Abstract
Identifying comorbidities in polymyalgia rheumatica/giant cell arteritis (PMR/GCA) is crucial for patients’ outcomes. The present study aimed to evaluate the impact of the inflammatory process and glucocorticoid treatment on aortic arterial stiffness and body composition in PMR/GCA. 77 patients with newly diagnosed PMR/GCA were treated with oral glucocorticoids and followed for 40 weeks. Aortic pulse wave velocity (PWV) was measured at baseline and during the follow-up period and compared to the results of temporal artery biopsy (TAB) and 18F-FDG PET/CT. Body composition was assessed by total body DXA at baseline and the end of the study. Of 77 patients (49 (63.6%) female, mean of age: (71.8 ± 8.0)), 64 (83.1%) had pure PMR, 10 (13.0%) concomitant PMR and GCA, and 3 (3.9%) pure GCA. Compared to baseline values, aortic PWV was initially decreased at week 16 (p = 0.010) and remained lower than baseline at week 28 (p = 0.002) and week 40 (p < 0.001), with no association with results of TAB and 18F-FDG PET/CT. Aortic PWV was significantly associated with age, male gender, left systolic and diastolic blood pressure, right diastolic blood pressure, and CRP. Total bone mineral content (BMC) was decreased in both genders (p < 0.001), while fat mass (FM) was significantly increased (p < 0.001). However, lean body mass did not significantly change during the study. Changes in FM were correlated with cumulative prednisolone dose (rho: 0.26, p = 0.031). Glucocorticoid treatment of patients with PMR/GCA had several prognostic impacts. Arterial stiffness was decreased due either to the treatment or a reduction in the inflammatory load. Additionally, treatment led to changes in body composition, including a decrease in BMC and FM excess.
Similar content being viewed by others
Introduction
The recent European League Against Rheumatism (EULAR) recommendations for the management of polymyalgia rheumatica (PMR) and giant cell arteritis (GCA) underline the importance of screening for disease- and treatment-related comorbidities such as osteoporosis (and particularly recent fractures), cardiovascular disease, diabetes and dyslipidaemia1,2. These comorbidities are commonly observed in PMR/GCA and present additional challenges for clinicians. Identifying comorbidities at the time of diagnosis, before initiation of the glucocorticoid treatment, and underlying mechanisms and long-term complications is crucial and has been the subject of growing research efforts.
Arterial stiffness is an early and recognized risk factor for atherosclerosis3,4. It is a significant predictor for future cardiovascular disease as well as all-cause mortality, even in those without overt cardiovascular disease4,5. Arterial stiffness is caused by a generalized process of vascular aging that involves small to large arteries, and is increased in the presence of traditional cardiovascular risk factors4,6. Accumulating evidence suggests that the inflammatory process shares several pathogenic mechanisms with atherosclerosis and has a role in the initiation and progression of atherosclerosis7,8,9. This process in PMR/GCA can be explained by a complex interaction between inflammation, glucocorticoid treatment, and other individual risk factors, e.g. dyslipidemia, hypertension, obesity, and smoking10. Arterial stiffness is most frequently defined by aortic pulse wave velocity (PWV) and augmentation index (AIx) measured non-invasively using applanation tonometry, which is considered the gold standard technique to assess arterial stiffness11.
Another consequence of the inflammatory process together with glucocorticoid treatment is a decline in bone mass as well as a change in body composition12,13,14. Body composition refers to a three-compartment human body model consisting of bone mineral content (BMC), fat mass (FM), and lean body mass (LBM)15. While loss of bone mass puts patients at risk of future fractures, a decrease in LBM may contribute to the general debilitation of patients due to an increased fall tendency16. Glucocorticoid treatment alters energy metabolism by increasing protein breakdown and decreasing synthesis, promoting fat accumulation and fat redistribution from peripheral to central tissues, resulting in loss of LBM and increase in FM13,17. Increased FM is associated with cardiovascular disease and several metabolic morbidities, e.g. insulin resistance and type 2 diabetes mellitus18. Changes in body composition have previously been described in several rheumatic diseases, e.g. rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE)17,19,20,21,22. However, data on this topic in PMR/GCA are scarce.
In light of the above considerations, the present study aimed to evaluate the impact of glucocorticoid treatment and the inflammatory process in PMR/GCA on aortic arterial stiffness and body composition at diagnosis and during a 40-week follow-up period.
Materials and methods
Study design and setting
This is a longitudinal cohort study. The study was performed at the Diagnostic Center in collaboration with the Section of Rheumatology at Svendborg Hospital, Svendborg, Denmark, between February 2018 and December 2019. Total body dual energy X-ray (DXA) scans were undertaken at the osteoporosis clinic, Odense University Hospital. Ethical approval was granted by the Regional Ethics Committee of the Region of Southern Denmark (identification number: S-20160098) and the Danish Data Protection Agency (J.nr 16/40522). All examinations were performed in accordance with relevant local guidelines and the informed consent was obtained from all included patients. The study was also registered at ClinicalTrials.gov (Identifier: NCT02985424, first posted on 07/12/2016).
Participants
80 consecutive patients with newly suspected PMR, GCA, or concomitant PMR and GCA were included in the study. Inclusion and exclusion criteria have previously been described23,24. Briefly, PMR patients met the following criteria: 1. Age ≥ 50 years; 2. Bilateral shoulder or hip pain; 3. Morning stiffness > 45 min; 4. Elevated erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) and 5. Disease duration > 2 weeks. In case of Cranial-GCA (C-GCA), 1. Age ≥ 50 years; 2. Elevated ESR and/or CRP; 3. Scalp tenderness; 4. Vision disturbances; 5. Headache (new or changed); 6. Jaw claudication and 7. Tenderness of the temporal arteria were considered. Furthermore, patients with clinical suspicion of Large Vessel-GCA (i.e., upper extremity claudication and upper extremity blood pressure discrepancies) were also eligible for inclusion. Patients were excluded from the study if they met one of the below mentioned criteria:
-
1.
Infections, malignancy, or any other conditions that prednisolone was unsuitable to initiate.
-
2.
Contraindication to 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) i.e. blood glucose > 145 mg/dL after 6 h fasting.
-
3.
Initiation of steroid treatment more than 3 days prior to 18F-FDG PET/CT.
-
4.
Inability to provide informed consent.
-
5.
Patients with dementia or inability to communicate in Danish.
All patients were treated with oral glucocorticoids according to current national guidelines with 20–30 mg/day in the case of PMR and up to 75 mg/day when GCA was suspected23. In addition, all patients received calcium/vitamin D supplementation alone or together with bisphosphonates if there were signs of osteopenia or osteoporosis on DXA according to current national guidelines. Patients were seen at baseline (visit 1), week 4 (visit 2), week 16 (visit 3), week 28 (visit 4), and week 40 (visit 5). The follow-up period was 40 weeks because previous studies have reported that this time period is sufficient to ensure a certain diagnosis25,26.
Data collection
Patients’ demographics, clinical data, Charlson comorbidity index score27, and laboratory tests at baseline and follow-up visits were collected and managed by means of REDcap (Research Electronic Data Capture), which is a secure, web-based software platform designed to support data capture for research studies at the Open Patient data Explorative Network28. Cardiovascular disease risk prediction was assessed by Framingham risk score at baseline, which classifies the patients into three risk categories: low (< 10% risk of an event in 10 years), intermediate (10% to 20%), and high (> 20%) according to age, gender, smoking status, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, antihypertensive treatment and presence of diabetes or known vascular disease29.
A unilateral temporal artery biopsy (TAB) was also performed in all included patients at baseline. TAB was considered positive if signs of active arteritis or healed arteritis were detected on pathologic examination.
Every included patient underwent an 18F-FDG PET/CT either before or in the case of GCA within 3 days of initiation of glucocorticoid treatment23,24. Based on previously described methodology, FDG uptakes in 8 paired articular/periarticular sites and 14 arterial segments were described visually based on a 4-point visual grading scale (VGS) with 0 = no uptake; 1 = slight but not negligible uptake, lower than liver uptake; 2 = intermediate uptake, equivalent to liver uptake; 3 = high-grade uptake, higher than liver uptake. Two pathologic cutoff values of VGS ≥ 3 and VGS ≥ 2 were used to analyze the results of 18F-FDG PET/CT24. Total PMR and GCA scores were defined as the sum of VGS at each articular/periarticular site or arterial segment.
Aortic PWV analysis
Aortic PWV (m/s) and AIx (%) were measured by applanation tonometry using the Sphygmocor device (AtCor Medical, Sydney, Australia) by the same observer, who was blinded to 18FDG-PET/CT results, in every patient to avoid any inter-observer variability. All PWV measurements were undertaken after at least 10 min’ rest. Aortic PWV was calculated by measuring the surface distance between the two recording sites, carotid and femoral, and recording the sequential waveforms at the right common carotid and femoral arteries. Pulse transit time was calculated with the aid of a simultaneously recorded electrocardiogram as a common reference. Aortic PWV was then calculated automatically from measurements of pulse transit time and the distance between the two sites, with higher levels suggesting greater arterial stiffness. AIx was defined as the difference between the first and second peaks of the central arterial waveform expressed as a percentage of the pulse pressure and standardized to a heart rate of 75 beats per minute (AIx75 (%)), since AIx is influenced by alteration in heart rate.
Total body DXA
Body composition was measured by total body DXA (Hologic Discovery QDR, Scanner ID: 82245) at baseline (visit 1), before or within 2 weeks after treatment initiation, and subsequently at week 40 (visit 5). FM, LBM, and BMC were assessed. FM was expressed as absolute numbers in grams and as a percentage of total body weight. Fat-free mass (FFM) was the sum of LBM and BMC. The fat mass index (FMI) and fat-free mass index (FFMI) were calculated as follows: FMI = FM/height2 and FFMI = FFM/height2. Data from a Swiss population of 5635 healthy adults (2986 men and 2649 women, age from 24 to 98 years) were used to categorize patients into the following groups: obese: FMI > 90th percentile, lean: FFMI < 10th percentile, wasted: FFMI < 10th percentile and FMI < 25th percentile, and cachectic: FFMI < 10th percentile and FMI > 25th percentile30. Osteopenia and osteoporosis were defined as a T score less than − 1.0 and greater than − 2.5 (− 1.0 < T score < − 2.5) and osteoporosis as a T score ≤ − 2.531.
Partial patient and public involvement
The present study was supported by a patient advisory group that provided input to the research questions. Patients partnered with us regarding the design of the study, the informational material to support the intervention, and the burden of the intervention from the patient’s perspective, for instance, number of blood samples and time between blood samples. However additional patient involvement was difficult due to the technical nature of the methods used in the study. All participants will be informed of the trial results by mail. The study results will be disseminated to the public through online media.
Statistical analysis
Data are presented as frequencies (percentages), mean ± standard deviation (SD), or median (interquartile range (IQR)) depending on data type and distribution. Correlation analysis was performed by Pearson’s correlation (r) or Spearman’s rank correlation (rho). A comparison of two paired binary variables was performed using McNemar’s test. The comparison of continuous variables was performed by Student’s t-test or Wilcoxon rank-sum test (Mann–Whitney U test), if unpaired, and Wilcoxon signed-rank test or paired Student’s t-test, if paired, depending on the assumption of normally distributed data. The Kruskal–Wallis test or analysis of variance was used when more than two groups were compared. Analysis of PWV repeated measurements over time was done by means of mixed linear model considering aortic PWV as the outcome variable and age, gender, clinical diagnosis, blood pressure, and CRP as fixed factors. Data from a European cross-sectional study in 11,092 individuals (Mean age (year) ± SD: 50 ± 17, Gender Male/Female: 5520/5572) without overt cardiovascular disease and diabetes mellitus were used as reference values for PWV in a subgroup of our patients without these comorbidities32. A p value was considered as significant if p < 0.05. No method of imputation was used for missing data. Statistical analysis was performed using STATA version 16.0 (StataCorp, College Station, TX, USA).
Results
Of 80 included patients, 3 patients were diagnosed with seronegative RA during the follow-up period. Statistical analyses were performed in 77 patients. Among 77 patients, 64 (83.1%) patients were diagnosed with pure PMR, 10 (13.0%) patients with concomitant PMR and GCA, and 3 (3.9%) with pure GCA, the clinical diagnoses being confirmed during the follow-up period. The mean age of the patients was 71.8 ± 8.0 years and 49 (63.6%) were women. Baseline demographic data have previously been published in detail24,33. Clinical data together with laboratory results at visit 1 to visit 5 are summarized in Table 1. The total number of relapse during the study and cumulative prednisolone dose were (mean ± SD: 0.6 ± 0.9, median (IQR): 0 (0 to 1), min: 0, max: 5) and (mean ± SD: 2995.1 ± 1269.7, median (IQR): 2438.7 (2193.7 to 3420), min: 1226.25, max: 7511.25), respectively. 69 (89.6%) patients out of 77 completed the study. The numbers of patients who withdrew from the study together with the reason for withdrawal are summarized in Supplementary Table S1 (disposition table). Of 70 patients who agreed to performance of TAB, TAB was positive in 7 (10%) patients: active arteritis n = 4 (5.7%) and healed arteritis n = 3 (4.3%).
Framingham risk score
Supplementary Table S2 summarized the cardiovascular risk factors for the patients. The risk stratification showed that 19.7%, 36.8% and 43.4% of the included patients were classified as of low, intermediate and high -risk at baseline, respectively.
18F-FDG PET/CT results
The means ± SDs of the total PMR and GCA scores were 12.5 ± 5.9 and 0.8 ± 2.0, respectively. With a pathologic cutoff value of ≥ 3, 55 (71.4%) patients had signs of PMR activity, 2 (2.6%) GCA activity, 1 (1.3%) PMR and GCA activity, and 19 (24.7%) had neither PMR nor GCA activity. On the other hand, with a pathologic cutoff value of ≥ 2, 58 (75.3%) patients showed PMR activity, 3 (3.9%) GCA activity, 9 (11.7%) PMR and GCA activity, and 7 (9.1%) had neither PMR nor GCA activity24.
Aortic PWV analysis
Male patients had a higher aortic PWV than female patients at baseline (12.9 (11.2 to 14.45) vs 11.5 (10.5 to 12.9) p = 0.023). Results of aortic PWV analysis at visit 1 to visit 5 are summarized in Table 2. Aortic PWV did not show any significant correlation with baseline AIx (rho = 0.15, p = 0.21) and AIx75 (rho: 0.12, p = 0.30) (Supplementary Table S3).
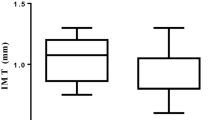
Compared to the baseline (visit 1), aortic PWV was initially decreased at week 16 (visit 3, p = 0.010) and remained below baseline at week 28 (visit 4, p = 0.002) and week 40 (visit 5, p < 0.001) (Fig. 1). No significant difference was found between baseline aortic PWV (visit 1) and aortic PWV at week 4 (visit 2, p = 0.20). AIx and AIx75 did not significantly differ from baseline values during the follow-up period (Supplementary Fig. S2A,B).
Box plot of aortic PWV at baseline (visit 1), week 4 (visit 2), week 16 (visit 3), week 28 (visit 4), and week 40 (visit 5) including p values from pairwise comparisons. (The figure is graphed using Stata version 16.0 (StataCorp LLC, College Station, TX, USA, https://www.stata.com/)).
There were no significant differences in aortic PWV between patients with low, intermediate and high future cardiovascular risk at baseline (p = 0.09), week 4 (p = 0.11) and week 28 (p = 0.053), although it was tended to be higher in the intermediate- and high-risk patients (Supplementary Fig. S3). At week 16 and week 40, between groups analyses showed that aortic PWV was significantly higher in patients with high (high- vs low-risk p = 0.026 (week 16) and 0.003 (week 40)) and intermediate future cardiovascular risk (intermediate- vs low-risk p = 0.010 (week 16) and 0.009 (week 40)) compared to low-risk patients.
With regard to TAB and 18F-FDG PET/CT results, there were no significant differences in AIx, AIx75, and aortic PWV between the groups (Supplementary Table S4). Furthermore, baseline aortic PWV, Aix, and AIx75 did not show any significant correlation with total PMR and GCA scores (Supplementary Table S5).
In the most robust mixed model analysis, aortic PWV was significantly associated with age (coefficient: 0.096, p < 0.001), male gender (coefficient: 1.040, p = 0.009), left systolic blood pressure (coefficient: 0.016, p = 0.036), left diastolic blood pressure (coefficient: 0.030, p = 0.016), right diastolic blood pressure (coefficient: 0.023, p- = 0.041), and CRP (coefficient: 0.005, p- = 0.029) (Table 3). Framingham risk score was not significantly associated with aortic PWV in the regression model. Besides, when the results of TAB and 18F-FDG PET/CT (cutoff values ≥ 3 and ≥ 2) were sequentially added to the model, they did not achieve statistical significance.
Comparison of the median of baseline PWV values in a subgroup of our patients without overt cardiovascular disease and diabetes mellitus (n = 65) with values in age- and blood pressure-matched individuals from the European reference population32, suggested that the baseline aortic PWV was probably higher in PMR/GCA patients than in the reference population (Supplementary Table S6, Supplementary Fig. S4). However, a definite comparison could not be made because of too few observations in several groups and lack of a formal control group.
Total body DXA measurements
Results of total body DXA at baseline (visit 1) and week 40 (visit 5) are summarized in Table 4. Total BMC was significantly decreased in both male (p ≤ 0.001) and female (p ≤ 0.001) patients. Additionally, FM was significantly increased in both male (p ≤ 0.001) and female (p ≤ 0.001) patients. However, LBM did not significantly change during the study in male (p = 0.17) and female (p = 0.67) patients. Changes in FM were significantly correlated with cumulative prednisolone dose (rho: 0.26, p = 0.031) and was greater in male patients (2822.9 (2263 to 4956) vs 2138.5 (254.6 to 3486.9) p = 0.049). There were no significant correlations between cumulative prednisolone dose and changes in total BMC (rho: − 0.17, p = 0.16) and LBM (rho: − 0.15, p = 0.22).
The prevalence of obese (p = 0.039) patients increased significantly during follow-up period (Table 5). Besides, the prevalences of osteopenia (p = 0.12), underlean (p = 0.62), wasted (p = 1.00) and cachexia (p = 1.00) were slightly increased, but it did not reach statistical significance.
21 (28%) patients were treated with calcium/vitamin D supplementation alone, while 54 (72%) patients were treated with bisphosphonates in combination with calcium/vitamin D supplementation. Changes in total BMC (− 104.3 (− 211.0 to − 67.8) vs − 74.3 (− 122.7 to − 38.9), p = 0.08), hip BMC (− 0.3 (− 0.9 to 0.5) vs 0.3 (− 0.6 to 0.8), p = 0.14), and spine BMC (− 1.4 (− 4.4 to 1.0) vs 0.3 (− 0.8 to 1.32), p = 0.010) were negative and greater in those who were treated with calcium/vitamin D supplementation alone compared to those who received bisphosphonates in combination with calcium/vitamin D supplementation, although only changes in spine BMC reached statistical significance. Supplementary Fig. S5 illustrated changes in total BMC, as well as hip and spine T scores in those who were treated with calcium/vitamin D supplementation alone, compared to those who were treated with calcium/vitamin D supplementation together with bisphosphonates.
Discussion
To the best of the authors’ knowledge, this is the first study of its kind investigating two important comorbidities, namely arterial stiffness and body composition, in patients with PMR/GCA in a 40-week longitudinal study. The decrease in aortic PWV was initially seen at week 16 (visit 3) and remained below the baseline value at week 28 (visit 4) and week 40 (visit 5). However, AIx and AIx75 did not change significantly during the study. In addition, the decrease in aortic PWV was independent of TAB and 18F-FDG PET/CT results. In the mixed model regression analysis, aortic PWV was significantly associated with age, male gender, left systolic blood pressure, left diastolic blood pressure, right diastolic blood pressure, and CRP. Comparison of the baseline PWV analysis in the present cohort with the age- and blood pressure-matched individuals from the European reference population suggested that the baseline aortic PWV in PMR/GCA patients tended to be higher than that in the reference population.
With regard to body composition, BMC decreased significantly in both genders during the study. Additionally, there was a significant increase in FM in both genders, which was significantly correlated with cumulative prednisolone dose. However, LBM did not change significantly during the study. Prevalence of obese patients was significantly higher at the end of the study (week 40) than at baseline. Those who were treated with calcium/vitamin D supplementation alone had a greater loss of total, hip, and spine BMC than those treated with supplementation and bisphosphonates, although only changes in spine BMC reached statistical significance.
PMR and GCA are inflammatory rheumatic diseases that frequently overlap each other. They share several features for instance genetic background and clinical manifestations and it is believed that PMR and GCA belong to the same disease entity, although it is difficult to draw a definite conclusion34. In this regard several authors suggest a disease spectrum where PMR is at one side of the spectrum, representing a milder subset, and GCA is at the other end of the spectrum, representing a severe subset of the disease35. Imaging findings also support this model by illustrating the synchronous findings of PMR and GCA in the patients36.
Previous research on arterial stiffness in PMR and/or large vessel vasculitis, GCA and Takayasu’s arteritis, is limited to a few studies37,38,39,40,41,42. In a study by Pieringer et al. in 13 patients with newly diagnosed PMR and 30 age- sex-matched subjects as a control group, AIx tended to be higher in the PMR group and improved significantly from 28.5 to 25.3 (p = 0.006) after 4 weeks of treatment with 25 mg/day prednisolone37. In another study by Schillaci et al. in 39 patients with PMR and 39 age-, sex-, and blood pressure-matched healthy subjects, aortic PWV was higher in patients with PMR than in control subjects (12.4 ± 4 vs 10.2 ± 2 m/s, p ≤ 0.01)38. Both aortic PWV (from 11.8 ± 3 to 10.5 ± 3 m/s, p = 0.015) and AIx75 (from 34 ± 7 to 29 ± 8, p = 0.012) were decreased after 4 weeks of treatment with prednisone at a dose of 15 mg/day, and the change in aortic PWV was significantly correlated with the percentage change in plasma CRP. Our results support these findings, although with a few differences. In our study, the decrease in aortic PWV was first seen after 16 weeks from diagnosis without any changes in AIx or AIx75 being found. This is probably due to the difference in the patient populations because patients in Schillaci et al.’s study were selected from PMR patients without risk factors that would interfere with large artery properties: diabetes mellitus, overt cardiovascular, renal disease, smokers, and patients receiving treatment with antihypertensive drugs or any vasoactive drugs. We collected real-world data in a patient population from a general rheumatology clinic that included patients with both PMR and GCA with comorbid conditions, which minimized possible selection bias. The effects of glucocorticoids on atherosclerosis and cardiovascular events are debated and not well understood. Although glucocorticoids have a well-recognized impact on insulin resistance, lipid profile, blood sugar, blood pressure, and obesity, all well-known risk factors for cardiovascular disease, an anti-atherosclerotic effect of glucocorticoid has been identified in animal models43,44,45. The underlying anti-atherosclerotic mechanism of glucocorticoids is thought to be related to the anti-inflammatory effect of glucocorticoids46. Whether the benefit of glucocorticoids on arterial stiffness by reducing the inflammatory state is a short-term effect or remains after cessation of treatment is a matter of interest and needs further research.
In our study, over 40% of the patients were categorized as a high-risk group for future cardiovascular events. In addition, aortic PWV was tended to be higher in the patients with high and intermediate future cardiovascular risk compared to the low risk patients. In this regard, in a retrospective, population-based incidence cohort comparing GCA with a cohort of age-, sex- and calendar year-matched patients without GCA, the overall Framingham risk score in the GCA cohort did not significantly differ from the non-GCA cohort47. In the present cohort, it was not possible for us to further elaborate on the results due to the lack of a control group. It is worth mentioning that, the evidence regarding the utility of Framingham score in PMR and GCA is scarce and additionally, it may underestimate the cardiovascular risk in the patients, as it is initially developed for predicting cardiovascular risk in the general population and its value is not well investigated in the patients with inflammatory diseases48,49.
Glucocorticoids are partly responsible for the loss of bone mass and changes in body composition seen in several rheumatic diseases like SLE or RA17,19,20,21,22. They have negative effects on BMC and are the principal cause for secondary osteoporosis50. The mechanism behind is mainly related to enhancing bone resorption by increasing the life span of osteoclasts together with decreasing bone formation50. Due to standard bone protection, calcium/vitamin D and/or bisphosphonates started together with glucocorticoid treatment, hip and spine T scores remained fairly stable during the study period. Furthermore, as we reported earlier, the adherence to osteoporosis medications in our PMR/GCA population was high51,52. Although previously identified, the effect of glucocorticoids, or the diseases themselves, on body composition in rheumatic diseases is often neglected53. Treatment with glucocorticoids can lead to an increase in FM and central obesity. These are unfavourable conditions that can induce activation of the inflammatory response by producing pro-inflammatory cytokines, such as TNF-a and IL-654. Also, decreased physical function due to the disease itself, causes deterioration of the patient’s general condition and puts the patient at an increased risk of metabolic syndrome and cardiovascular events. Our results demonstrated a significant increase in FM in both genders, changes in FM being correlated with the cumulative prednisolone dose. These findings are in line with a previous study by Nordborg et al. in 24 patients with GCA (19 women and 5 men, 16 TAB positive) showed a significant increase in total body fat at 3, 6, 12, and 24 months compared to baseline values. Cumulative prednisolone dose was significantly correlated with FM in Nordborg et al.’s study55.
Our study has some limitations. Firstly, our patient population had several comorbid diseases, e.g. diabetes mellitus and hypertension. Although this minimized the selection bias, the comorbidities may have served as confounding factors and had varied impacts on our results. Secondly, the present study was observational by design and not a randomized controlled trial. Whether the changes in PWV and body composition are related to glucocorticoid use or the reduction in inflammatory load cannot be distinguished. However, due to the possible severe consequence of a placebo-controlled trial in PMR, this option was not applicable. Thirdly, cachexia in RA was first defined in 1992 and is different from the cachexia seen in cancer diseases56. Whether this term can be used interchangeably in PMR and GCA is unknown and needs to be investigated. The major strengths of our study were a relatively long follow-up period and the prospective design and the application and comparison of 18F-FDG PET/CT and aortic PWV analysis in terms of arterial stiffness. Furthermore, the study population represented a real-life PMR and GCA population with varied phenotypes of the disease, and thus our study has a high degree of generalizability.
In conclusion, our data demonstrated that treatment with glucocorticoids in patients with PMR/GCA had several prognostic impacts. Glucocorticoid treatment caused a decline in arterial stiffness, and this decline was independent of TAB and 18F-FDG PET/CT results. Additionally, it resulted in a decrease in BMC and an increase in FM. Anti-osteoporotic medications were beneficial and prevent further bone loss and should be initiated together with glucocorticoids. Lifestyle advice to reduce cardiovascular risk has also a preventive role in the management of the patients.
Data availability
On request, an anonymised dataset will be shared. Provided permission is given from the Danish Data Protection Agency.
References
Dejaco, C. et al. 2015 recommendations for the management of polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheumatol. 67, 2569–2580 (2015).
Hellmich, B. et al. 2018 update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 79, 19–30 (2020).
Laurent, S. et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37, 1236–1241 (2001).
Roman, M. J. et al. Arterial stiffness in chronic inflammatory diseases. Hypertension 46, 194–199 (2005).
Redheuil, A. et al. Proximal aortic distensibility is an independent predictor of all-cause mortality and incident CV events: The MESA study. J. Am. Coll. Cardiol. 64(24), 2619–2629. https://doi.org/10.1016/j.jacc.2014.09.060 (2014).
Aboyans, V., Lacroix, P. & Criqui, M. H. Large and small vessels atherosclerosis: Similarities and differences. Prog. Cardiovasc. Dis. 50, 112–125 (2007).
Hansson, G. K. Inflammatory mechanisms in atherosclerosis. J. Thromb. Haemost. 7, 328–331 (2009).
Shoenfeld, Y. et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 112, 3337–3347 (2005).
Libby, P., Ridker, P. M. & Maseri, A. Inflammation and atherosclerosis. Circulation 105, 1135–1143 (2002).
Sanjadi, M. et al. Atherosclerosis and autoimmunity: A growing relationship. Int. J. Rheum. Dis. 21, 908–921 (2018).
Laurent, S. et al. European Network for non-invasive investigation of large arteries. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 27, 2588–2605. https://doi.org/10.1093/eurheartj/ehl254 (2006).
Lane, N. E. Glucocorticoid-induced osteoporosis: New insights into the pathophysiology and treatments. Curr. Osteoporos. Rep. 17, 1–7 (2019).
McKay, L. I. & Cidlowski, J. A. Physiologic and pharmacologic effects of corticosteroids. In Holland-Frei Cancer Medicine 6th edn (eds Kufe, D. W., Pollock, R. E., Weichselbaum, R. R. et al.) (BC Decker, 2003).
Roubenoff, R. et al. Rheumatoid cachexia: Cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J. Clin. Investig. 93, 2379–2386 (1994).
Pietrobelli, A., Formica, C., Wang, Z. & Heymsfield, S. B. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am. J. Physiol. 271, E941–E951 (1996).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on sarcopenia in older people. Age Ageing 39, 412–423 (2010).
Engvall, I. L. et al. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand. J. Rheumatol. 37, 321–328 (2008).
Piché, M. E., Poirier, P., Lemieux, I. & Després, J. P. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: An update. Prog. Cardiovasc. Dis. 61, 103–113 (2018).
Liyanage, A., Lekamwasam, S., Dissanayake, S. P. & Munidasa, D. Factors that determine body composition of female systemic lupus erythematosus (SLE) patients in Sri Lanka: A comparative study using dual-energy x-ray absorptiometry. Lupus 22, 972–976 (2013).
Mok, C. C., To, C. H. & Ma, K. M. Changes in body composition after glucocorticoid therapy in patients with systemic lupus erythematosus. Lupus 17, 1018–1022 (2008).
Müller, R. et al. Factors associated with low lean mass in early rheumatoid arthritis: A cross-sectional study. Medicina (Kaunas) 55, 730 (2019).
Elkan, A. C., Håkansson, N., Frostegård, J., Cederholm, T. & Hafström, I. Rheumatoid cachexia is associated with dyslipidemia and low levels of atheroprotective natural antibodies against phosphorylcholine but not with dietary fat in patients with rheumatoid arthritis: A cross-sectional study. Arthritis Res. Ther. 11, R37 (2009).
Emamifar, A. et al. Polymyalgia rheumatica and giant cell arteritis-three challenges-consequences of the vasculitis process, osteoporosis, and malignancy: A prospective cohort study protocol. Medicine (Baltimore) 96, e7297 (2017).
Emamifar, A. et al. The utility of 18F-FDG PET/CT in patients with clinical suspicion of polymyalgia rheumatica and giant cell arteritis: A prospective, observational, and cross-sectional study. ACR Open Rheumatol. 2, 478–490. https://doi.org/10.1002/acr2.11163 (2020).
Luqmani, R. et al. the role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): A diagnostic accuracy and cost-effectiveness study. Health Technol. Assess. 20, 1–238 (2016).
Sammel, A. M. et al. Diagnostic accuracy of positron emission tomography/computed tomography of the head, neck, and chest for giant cell arteritis: A prospective, double-blind, cross-sectional study. Arthritis Rheumatol. 71, 1319–1328 (2019).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383 (1987).
Open Patient data Explorative Network (accessed 6 December 2019); https://open.rsyd.dk/index_en.html.
D’Agostino, R. B. Sr. et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 117, 743–753. https://doi.org/10.1161/CIRCULATIONAHA.107.699579 (2008).
Schutz, Y., Kyle, U. U. & Pichard, C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int. J. Obes. Relat. Metab. Disord. 26, 953–960 (2002).
Rubin, K. H. et al. Effectiveness of a two-step population-based osteoporosis screening program using FRAX: The randomized risk-stratified osteoporosis strategy evaluation (ROSE) study. Osteoporos. Int. 29, 567–578 (2018).
Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “Establishing normal and reference values”. Eur. Heart J. 31, 2338–2350 (2010).
Emamifar, A. et al. Prevalence of newly diagnosed malignancies in patients with polymyalgia rheumatica and giant cell arteritis, comparison of 18F-FDG PET/CT scan with chest X-ray and abdominal ultrasound: Data from a 40 week prospective, exploratory, single centre study. J. Clin. Med. 9(12), 3940. https://doi.org/10.3390/jcm9123940 (2020).
Cantini, F. et al. Are polymyalgia rheumatica and giant cell arteritis the same disease?. Semin. Arthritis Rheum. 33(5), 294–301. https://doi.org/10.1016/j.semarthrit.2003.09.008 (2004).
Dejaco, C., Duftner, C., Buttgereit, F., Matteson, E. L. & Dasgupta, B. The spectrum of giant cell arteritis and polymyalgia rheumatica: Revisiting the concept of the disease. Rheumatology (Oxford) 56, 506–515. https://doi.org/10.1093/rheumatology/kew273 (2017).
Rehak, Z. et al. 18F-FDG PET/CT in polymyalgia rheumatica-a pictorial review. Br. J. Radiol. 90(1076), 20170198. https://doi.org/10.1259/bjr.20170198 (2017).
Pieringer, H., Stuby, U., Hargassner, S. & Biesenbach, G. Treatment with corticosteroids reduces arterial stiffness in patients with polymyalgia rheumatica as measured with pulse wave analysis. Ann. Rheum. Dis. 67, 279 (2008).
Schillaci, G. et al. Aortic stiffness is increased in polymyalgia rheumatica and improves after steroid treatment. Ann. Rheum. Dis. 71, 1151–1156 (2012).
Salles Rosa Neto, N. et al. Determinants of arterial stiffness in female patients with Takayasu arteritis. J. Rheumatol. 41, 1374–1378 (2014).
Ng, W. F. et al. Takayasu’s arteritis: A cause of prolonged arterial stiffness. Rheumatology (Oxford) 45, 741–745 (2006).
Wang, X. & Dang, A. Prognostic value of brachial-ankle pulse wave velocity in patients with takayasu arteritis with drug-eluting stent implantation. Arthritis Care Res. (Hoboken) 67, 1150–1157 (2015).
He, Y., Cheng, N., Dang, A. & Lv, N. Association between increased arterial stiffness measured by brachial-ankle pulse wave velocity and cardiovascular events in patients with Takayasu’s arteritis. Clin. Exp. Rheumatol. 37, 65–71 (2019).
Asai, K. et al. Dexamethasone-induced suppression of aortic atherosclerosis in cholesterol-fed rabbits. Possible mechanisms. Arterioscler. Thromb. 13, 892–899 (1993).
Makheja, A. N., Bloom, S., Muesing, R., Simon, T. & Bailey, J. M. Anti-inflammatory drugs in experimental atherosclerosis. 7. Spontaneous atherosclerosis in WHHL rabbits and inhibition by cortisone acetate. Atherosclerosis 76, 155–161 (1989).
Lobatto, M. E. et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol. Pharm. 7, 2020–2029 (2010).
Bailey, J. M. & Butler, J. Anti-inflammatory drugs in experimental atherosclerosis. I. Relative potencies for inhibiting plaque formation. Atherosclerosis 17, 515–522 (1973).
Udayakumar, P. D., Chandran, A. K., Crowson, C. S., Warrington, K. J. & Matteson, E. L. Cardiovascular risk and acute coronary syndrome in giant cell arteritis: A population-based retrospective cohort study. Arthritis Care Res. (Hoboken) 67(3), 396–402. https://doi.org/10.1002/acr.22416 (2015).
Wilson, P. W. et al. Prediction of coronary heart disease using risk factor categories. Circulation 97(18), 1837–1847. https://doi.org/10.1161/01.cir.97.18.1837 (1998).
Chung, C. P., Oeser, A., Avalos, I., Raggi, P. & Stein, C. M. Cardiovascular risk scores and the presence of subclinical coronary artery atherosclerosis in women with systemic lupus erythematosus. Lupus 15(9), 562–569. https://doi.org/10.1177/0961203306071870 (2006).
Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 61, 7–16 (2018).
Emamifar, A. et al. Level of adherence to prophylactic osteoporosis medication amongst patients with polymyalgia rheumatica and giant cell arteritis: A cross-sectional study. Int. J. Rheumatol. 2015, 783709 (2015).
Emamifar, A. et al. High level of adherence to osteoporosis prophylaxis medications in steroid-treated polymyalgia rheumatica (pmr)/giant cell arteritis (gca) patients: A prospective cohort study. IOF Regional 7th Asia-Pacific Osteoporosis Conference Sydney, Australia 2018-posters abstracts. Osteoporos. Int. 30, 55–114 (2019).
Konijn, N. P. et al. The short-term effects of two high-dose, step-down prednisolone regimens on body composition in early rheumatoid arthritis. Rheumatology (Oxford) 55, 1615–1622 (2016).
Tilg, H. & Moschen, A. R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 6, 772–783 (2006).
Nordborg, E., Schaufelberger, C. & Bosaeus, I. The effect of glucocorticoids on fat and lean tissue masses in giant cell arteritis. Scand. J. Rheumatol. 27, 106–111 (1998).
Roubenoff, R., Roubenoff, R. A., Ward, L. M., Holland, S. M. & Hellmann, D. B. Rheumatoid cachexia: Depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. J. Rheumatol. 19, 1505–1510 (1992).
Acknowledgements
We would like to thank Mrs. Merete Birkholm Hansen for her contribution to study coordination. We would like to thank Mrs. Maryam Mousavi for her contribution to data management. And finally, we would like to thank senior consultants Søren Andreas Just, Anders Jørgen Svendsen, Wolf-gang Böhme, Dr. Susan Due Kay and Dr. Rasmus Hviid Larsen for their professional input.
Funding
The present study is funded by the Region of Southern Denmark, The Danish Rheumatism Association, Department of Medicine at Svendborg Hospital, University of Southern Denmark, Odense University Hospital and A.P Møller Fonden.
Author information
Authors and Affiliations
Contributions
A.E., T.E., S.H., O.G., I.M.J.H. and P.T.R. contributed to conceptualization, data curation, formal analysis, Investigation, methodology and validation. A.P.H., Z.A.F. and P.S.H. contributed to data curation, formal analysis, investigation and validation. A.E. drafted the first version of the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emamifar, A., Ellingsen, T., Hermann, A.P. et al. Prognostic impacts of glucocorticoid treatment in patients with polymyalgia rheumatica and giant cell arteritis. Sci Rep 11, 6220 (2021). https://doi.org/10.1038/s41598-021-85857-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85857-4
This article is cited by
-
Predictors of complete 24-month remission and flare in patients with polymyalgia rheumatica
Clinical and Experimental Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.