Abstract
There has been controversy over the cardiovascular safety of domperidone, attributable to the lack of a well-designed study as well as inconsistent results. This study aimed to examine the risk of severe domperidone-induced ventricular arrhythmia (VA), compared to mosapride, itopride, or non-use of all three prokinetics, in the general population. We conducted a population-based, self-controlled case series analysis. Enrolled subjects were individuals who were diagnosed with severe VA and were prescribed domperidone, mosapride, or itopride from 2003 to 2013 in the National Health Insurance Service-National Sample Cohort. The incidence rate ratio for severe VA was measured during exposure to prokinetics and compared with unexposed periods and itopride (no-proarrhythmic effect)-exposure periods, as control. A total of 2,817 subjects were included. Domperidone, mosapride, or itopride use was associated with increased risk of severe VA, compared with non-use (adjusted incidence rate ratios (IRR) of 1.342 (95% CI 1.096–1.642), 1.350 (95% CI 1.105–1.650), and 1.486 (95% CI 1.196–1.845), respectively). The risk of severe domperidone-induced VA was lower, compared to that of itopride [adjusted IRR of 0.548 (95% CI 0.345–0.870)]. Of the subjects who had been prescribed all three prokinetics, domperidone-exposure was associated with a lower risk of severe VA, compared to itopride-exposure (crude IRR, 0.571; 0.358–0.912). Mosapride-exposure did not show IRR difference for severe VA, compared to itopride-exposure. Domperidone, mosapride, or itopride use is associated with an increased risk of severe VA. However, the magnitude of association was modest and domperidone use does not increase further the risk, compared with other prokinetics.
Similar content being viewed by others
Introduction
Domperidone is effective for treating nausea and vomiting, delayed gastric emptying, and dyspepsia1,2 and is preferred to other commonly prescribed prokinetic agents such as metoclopramide and mosapride2. It is not associated with central nervous system side effects because it does not readily cross the blood–brain barrier1,2. Domperidone is more effective than other prokinetic agents in treating gastroparesis3,4. and exhibits concomitant central antiemetic activity through dopamine receptors within the chemoreceptor trigger zone5. Despite all these advantages, the clinical use of oral domperidone is currently limited to individuals with an FDA Investigational New Drug exemption (treatment-refractory gastroparesis), owing to the risk of severe ventricular arrhythmia (VA)2.
Cardiac safety concerns were raised in the 1980s, when several serious cardiac events were reported6,7,8,9. Intravenous domperidone has since been withdrawn from the market, with limited use of oral domperidone because of a possible association with cardiotoxicity10,11,12,13,14. Although case series and retrospective case–control studies exist on the association between domperidone use and severe VA, there has been no prospective study or randomized controlled study with an appropriate control. Thus, we aimed to investigate the cardiac safety issue of domperidone by conducting a self-controlled case series (SCCS) using a large population-based cohort and an appropriate control.
Results
We identified 2,817 patients with at least one incident of severe VA and exposure to domperidone, mosapride, or itopride between 2003 and 2013 (Fig. 1). The study medication at cohort entry was domperidone in 70.3%, mosapride in 70.9%, and itopride in 60.8% of the patients (Table 1). The median age at cohort entry was 47 years (45–49 years) and 46.0% of patients were males. Severe VA was observed in 2,487 cases during 25,130.7 person-year non-exposure periods (incidence rate, 9.9 cases per 100 person-years), 117 cases during 813.95 person-year domperidone-exposure periods (incidence rate, 14.4 cases per 100 person-years), 114 cases during 776.6 person-year mosapride-exposure periods (incidence rate 14.7 cases per 100 person-years), and 99 cases during 608.5 person-year itopride-exposure periods (incidence rate, 16.3 cases per 100 person-years) (Table 2). Severe VA crude IRRs for domperidone, mosapride, and itopride, compared to non-exposure were as follows: 1.345 (95% CI 1.100–1.646, p = 0.004), 1.541 (95% CI 1.266–1.877, p < 0.001), and 1.635 (95% CI 1.319–2.026, p < 0.001) (Table 3). When adjusted for confounders (age, sex, and co-morbidities), the association remained similar.
Of the 918 patients who had been prescribed all three prokinetics, severe VA was observed in 765 cases during the 8,332 person-year unexposed periods, 36 cases during the 397.73 person-year domperidone-exposure periods, 57 cases during the 384.1 person-year mosapride-exposure periods, and 60 cases during the 354.4 person-year itopride-exposure periods. Domperidone-exposure was not associated with increased risk of severe VA (crude IRR; 0.948, 0.672–1.338, p = 0.762), whereas mosapride and itopride-exposure were associated with increased risk of severe VA, compared to non-exposure. After adjusting for confounders (age, sex, and co-morbidities), these associations remained unchanged (Table 4).
Of the 2,817 patients who had been prescribed any of three prokinetics, severe VA was observed in 2,487 cases during the 25,130.7 person-year unexposed periods, 117 cases during the 814 person-year domperidone-exposure periods, 114 cases during the 776.6 person-year mosapride-exposure periods, and 99 cases during the 608.5 person-year itopride-exposure periods (Table 5). All prokinetic-exposure was associated with increased risk of severe VA, compared to non-exposure.
The IRRs of severe domperidone- and mosapride-induced VA were compared to itopride-exposure. Domperidone-exposure was associated with a lower risk of severe VA, compared to itopride-exposure (crude IRR, 0.512; 0.328–0.801; p = 0.003), whereas mosapride-exposure failed to show any significant IRR difference for severe VA, compared to itopride-exposure (crude IRR, 1.111; 0.772–1.598; p = 0.571) (Table 6). After adjusting for confounders (age and co-morbidities), these associations remained unchanged. Also, of the patients who had been prescribed all three prokinetics, domperidone-exposure was associated with a lower risk of severe VA, compared to itopride-exposure (crude IRR, 0.571; 0.358–0.912), whereas mosapride-exposure did not show any significant IRR difference for severe VA, compared to itopride-exposure (crude IRR, 0.985; 0.655–1.481) (Table 7). Similarly, of the patients who had been exposed to any prokinetics, domperidone-exposure was associated with a lower risk of severe VA, compared to itopride-exposure (IRR, 0.678; 0.462–0.997), whereas mosapride-exposure did not show significant IRR difference for severe VA, compared to itopride-exposure (crude IRR 0.950; 0.672–1.343) (Table 8).
Discussion
Initially, domperidone was commonly used to treat nausea, vomiting, and gastroparesis, owing to its effectiveness2. However, of late, given that several case reports and retrospective studies have reported an association between domperidone and severe VA, its use has been strictly restricted. Although some meta-analyses indicate that domperidone may not be associated with the risk of overall cardiovascular (CV) events and QT prolongation15,16,17, many clinicians hesitate to prescribe it to their patients. Thus, it is essential to conduct well-designed studies to clarify the association between domperidone and severe VA. We conducted a self-controlled case series based on a large population cohort, which could enable the adjustment of all fixed time-independent confounders during the observation period.
Our study suggested that domperidone may be associated with increased risk of severe VA. However, considering the weak association (adjusted IRR, 1.342), it is less likely to have a causal relationship18. This modest association, referred to as the “zone of bias,” can be easily susceptible to undetected biases, particularly residual confounding18,19. Therefore, the causal inference from observational studies must be carefully interpreted. Tables 6, 7 and 8 show that domperidone is less likely to be associated with severe VA, compared with itopride, known to be the safest prokinetic agent20. Thus, it can be inferred that the weak association between domperidone and severe VA could be largely attributed to undetected bias.
Published data on potential domperidone-induced adverse CV events are largely limited to case reports and case–control studies15,21,22,23. Given the nature of the retrospective study design, it is inherently impossible to avoid bias. We used the self-controlled case series design to effectively avoid confounding and bias, unlike the traditional case–control or cohort designs. In this study design, comparisons were made entirely intra-personally24. Therefore, it is self-controlled and accounts for any factors or characteristics that remain constant over the observation period24. Our study results are consistent with a recent meta-analysis on the cardiac safety profile of domperidone15. Serhat et al. conducted the meta-analysis using nine studies and reported that low-dose domperidone may not be associated with the overall risk of CV events and does not result in QT prolongation15.
Domperidone displays an affinity for the hERG/Kv11.1 channel and has been linked to drug induced long QT syndrome, torsades de pointes (TdP), and sudden cardiac death (SCD)5,25,26. However, an exaggerated QTc response, TdP, and/or SCD are rarely observed after its oral administration5. This phenomenon may be attributable to the vastly lower intra-cardiomyocyte concentrations of these medications, resulting from the combined effects of hepatic drug metabolism and intracellular drug accumulation on the bioavailability27,28,29. Practically, severe domperidone-induced VA rarely occurs, considering the frequency of domperidone prescription. Severe VA is likely to occur when multifactorial occurrences such as drug-drug interactions (co-administration of potent CYP3A4 inhibitors), patient-specific risk factors, and underlying genetic predisposition, are combined5. Thus, if domperidone is prescribed after careful consideration of drug interactions and underlying disease, the risk of severe VA would be minimal.
An important advantage of this study is the use of the self-controlled case series design, which cancels out time-invariant factors. Furthermore, we analyzed the incidence rate ratio of severe VA for other commonly prescribed prokinetics; itopride and mosapride. We equally compared domperidone-induced severe VA risk to that of itopride, for the first time. When prokinetics were prescribed for early symptoms of undiagnosed heart disease, it was possible to exaggerate the association between prokinetics and severe VA. However, we could effectively adjust this potential bias by comparing other prokinetics. Although only a few studies have used case–control design with incomplete controls, there have been no studies using a robust study design with appropriate controls or prospective studies. We also used a large nationwide cohort representing the general population. We followed-up 2,817 severe VA patients extracted from over 1 million subjects for up to 11 years.
As a limitation, the current study failed to investigate the association between prokinetic dose and severe VA. Secondly, it was unfeasible to take concomitant medication related to QT prolongation into consideration. However, given that we adopted a self-controlled case series design while using other prokinetics as control, it was possible to adjust for confounding factors.
Our study is indicative of insufficient evidence to support any association between the use of domperidone and increased severe VA risk, compared to other prokinetics. Thus, domperidone can be safely prescribed to patients in need of prokinetics.
Methods
Data source
Data was obtained from the National Health Insurance Service National Sample Cohort (NHIS-NSC) in South Korea30. The NHIS-NSC is a population-based retrospective sample cohort and consists of 1,025,340 individuals who were selected as representatives of the South Korean population, by stratified random sampling from 46,605,433 individuals who were eligible for the National Health Insurance Service in 2002. The cohort contains the de-identified information on personal information, disease diagnosis, drug prescription, medical treatment and death status from January 1st, 2002 till December 31st, 2013. Infants were included annually into the cohort as replacements for individuals who had been excluded due to death or disqualification from the national health insurance owing to situations such as emigration. The study was performed in accordance with the ethical guidelines of the Declaration of Helsinki. The protocol of this study was approved by the Institutional Review Board (No. 2018-11-047) at Samsung Medical Center. Since this study was based on retrospective analysis of existing data, the requirement of obtaining informed consent was waived by the Institutional Review Board.
Definition
International Classification of Diseases, Tenth Revision (ICD-10) code were used to define severe VA. Patients who were diagnosed with I47.2 (ventricular tachycardia), I49.0 (ventricular fibrillation and flutter), and I49.8 (other specified cardiac arrhythmias) were classified as having severe VA.
Study population
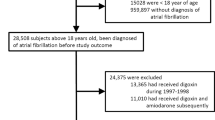
Figure 1 shows a flow chart for the selection of the study population. Eligible subjects were individuals with at least one severe VA diagnosis in the NHIS-NSC. To ensure that all study subjects had at least one severe VA-free year, we eliminated subjects who were diagnosed with severe VA within the same year of inclusion into the NHIS-NSC. Furthermore, we excluded subjects with prescribed prokinetic agents such as domperidone, itopride, and mosapride during the last month of the year of inclusion into the NHIS-NSC, to ensure at least one prokinetic agent-free month. Subjects were further excluded if they had any disqualification during the course of the observations.
Selected subjects were then prescribed any prokinetic agent during the observation period. Exposed periods and unexposed periods were identified for each agent. After eliminating overlapping exposure periods, we further excluded subjects whose observation period no longer contained the 1st incident severe VA diagnosis or any exposed period. Subjects were equally eliminated if the 1st incident diagnosis occurred within the first 2 days of exposure, to ensure corresponding incidence.
Study design
The SCCS method is a case-only design in which a cohort consists only of cases (individuals with the outcome or adverse event of interest)31. The aim of the SCCS method was to investigate the association between the outcome and exposure of interest, by comparing the incidence rate during risk-increased periods due to exposure (exposed periods), with the incidence rate at other times (unexposed periods), intra-personally24. The advantages of this method are two-folds. Firstly, the possible confounding effects of any time-invariant covariates are automatically prevented. Secondly, no separate control selection is required. It can be beneficial in situations where finding suitable controls is challenging.
The outcome of interest was the incidence of severe VA. For individuals with multiple incidences, only the 1st incident diagnosis was included in analyses because the exposure pattern could be modified after incidence occurrence. However, the information on multiple incidences was used to define outcome periods and observation periods.
For individuals with a single incidence, the outcome period was defined as 1 month post-incidence (30 days from the date of the incident diagnosis). For those with multiple incidences, the outcome period for the 1st incidence was defined as post 1-month after the 1st incident diagnosis. If this period overlapped with the post 1-month period(s) after the other incident diagnoses, the outcome period was extended by combining all overlapped periods. The last follow-up date was defined as the earliest date between December 31st, 2013 and the date of death, disqualification, or the 2nd incident diagnosis, as relevant. We defined the observation period as the time from the first date in the year after inclusion into the cohort until the last follow-up date, excluding the outcome period. The observation period was further partitioned into the exposed and unexposed periods.
The exposed period consisted of two parts: the agent-exposed time and the residual time (Fig. 2). We defined the agent-exposed time as the total consecutive days in single or multiple prescriptions for each agent. Taking possible residual efficacy into account, the 30 days following agent-exposed time were considered as the residual time. If exposed periods for different agents overlapped, the first period was shortened by defining its last date as the first date of the 2nd period, and the following periods removed as unobserved (Fig. 3), to avoid confounding effects from different agents. All time intervals other than the exposed periods within the whole observation period were defined as unexposed periods.
Pictorial representation of the study design. This figure illustrates three individuals prescribed prokinetics during their observation period. All subjects included in the analyses had an incident of severe ventricular arrhythmia (VA) and at least one prescription for domperidone, mosapride, or itopride. The observation period consists of the exposed and unexposed period. The colored exposed period indicates the agent-exposed time (blue, green, or yellow) or residual time (gray). The uncolored time interval between the exposed periods represents the unexposed period. Severe VA can occur during the exposed period (agent-exposed time and the residual time) or the unexposed period. The date of the incident diagnosis indicates the starting point of the outcome period (red). The last follow-up date was defined as the time between the earliest date (including December 31st, 2013) and the date of death, disqualification, or the 2nd incident diagnosis, as relevant.
An example of periods eliminated from analyses: (a) partial observation period with overlapped exposed times and (b) whole outcome period. To avoid possible confounders, such as residual effect created by other agents and the outcome event, sections of the exposed and outcome periods were eliminated from the analyses. Where exposed periods for different agents overlapped, the 1st period was reduced by defining its last date as the first date of the 2nd period.
Statistical analyses
The conditional Poisson regression was used to estimate the incidence rate ratios at 95% confidence intervals (CI), which indicated the relative risks of exposure to prokinetic agents. While controlling the time-invariant covariates by the SCCS design, we further adjusted for time-dependent covariates: age group (< 50 versus ≥ 50) and the history of severe VA-related diseases such as structural heart disease, hypertension, diabetes mellitus, and dyslipidemia.
Data preparation and statistical analyses were conducted with R software (version 3.5.0, Vienna, Austria)32 and its packages, including gnm33. A P value < 0.05 was considered statistically significant.
Abbreviations
- VA:
-
Ventricular arrhythmia
- SCCS:
-
Self-controlled case series
- NHIS-NSC:
-
National Health Insurance Service National Sample Cohort
- CI:
-
Confidence intervals
- CV:
-
Cardiovascular
- TdP:
-
Torsades de pointes
- SCD:
-
Sudden cardiac death
References
Barone, J. A. Domperidone: A peripherally acting dopamine2-receptor antagonist. Ann. Pharmacother. 33, 429–440. https://doi.org/10.1345/aph.18003 (1999).
Reddymasu, S. C., Soykan, I. & McCallum, R. W. Domperidone: Review of pharmacology and clinical applications in gastroenterology. Am. J. Gastroenterol. 102, 2036–2045. https://doi.org/10.1111/j.1572-0241.2007.01255.x (2007).
Patterson, D., Abell, T., Rothstein, R., Koch, K. & Barnett, J. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am. J. Gastroenterol. 94, 1230–1234. https://doi.org/10.1111/j.1572-0241.1999.00456.x (1999).
Dumitrascu, D. L. & Weinbeck, M. Domperidone versus metoclopramide in the treatment of diabetic gastroparesis. Am. J. Gastroenterol. 95, 316–317. https://doi.org/10.1111/j.1572-0241.2000.01721.x (2000).
Giudicessi, J. R., Ackerman, M. J. & Camilleri, M. Cardiovascular safety of prokinetic agents: A focus on drug-induced arrhythmias. Neurogastroenterol. Motil. 30, e13302. https://doi.org/10.1111/nmo.13302 (2018).
Giaccone, G., Bertetto, O. & Calciati, A. Two sudden deaths during prophylactic antiemetic treatment with high doses of domperidone and methylprednisolone. Lancet 2, 1336–1337. https://doi.org/10.1016/s0140-6736(84)90841-9 (1984).
Joss, R. A., Goldhirsch, A., Brunner, K. W. & Galeazzi, R. L. Sudden death in cancer patient on high-dose domperidone. Lancet 1, 1019. https://doi.org/10.1016/s0140-6736(82)92016-5 (1982).
Osborne, R. J., Slevin, M. L., Hunter, R. W. & Hamer, J. Cardiotoxicity of intravenous domperidone. Lancet 2, 385. https://doi.org/10.1016/s0140-6736(85)92515-2 (1985).
Roussak, J. B., Carey, P. & Parry, H. Cardiac arrest after treatment with intravenous domperidone. Br. Med. J. (Clin. Res. Ed) 289, 1579, https://doi.org/10.1136/bmj.289.6458.1579 (1984).
Arana, A. et al. Risk of out-of-hospital sudden cardiac death in users of domperidone, proton pump inhibitors, or metoclopramide: A population-based nested case-control study. Drug Saf. 38, 1187–1199. https://doi.org/10.1007/s40264-015-0338-0 (2015).
Straus, S. M. et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur. Heart J. 26, 2007–2012. https://doi.org/10.1093/eurheartj/ehi312 (2005).
van Noord, C., Dieleman, J. P., van Herpen, G., Verhamme, K. & Sturkenboom, M. C. Domperidone and ventricular arrhythmia or sudden cardiac death: A population-based case-control study in the Netherlands. Drug Saf. 33, 1003–1014. https://doi.org/10.2165/11536840-000000000-00000 (2010).
Chen, H. L. & Hsiao, F. Y. Domperidone, cytochrome P450 3A4 isoenzyme inhibitors and ventricular arrhythmia: A nationwide case-crossover study. Pharmacoepidemiol. Drug Saf. 24, 841–848. https://doi.org/10.1002/pds.3814 (2015).
Johannes, C. B., Varas-Lorenzo, C., McQuay, L. J., Midkiff, K. D. & Fife, D. Risk of serious ventricular arrhythmia and sudden cardiac death in a cohort of users of domperidone: A nested case-control study. Pharmacoepidemiol. Drug Saf. 19, 881–888. https://doi.org/10.1002/pds.2016 (2010).
Bor, S., Demir, M., Ozdemir, O. & Yuksel, K. A meta-analysis on the cardiac safety profile of domperidone compared to metoclopramide. United Eur. Gastroenterol. J. 6, 1331–1346. https://doi.org/10.1177/2050640618799153 (2018).
Field, J. et al. Effect of chronic domperidone use on QT interval: A large single center study. J. Clin. Gastroenterol. 53, 648–652. https://doi.org/10.1097/MCG.0000000000001183 (2019).
Biewenga, J. et al. Absence of QTc prolongation with domperidone: A randomized, double-blind, placebo- and positive-controlled thorough QT/QTc study in healthy volunteers. Clin. Pharmacol. Drug Dev. 4, 41–48. https://doi.org/10.1002/cpdd.126 (2015).
Vaezi, M. F., Yang, Y. X. & Howden, C. W. Complications of proton pump inhibitor therapy. Gastroenterology 153, 35–48. https://doi.org/10.1053/j.gastro.2017.04.047 (2017).
Grimes, D. A. & Schulz, K. F. False alarms and pseudo-epidemics: The limitations of observational epidemiology. Obstet. Gynecol. 120, 920–927. https://doi.org/10.1097/AOG.0b013e31826af61a (2012).
Huang, X., Lv, B., Zhang, S., Fan, Y. H. & Meng, L. N. Itopride therapy for functional dyspepsia: A meta-analysis. World J. Gastroenterol. 18, 7371–7377. https://doi.org/10.3748/wjg.v18.i48.7371 (2012).
Buffery, P. J. & Strother, R. M. Domperidone safety: A mini-review of the science of QT prolongation and clinical implications of recent global regulatory recommendations. N. Z. Med. J. 128, 66–74 (2015).
Leelakanok, N., Holcombe, A. & Schweizer, M. L. Domperidone and risk of ventricular arrhythmia and cardiac death: A systematic review and meta-analysis. Clin. Drug Investig. 36, 97–107. https://doi.org/10.1007/s40261-015-0360-0 (2016).
Ortiz, A. et al. Cardiovascular safety profile and clinical experience with high-dose domperidone therapy for nausea and vomiting. Am. J. Med. Sci. 349, 421–424. https://doi.org/10.1097/MAJ.0000000000000439 (2015).
Petersen, I., Douglas, I. & Whitaker, H. Self controlled case series methods: An alternative to standard epidemiological study designs. BMJ 354, i4515. https://doi.org/10.1136/bmj.i4515 (2016).
Claassen, S. & Zunkler, B. J. Comparison of the effects of metoclopramide and domperidone on HERG channels. Pharmacology 74, 31–36. https://doi.org/10.1159/000083234 (2005).
Hondeghem, L. M. Domperidone: Limited benefits with significant risk for sudden cardiac death. J. Cardiovasc. Pharmacol. 61, 218–225. https://doi.org/10.1097/FJC.0b013e31827afd0d (2013).
Simard, C. et al. Identification of the cytochrome P450 enzymes involved in the metabolism of domperidone. Xenobiotica 34, 1013–1023. https://doi.org/10.1080/00498250400015301 (2004).
Couture, L., Nash, J. A. & Turgeon, J. The ATP-binding cassette transporters and their implication in drug disposition: A special look at the heart. Pharmacol. Rev. 58, 244–258. https://doi.org/10.1124/pr.58.2.7 (2006).
Michaud, V., Frappier, M., Dumas, M. C. & Turgeon, J. Metabolic activity and mRNA levels of human cardiac CYP450s involved in drug metabolism. PLoS ONE 5, e15666. https://doi.org/10.1371/journal.pone.0015666 (2010).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 46, e15. https://doi.org/10.1093/ije/dyv319 (2017).
Farrington, P., Whitaker, H. & Weldeselassie, Y. G. Self-Controlled Case Series Studies: A Modelling Guide with R. (Chapman and Hall/CRC, 2018).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, 2018). https://www.R-project.org/. Accessed 1 May 2019.
Turner, H. & Firth, D. Generalized Nonlinear Models in R: An Overview of the gnm Package (2007). https://cran.r-project.org/web/packages/gnm/.../gnmOverview.pdf . Accessed 1 May 2019.
Author information
Authors and Affiliations
Contributions
B.G.S.: study concept and design; data acquisition, analyses, and interpretation; and drafting of the manuscript; Y.C.L.: study concept and design; data acquisition, analyses, and interpretation; and drafting of the manuscript; K.K.: study design; data interpretation; and critical manuscript revision for important intellectual content; H.L.: data acquisition and critical manuscript revision for important intellectual content; H.J.S.: data acquisition and critical manuscript revision for important intellectual content; P.-L.R.: data acquisition and critical manuscript revision for important intellectual content; Y.W.M.: data acquisition and study supervision. All the authors approve the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, B.G., Lee, Y.C., Min, Y.W. et al. Risk of domperidone induced severe ventricular arrhythmia. Sci Rep 10, 12158 (2020). https://doi.org/10.1038/s41598-020-69053-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69053-4
This article is cited by
-
Mosapride stimulates human 5-HT4-serotonin receptors in the heart
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.