) and that of hydroxychloroquine.
Software
Data management was performed using Azure data studio version 1.19.0, and all statistical analyses were conducted using Python version 3.7 and the Python package Statsmodel version 0.11.1. The Python packages Matplotlib version 3.2.2, and seaborn Python packages version 0.10.0 were also used for visualization of the data and results.
Results
Baseline characteristics
The total number of subjects included in the final analysis was 444,575. Among them, the QT prolongation case group had 58,258 subjects and the control group had 386,317 subjects. Of the total patients, 218,997 were men and 225,578 were women. 1417 patients received hydroxychloroquine within 7 days before their ECG examination date. The baseline characteristics of the patient group are summarized in Table 1.
Drug–drug interaction analysis
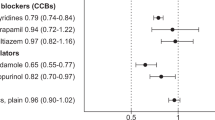
We analyzed drugs through iterative multiple regression analysis with the interaction terms. As shown in Table 2, the ORs for the interaction terms were significant in the analysis of trimebutine, tacrolimus, tramadol, rosuvastatin, cyclosporin, sulfasalazine, rofecoxib, diltiazem, piperacillin/tazobactam, isoniazid, clarithromycin, and furosemide.
In 8 of these 12 drugs (trimebutine, tramadol, rosuvastatin, cyclosporin, sulfasalazine, rofecoxib, diltiazem, and isoniazid), DDIs were present in the direction of increasing the risk of QT prolongation, even though the risk of QT prolongation was not observed with the use of these drug alone. In piperacillin, clarithromycin, and furosemide, we observed a statistically significantly higher risk of QT prolongation even when they were used alone, where DDIs were also observed. The results of the analysis of all medications are included in Supplementary Table S3 online. The full result including ORs of all covariates on tramadol is presented in Supplementary Table S4 online as an example of iterative multiple logistic regression.
In each regression model, the average logit value of the intercept was − 2.7. This corresponds to a QT prolongation probability of 0.07 when all other variables were set as 0 (i.e., having no risk factor and no exposure to both hydroxychloroquine and target drug). The average logit value when using hydroxychloroquine alone without any other risk factors was − 2.5 (QT prolongation probability of 0.08). As shown in Fig. 2, the logit value (probability of QT prolongation occurrence) increased when hydroxychloroquine and the target drug were administered at the same time compared to expected additive effect of the two drugs in all 12 drugs. This means that all 12 drugs have DDIs in the direction of increasing the risk of QT prolongation.
Interaction plots for 12 drugs that showed significant DDI with hydroxychloroquine. These plots show how the logit value (Y-axis) changes when the target drug (X-axis) is used alone and in combination with hydroxychloroquine. The 12 drugs plotted were drugs with statistically significant drug-drug interaction (DDI) in the iterative multiple logistic regression analysis. If there is a DDI, the two graphs have non-parallel slopes. In particular, when the interval between slopes increases, DDI exists in the direction of increasing QT prolongation. The interval between slopes increases in all 12 drugs.
Subgroup analysis
We divided the patient group into four subgroups according to sex and age. We then investigated whether the drugs used in each subgroup showed significant (p < 0.05) DDIs with hydroxychloroquine. We found that five drugs (tramadol, trimebutine, nifedipine, sulfasalazine, and rofecoxib) showed significant (p < 0.05) DDIs with hydroxychloroquine in the female subgroup, and three drugs (tacrolimus, paracetamol, and propacetamol) showed significant (p < 0.05) DDIs in the male patient group. Nine drugs (rosuvastatin, tacrolimus, sulfasalazine, trimebutine, ranitidine, ranitidine, diltiazem, celecoxib, paracetamol, and aceclofenac) showed DDIs with hydroxychloroquine in the group under 60 years of age. In the elderly group over 60 years old, no drug was significant (p < 0.05), but five drugs (clopidogrel, bisoprolol, furosemide, tramadol, and trimebutine) implied the possibility of a DDI (p < 0.1). The ten drugs with the highest probability of interaction for each subgroup are described in Table 3.
Discussion
Using EMRs obtained at a tertiary hospital, we investigated DDIs between hydroxychloroquine and 118 other drugs. We observed significant (p < 0.05) DDIs in 12 drugs. Among them, piperacillin/tazobactam, clarithromycin, and furosemide showed a risk of QT prolongation in individual treatment, and a DDI in the direction of increasing QT prolongation risk was also observed. However, for eight drugs (trimebutine, tramadol, rosuvastatin, cyclosporin, sulfasalazine, rofecoxib, diltiazem, and isoniazid), DDI was present in the direction of increasing the risk of QT prolongation, even though the QT prolongation risk of individual drugs was not significant (p < 0.05).
It is well known that hydroxychloroquine can cause QT prolongation14,15. With the concern that DDIs between hydroxychloroquine and other drugs may exacerbate side effects such as this, several studies on the DDI of hydroxychloroquine have been investigated14,16,17. However, existing studies have selectively studied the DDI between hydroxychloroquine and some drugs that captured the attention of clinicians, such as immunosuppressants or antibiotics16,17,18,19.
In the case of patients with severe COVID-19, dozens of drugs are prescribed simultaneously with hydroxychloroquine, not only immunosuppressants or antibiotics. These drugs are medications that are taken regularly to treat chronic underlying diseases or are prescribed to relieve the patient's symptoms without specific indications. Most of these drugs are known to have minimal effects on QT prolongation when administered individually, but there is a risk of prolonging the QT interval indirectly through DDI with hydroxychloroquine. To overcome this problem, we conducted a DDI study not only on selected drugs but for all drugs that were prescribed concurrently with hydroxychloroquine in the chosen period. Consequently, the risk of prolonging the QT interval through DDI was observed in 12 drugs, even though the risk of QT prolongation in individual prescriptions was observed in only three drugs.
According to the therapeutic class of these drugs, three antibiotics (clarithromycin, piperacillin, and isoniazid) showed DDIs, and the mechanisms of these three antibiotics were also varied. Clarithromycin is a macrolide, such as azithromycin. Therefore, a DDI between clarithromycin and hydroxychloroquine suggests the possibility of a DDI in the combined therapy of azithromycin and hydroxychloroquine, which was suggested as a COVID-19 treatment20. In this study, azithromycin was not included since there was not a sufficient number of patients who used the drug concurrently with hydroxychloroquine. DDI studies for azithromycin would be required later using a more massive clinical database so this can be investigated further.
Besides, other antibiotics can also be lethal in diseases such as COVID-19, which is deeply associated with respiratory disease. Piperacillin is a drug commonly prescribed in the hospitalization of patients in intensive care units (ICU) due to respiratory diseases such as pneumonia21. Isoniazid is a drug that is used to treat tuberculosis, a disease that has a harmful effect on the lungs in the long term. As with COVID-19, tuberculosis is more common in developing countries22. Therefore, if patients in developing countries have to treat tuberculosis and COVID-19 simultaneously, delicate QT interval monitoring is required.
Rofecoxib showed DDI with hydroxychloroquine in our main analysis, and several NSAIDs, such as celecoxib and paracetamol, showed interactions with hydroxychloroquine in the main analysis and in the young female subgroup analysis. Meanwhile, trimebutine, a spasmolytic drug commonly used for indigestion, showed DDI in the main analysis and in the young female subgroup analysis. These drugs are prescribed extensively and are often administered even in non-essential situations. Formerly, the side effects of these drugs are known to be minor. As a result, doctors are not cautious about these drugs, and they are often used as over-the-counter medicines. However, this study showed the possibility of DDI between these drugs and hydroxychloroquine, which could cause QT prolongation. Individually non-dangerous drugs could cause drug side effects due to DDI in environments where large amounts of drugs are co-administered, such as COVID-19. Unnecessary routine prescriptions should be reduced, and appropriate alternative drugs should be selected.
Tramadol, one of the most commonly prescribed opioids, also showed DDI interaction with hydroxychloroquine. The risk of QT prolongation in opioids has already been reported23. In particular, after COVID-19, it became accessible to purchase drugs on a non-face-to-face basis, and so opioid use disorder is rapidly increasing24,25. If patients with opioid use disorder are infected with COVID-19, it would be essential to prevent fatal cardiotoxic adverse effects through precise QT monitoring.
In the subgroup analysis, the drugs that showed DDIs differed substantially by subgroup. Only four drugs showed significant (p < 0.05) interactions in two or more subgroups among the drugs analyzed. These results suggest that the interaction of hydroxychloroquine with other drugs may vary by age and sex. However, the difference in the drugs showing interactions among subgroups could be due to the difference in prescription patterns for each subgroup. In the future, studies that focus on specific subgroups would require additional statistical methods to compensate for these prescription patterns.
It is common to manage DDIs in clinical practice, but the mechanisms of DDIs are not adequately understood. One possible hypothesis for the interaction of drugs is through the activity of the CYP 450 enzyme26. In the risk of QT prolongation interaction observed in this study, 7 out of 12 drugs had inhibitory effects on the metabolic pathway of hydroxychloroquine. CYP 450 2C8, and 3A4/5, especially clarithromycin and diltiazem, are known potent inhibitors of CYP 450 3A427. In addition, tramadol is also known to share the CYP 450 3A4 enzyme with hydroxychloroquine28. These drugs may interfere with the metabolism of hydroxychloroquine, leading to QT prolongation by raising the hydroxychloroquine concentration to more than necessary.
This study has some limitations. First, it used a single institutional database. In the future, a multi-center study would be required to further generalize the results of this DDI study. Second, the drug dose information was not included in this study. If the drug dose information is used in future studies, it will be possible to investigate DDI changes according to the drug dose.
Conclusion
The current study investigated DDIs with hydroxychloroquine in 118 drugs using real-world data. We found statistically significant DDI in 12 drugs (trimebutine, tacrolimus, tramadol, rosuvastatin, cyclosporin, sulfasalazine, rofecoxib, diltiazem, piperacillin/tazobactam, isoniazid, clarithromycin, and furosemide) and the direction of the DDI was toward increasing the risk of QT prolongation. In eight drugs (trimebutine, tramadol, rosuvastatin, cyclosporin, sulfasalazine, rofecoxib, diltiazem, and isoniazid), DDI was present in the direction of increasing the risk of QT prolongation, even though the risk of QT prolongation was not observed with the use of those drugs alone.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Administration USFaD. Who coronavirus disease (covid-19) dashboard. 2020.
Colson, P., Rolain, J.-M., Lagier, J.-C., Brouqui, P. & Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight covid-19. Int. J. Antimicrob. Agents 20, 105932 (2020).
Gao, J., Tian, Z. & Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of covid-19 associated pneumonia in clinical studies. Biosci. Trends 20, 20 (2020).
Mubagwa, K. Cardiac effects and toxicity of chloroquine: A short update. Int. J. Antimicrob. Agents 20, 106057–106057 (2020).
Roden, D. M., Harrington, R. A., Poppas, A. & Russo, A. M. Considerations for drug interactions on QTC in exploratory covid-19 (coronavirus disease 2019) treatment. Circulation 20, 20 (2020).
Molina, J. M. et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe covid-19 infection. Med. Mal. Infect. 50, 30085–30088 (2020).
Chorin, E. et al. QT interval prolongation and torsade de pointes in patients with covid-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm 20, 20 (2020).
Mercuro, N. J. et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (covid-19). JAMA Cardiol. 20, 20 (2020).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann. Rheum. Dis. 73, 492–509 (2014).
Fanouriakis, A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 78, 736–745 (2019).
Pons-Estel, B. A. et al. First Latin American clinical practice guidelines for the treatment of systemic lupus erythematosus: Latin American Group for the Study of Lupus (GLADEL, Grupo Latino Americano de Estudio del Lupus)-Pan-American League of Associations of Rheumatology (PANLAR). Ann. Rheum. Dis. 77, 1549–1557 (2018).
Kim, Y.-G. et al. Ecg-view ii, a freely accessible electrocardiogram database. PLoS One 12, e0176222 (2017).
Chung, D. et al. Construction of an electrocardiogram database including 12 lead waveforms. Healthc. Inform. Res. 24, 242–246 (2018).
Ducharme, J. & Farinotti, R. Clinical pharmacokinetics and metabolism of chloroquine. Clin. Pharmacokinet. 31, 257–274 (1996).
Stas, P., Faes, D. & Noyens, P. Conduction disorder and QT prolongation secondary to long-term treatment with chloroquine. Int. J. Cardiol. 127, e80–e82 (2008).
Paterson, D. L. & Singh, N. Interactions between tacrolimus and antimicrobial agents. Clin. Infect. Dis. 25, 1430–1440 (1997).
Nwankwo, J. O., Garba, M. A., Chinje, C. E., Mgbojikwe, L. O. & Emerole, G. O. Possible chloroquine-induced modification of n-acetylation of isoniazid and sulphadimidine in the rat. Biochem. Pharmacol. 40, 654–659 (1990).
Pukrittayakamee, S. et al. Pharmacokinetic interactions between primaquine and chloroquine. Antimicrob. Agents Chemother. 58, 3354–3359 (2014).
Miller, A. K. et al. Pharmacokinetic interactions and safety evaluations of coadministered tafenoquine and chloroquine in healthy subjects. Br. J. Clin. Pharmacol. 76, 858–867 (2013).
Omura, S. Macrolide Antibiotics: Chemistry, Biology, and Practice (Elsevier, 2002).
Perry, C. M. & Markham, A. Piperacillin/tazobactam. Drugs 57, 805–843 (1999).
Saunders, M. J., & Evans, C. A. Covid-19, tuberculosis, and poverty: Preventing a perfect storm. 2020.
Behzadi, M., Joukar, S. & Beik, A. Opioids and cardiac arrhythmia: A literature review. Med. Princ. Pract. 27, 401–414 (2018).
Sun, Y. et al. Challenges to opioid use disorders during covid-19. Am. J. Addict. 29, 174 (2020).
Alexander, G. C., Stoller, K. B., Haffajee, R. L., & Saloner, B. An epidemic in the midst of a pandemic: Opioid use disorder and covid-19. 2020.
Sinz, M., Wallace, G. & Sahi, J. Current industrial practices in assessing cyp450 enzyme induction: Preclinical and clinical. AAPS J. 10, 391–400 (2008).
Mayhew, B. S., Jones, D. R. & Hall, S. D. An in vitro model for predicting in vivo inhibition of cytochrome p450 3a4 by metabolic intermediate complex formation. Drug Metab. Dispos. 28, 1031–1037 (2000).
Lassen, D., Damkier, P. & Brøsen, K. The pharmacogenetics of tramadol. Clin. Pharmacokinet. 54, 825–836 (2015).
Giudicessi, J. R., Noseworthy, P. A., Friedman, P. A. & Ackerman, M. J. Urgent guidance for navigating and circumventing the QTC-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (covid-19). Mayo Clin. Proc. 20, 20 (2020).
Acknowledgements
This research was supported by a Grant (19182MFDS406) from the Ministry of Food and Drug Safety in 2020. This research was also supported by a government-wide R&D Fund project for infectious disease research (GFID), Republic of Korea (Grant number: HG18C0067). This work was also supported by the new faculty research fund of Ajou University School of Medicine.
Author information
Authors and Affiliations
Contributions
D.Y., B.C., Y.K., and T.K. conducted the research. B.C. performed data analysis and statistical analysis, interpretation of results and wrote the manuscript. Y.K. performed data analysis and wrote the manuscript. T.K. performed data analysis and interpretation of results. W.C., Y.J., J.P., and H.L. contributed to interpretation of results. B.P. contributed to statistical analysis. D.Y. revised the manuscript for important intellectual content and read and approved the final manuscript. All authors contributed meaningfully to this manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, B.J., Koo, Y., Kim, T.Y. et al. Risk of QT prolongation through drug interactions between hydroxychloroquine and concomitant drugs prescribed in real world practice. Sci Rep 11, 6918 (2021). https://doi.org/10.1038/s41598-021-86321-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86321-z
This article is cited by
-
Opioids-Induced Long QT Syndrome: A Challenge to Cardiac Health
Cardiovascular Toxicology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.