Abstract
Fertilizer-induced changes in soil nutrients regulate nitrogen (N) fixation in the terrestrial biosphere, but the influences of N and phosphorus (P) fertilization on the diazotroph communities in successive crop seasons were unclear. In this study, we assessed the effects of N and P (high vs. low doses) on the abundance and structure of N2-fixation communities after wheat and soybean harvest in a long-term (34 and 35 years) fertilization experiment. In both seasons, long-term N addition significantly decreased the abundance of nifH genes and 16S rDNA; in addition, high doses of N and P fertilizer decreased the richness of diazotrophs, whereas low doses did not. The proportion of the dominant genus, Bradyrhizobium, in the soybean season (86.0%) was higher than that in the wheat season (47.9%). Fertilization decreased diazotroph diversity and the relative abundance of Bradyrhizobium in the wheat season, but had insignificant effects in the soybean season. The addition of N, but not P, significantly changed the communities of both diazotrophs (at the genus level) and rhizobia (at the species level) in the two seasons. Soil pH was positively associated with nifH abundance and diazotrophic richness; soil NO3− content was negatively correlated with diazotrophic richness and positively correlated with diversity. Soil pH and NO3− content were the two main drivers shaping the soil diazotrophic community. Overall, long-term inorganic N had a greater influence than P on both diazotrophic abundance and community composition, and diazotrophic diversity was more clearly affected by fertilization in the wheat season than in the soybean season.
Similar content being viewed by others
Introduction
Nitrogen (N) is the most essential element limiting productivity in terrestrial ecosystems1. The global N input into agricultural systems from synthetic fertilizer has increased more than 40-fold since 19302. According to the National Bureau of Statistics of China, in Heilongjiang Province in Northeast China, where the total grain output was 75.05 billion kg in 2018, accounting for more than 10% of China's total grain output, erosion of its iconic black soil occurrs because of excessive application of chemical fertilizer (http://www.xinhuanet.com). The use of such large amounts of N, organic fertilizer, and organic fertilizer combined with chemical fertilizer has posed severe challenges to the soil microbial community, including bacteria3, fungi4,5, acidobacteria6, arbuscular mycorrhizal fungi7,8, diazotrophs9, and ammonia oxidizing archaea10, in black soil in northeast China.
Biological N fixation is an important ecological process11 on earth that is responsible for fixation of as much as 100 Tg N year−1 from the atmosphere globally, thus contributing more than 97% to the N input in natural terrestrial ecosystems12. Only microorganisms in the bacterial and archaeal domains are known to be capable of fixing atmospheric dinitrogen, a process termed diazotrophy, and nifH is a suitable marker for investigating the diversity and composition of diazotroph communities13. In agricultural systems, diazotrophs are sensitive to fertilizer management practices14, and large amounts of fertilizer might relegate N fixers to second place and may have long-term consequences for diazotrophs and ecosystem processes in the future15. Long-term chemical N fertilizer application has been shown to drastically decrease N fixation11, decrease diversity, and alter the community structure16 and assembly processes17 of soil diazotrophs. Taxa in the genera Bradyrhizobium and Burkholderia have been shown to have a positive association, whereas Geobacter and Anaeromyxobacter have been shown to have a negative association, with N fertilization for four decades11.
Soybean is the most important leguminous food crop18 and is often used as a rotation crop because it is excellent in improving soil structure and fertility19. Rainfed wheat-soybean-maize rotations account for more than half of China’s food production, particularly in northeast China20. The abundance and composition of the diazotrophic community in the soil are related to several factors, including fertilization regimes11, soil moisture and temperature21, and vegetation types22. Soil physicochemical properties are affected by specific plant species through litterfall, root phenes and exudates containing different nutrients, which influence the soil microenvironment and subsequently affect the diazotrophic community23. However, the differences in the diazotrophic community abundance and composition between soils in two continuous crop seasons (wheat and soybean, a nonlegume and legume, respectively) under N fertilization regimes are unclear.
Here, we investigated the response of the diazotrophic community assembly in wheat and soybean seasons to five long-term (34 and 35 years) inorganic fertilizer treatments. One approach was no fertilizer added (CK), and the other four approaches involved the addition of artificial fertilizer treatments: low N (N1), low N plus low P (N1P1), high N (N2) and high N plus high P (N2P2). We performed pyrosequencing and real-time PCR based analysis of nifH sequences obtained from long-term fertilization trials located at the Heilongjiang Academy of Agricultural Sciences, China. In this study, using experimental gradients of N and P, we addressed the following specific questions: (i) Can N and P addition have different effects on the abundance and composition of the soil diazotrophic community and the dominant phyla/classes/genera in the wheat and soybean seasons? (ii) would shifts in specific bacterial taxa correspond to the fertilization regimes or plants?
Results
Effects of long-term fertilization on the abundance of bacteria and diazotrophs
The abundances of the nifH gene (Fig. 1A) and 16S rDNA (Fig. 1B) were all significantly lower in the N input soils (N1, N1P1, N2, and N2P2) than in unfertilized soils in the two seasons. The diazotroph to bacteria ratio showed no clear differences among the five samples in the wheat season but was lower in N input soils (N1, N1P1, N2, and N2P2) than in unfertilized soil in the soybean season (Fig. 1C).
Effect of fertilization on diazotrophic diversity
A significantly lower Chao index of the nifH communities was measured with high fertilizer treatments (N2 and N2P2), whereas the results were not significantly different between low fertilizer treatments (N1 and N1P1) and unfertilized soil in the two seasons (Fig. 2A). Fertilization was considered to have an insignificant effect on the Shannon index at the P < 0.05 level (P = 0.625) in the soybean season, whereas the index was lower with N1, N1P1, and N2P2, as compared with CK, in the wheat season (Fig. 2B). The Chao and Shannon indices were lower with N2P2 than with N2 in the two seasons.
Relationship between soil chemical properties and α-diversity and gene copies
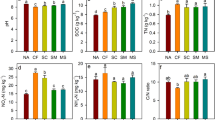
A t-test comparison across all treatments in both crop seasons revealed a significant decline in soil pH (Table 1). In contrast, the concentrations of NO3−, Avail P, Avail K, TN, and OM, which were clearly higher in soil with fertilizer treatments (N1, N1P1, N2, and N2P2) than in unfertilized soil (Table 1). Ammonium (NH4+) was greater under theN and P fertilizer treatments than CK, whereas no statistically significant differences were observed for plots fertilized during the soybean season, regardless of fertilization (Table 1).
Soil pH showed a highly significant (P < 0.01) and positive linear relationship with the number of nifH gene copies (r = 0.64, Supplementary Fig. S1A) and the Chao index (r = 0.628, Supplementary Fig. S1C). The oil NO3- content had a significant (P < 0.01) negative relationship with the number of 16S rDNA copies (r = − 0.595, Supplementary Fig. S1B) and the Chao index (r = − 0.59, Supplementary Fig. S1D), but had a significant (P < 0.01) and positive relationship with the Shannon index (r = − 0.527, Supplementary Fig. S1E). Other soil properties including the soil content of NH4+, Avail P, Avail K, TN and OM were not significantly associated with α diversity indices, and nifH gene or 16S rDNA copy numbers.
Regional distributions in the diazotrophic populations
We constructed a phylogenetic tree with the dominant diazotrophic phylotypes, which were generated for the 180,600 nifH sequences collected from the 30 soil samples. A large number of the nifH gene sequences were affiliated with Proteobacteria, which accounted for 86.5% of the bacteria in wheat soil and 98.5% in soybean soil (on average), followed by Cyanobacteria (0.13–27.8%), Firmicutes (0–11.26%) and Verrucomicrobia (0–6.2%) (Supplementary Table S3). For the phylum Proteobacteria, the classes Alphaproteobacteria, Betaproteobacteria, Deltaproteobacteria, and Gammaproteobacteria, were dominant in all soil samples (Fig. 3 A ①, ②, ③ and ④, respectively). The dominant family was Bradyrhizobiaceae, which was more abundant in the soybean season (71.5–89.5%) than in the wheat season (16.04–76.3%) (Supplementary Table S3).
Phylogenetic tree and clustering analysis based on nitrogen-fixing microorganisms. (A) Phylogenetic tree displaying the taxonomic information on soil diazotrophic phylotypes. (B,C) show the results of clustering analysis based on nitrogen-fixing microorganisms (at the genus level) in soils of the wheat and soybean seasons, respectively.
We found six genera belonging to rhizobia: Bradyrhizobium, Methylobacterium, Ensifer (formerly Sinorhizobium), Azorhizobium, Burkholderia, and Azotobacter (Fig. 4A). Interestingly, the average abundance of the genus Bradyrhizobium was much higher in the soybean season (86.0%) than in the wheat season (47.9%) (Supplementary Table S4). In the wheat season, Bradyrhizobium abundance under N1, N1P1, and N2 was 64.4%, 12.2% and 4.8% higher, respectively, whereas it was 65.4% lower under N2P2 than CK (Supplementary Table S4). However, there was no clear difference in Bradyrhizobium abundance in the soybean season (Supplementary Table S4). The soil NO3- content was negatively correlated with the relative abundances of Bradyrhizobium (r = − 0.627, P < 0.01) and Azotobacter (r = − 0.371, P < 0.05), but positively (r = 0.716, P < 0.05) correlated with the relative abundance of Methylobacterium (r = 0.716, P < 0.01) (Fig. 4A). The soil NH4+ content was negatively correlated with the relative abundance of Burkholderia (r = − 0.612, P < 0.01) and Azotobacter (r = − 0.390, P < 0.05), and positively correlated (r = 0.516, P < 0.05) with Azorhizobium (Fig. 4A).
Correlation and cluster analysis at the species level. (A) Correlationships between microbial abundance, genera belonging to rhizobia and soil chemical properties. (B,C) show the results of clustering analysis based on rhizobia (at the species level) in soils of the wheat and soybean seasons, respectively. (D) Shows the relative abundance of Bradyrhizobium diazoefficiens in soil samples and their correlation with N fertilizer. W soil samples in the wheat season, S soil samples in the soybean season.
The abundance of the dominant species, Bradyrhizobium diazoefficiens, was negatively correlated with soil NO3− (r = − 0.563, P < 0.05) and NH4+ (r = − 0.764, P < 0.01) contents, and positively associated with soil pH (r = − 0.636, P < 0.05) (Fig. 4A). In addition, the relative abundance of this species was significantly and negatively correlated with the N-level in the wheat season, whereas no significant relationship was observed in the soybean season (Fig. 4D).
The clustering results showed that the nifH communities were significantly separated by N addition treatments in the wheat (Fig. 3B) and soybean (Fig. 3C) seasons at the genus level. Similarly, the rhizobial group also showed the same trend at the species level (Fig. 4B,C). In the wheat season, compared with CK, Bradyrhizobium (Fig. 3B) and Bradyrhizobium diazoefficiens (Fig. 4D) were significantly higher in the presence of low N but lower in the presence of high N. However, no significant difference was observed among samples in the soybean season.
NMDS at the OTU level
The NMDS results revealed that nifH community composition varied significantly (P< 0.05) with respect to N addition but not P addition. Across the two seasons, the phylogenetic structure of the nifH communities shifted in similar ways. The nifH communities in soils in the wheat season did not differ significantly from those in the soybean season (Figure S2). Three separate groups were clearly observed along NMDS1 (accounting for 67.31% of the variation in nifH community): no fertilized soils (SCK and WCK, orange circles); low N and low N plus P fertilized soils (SN1, SN1P1, WN1, and WN1P1, green circles); and high N and high N plus P fertilized soils (SN2, SN2P2, WN2, and WN2P2, red circles, except for SN2 and SN2P2) (Supplementary Fig. S2). As the amount of added N increased, communities became more different from those in unfertilized soils in both seasons.
Environmental effects on diazotrophs
In the MRT, the dominant lineages were first split by NO3− content, which explained 26.0% of the variation in community structure (Fig. 5A). The tree explained 71.1% of the variance in the standardized diversity indices. At the second node, the split was determined by soil pH, which explained 17.8% of the variation. The communities were then split by pH and NO3−, accounting for 14.3% and 8.4% of the variation in the data, respectively (Fig. 5A). The results of RDA with Monte Carlo permutation tests showed that NO3−, pH, Avail P, and Avail K were significantly (P < 0.05) correlated with the changes in the composition of the N-fixing community, with contributions of 41.4%, 17.1%, 13.2%, and 9.2%, respectively (Supplementary Table S5). All samples were separated into two groups (wheat and soybean season: squares and circles, respectively) (Fig. 5B) along the RDA2 axis, except for SCK-3. Along the RDA1 axis, nifH communities under low N, and low N plus low P were clearly different from those under CK, and those under high N, and high N plus high P were more different in the two crop seasons. We found that climate factors (temperature and precipitation) were not significantly (P > 0.05 in MRT, P = 0.05 in RDA) correlated with the changes in the composition of the N-fixing community
The relationship between the N-fixing community composition and soil chemical properties. (A) Multivariate regression tree (MRT) analysis was performed to interpret the relationship between the nitrogen-fixing community composition and soil chemical properties at the class level. (B) Redundancy analysis (RDA) was performed to assess the relationship between the nitrogen-fixing community composition and soil chemical properties at the genus level. W soil samples in the wheat season, S soil samples in the soybean season.
Discussion
Fertilization decreased bacterial and diazotrophic abundance
The correlation between nifH abundance and fertilizer inputs was controversial in previous studies. In our study, the abundance of nifH in fertilizer treatments decreased in the two seasons, in agreement with conclusions reached by Zhang24 and Coelho25 validating Fan's11 hypothesis that N fixation and fixers would become less abundant over time in fertilized environments. Urea addition in the present study may have been particularly detrimental for obligate N fixers; their ability to downregulate fixation was limited, and thus they exhibited relatively narrow growth tolerance. For the first time, we reported that the diazotroph to bacteria ratio was more sensitive during the growth and development of soybean than that of wheat. The application of N fertilizers is generally expected to decrease the dependence of the ecosystem on free-living N- fixers26, and this finding might explain the slight decrease in nifH/16S rDNA in the wheat season. Although symbioses between some N fixing bacteria with soybean can provide N-fixers with an exclusive niche and contribute to their growth27, we found a clear decrease in nifH/16S rDNA in the soybean season, thus indicating that the symbiotic ability of N-fixers with the soybean decreased under a 35 year N fertilization regime. We concluded that the clear differences in this ratio in the two seasons could be explained by the interactions among individual plant strategies, in agreement with Sheffer's28 conclusion. NifH abundance under N1 and N2 did not show clear differences from those under N1P1 and N2P2, respectively, thus indicating that P did not have a significant effect on nifH gene copy number.
Most studies have found that an increase in N decreases soil pH29,30, and we found the same result; however, P had no such effect. In this study, we found that with increased acidification of black soil, the growth and reproduction of N-fixers was significantly inhibited. Other studies have found that the copy number of the nifH gene is strongly positively associated with soil NH4+31 and available K32, but negatively correlated with total N31.
Effects of fertilization on diazotrophic diversity
Many studies have found that N addition increases the richness of diazotrophs33,34. However, some studies have found that N has no such clear influence35. However, we observed significantly lower Chao index values for N fixers under the addition of high N, and high N plus high P in the two seasons (Fig. 2A). One possible reason for this finding is that N fixers have a strong advantage in N deficient conditions, but some species have difficulty surviving and may even die under intense increases in the concentration of available N in the soil microenvironment35.
Coelho’s36 study on another nonlegume crop, sorghum, showed that the diazotrophic Shannon diversity under high levels of N was lower than that under low levels of N, in agreement with the results in the wheat season in our study. However, the higher proportion of Bradyrhizobiaceae in the soybean season than in the wheat season might explain the lower diversity of nifH sequences in the soybean season. Furthermore, nodules formed by Bradyrhizobium and soybean roots increase the tolerance to various stresses, such as salt37, acidity38, drought39, insecticide40, and high aluminium38, thus potentially also explaining why nifH diversity was not significantly affected by N in the soybean season but was significant in the wheat season. We additionally found that P had clear inhibitory effects on the richness and diversity of diazotrophs.
Shannon indices of diazotrophic and nitrite-dependent anaerobic methane oxidation bacteria41 were positively correlated with NO3− content, thus indicating that the increase in NO3− was beneficial to the diversity of N cycling microorganisms. Santoscaton42 found that NO3− loads are associated with bacterial 16S rDNA abundance but not nifH gene abundance, similar to our results.
N fertilizer affects the structural composition of N-fixing bacteria
N but not P fertilizer had significant effects on diazotrophic community composition, which indicated that the level of N fertilizer was the most important factor affecting the structural composition of N-fixing bacteria in the black soil of Northeast China. This result was highly consistent with the response of diazotrophic bacterial25, ammonia-oxidizing archaeal43, bacterial44 and fungal5 communities to N fertilization regimes.
The process of nitrification in soil is performed partly by gram-negative bacteria in the family Bradyrhizobiaceae, in a process involving the conversion of NH4+ into NO2- and subsequently NO3–45. Therefore, the higher average concentration of NH4+ in soybean (42.08 mg kg−1) than in wheat (37.06 mg kg−1) soils, may lead to an increase in Bradyrhizobiaceae in the soybean season. The high abundance of Bradyrhizobiaceae in the soybean season could also be explained by stable symbiosis between leguminous plants (soybean) and rhizobia, although the roots of nonleguminous plants (wheat) can be colonized by rhizobia25. Furthermore, linear relationships between the cultivar and the bacterial community have been reported, such as genotype associations of maize with Azospirillum46, alfalfa cultivars with Sinorhizobium47, and sorghum cultivars with Paenibacillus48. Therefore, the results presented here emphasize the importance of cultivar type in selecting N-fixing strains for use as wheat and soybean inoculants.
N fertilizer affects the structural composition of rhizobia
Rhizobia, a collective name for the symbiotic N-fixing bacteria associated with legumes, comprise 14 genera1, six of which were found in the current study. The community structure of rhizobia was distinguished by N levels. Bradyrhizobium was reported to be more adapted to acidic soils49, while we found a lower abundance under N2P2 with a lower pH (4.79). Thus, we propose that Bradyrhizobium may use suitable amounts of available N to support their growth, whereas N fixation and N fixers will become increasingly less important when NO3- is excessive. This hypothesis is based on the negative correlation between Bradyrhizobium and the soil NO3− content (Fig. 4A). Ahmed50 concluded that soil NO3− has a negative effect on the activity of N-fixing rhizobia by inhibiting the function of the enzymes nitrogenase and leghaemoglobin.
The dominance of B. diazoefficiens over Bradyrhizobium sp., B. japonicum, and B. elkanii revealed a unique community structure of soybean rhizobia in the black soil, a finding not consistent with those of Yan51. The negative correlation between Bradyrhizobium diazoefficiens and N fertilizer in the wheat season rather than in the soybean season may be explained by the sensitivity of certain bacterial species present in plant types to N fertilizer. In addition, the relatively higher content of NO3− under N2 and N2P2 in the wheat season may have caused Bradyrhizobium diazoefficiens to become increasingly less important. The abundance of Bradyrhizobium diazoefficiens in the wheat season indicated that it is a genospecies whose growth is clearly inhibited by N fertilizer.
Effects of soil properties on the diazotrophic community
Researchers have confirmed that soil physicochemical characteristics affect the activity of N-fixers52. MRT and RDA results confirmed that the soil NO3− content was the most important contributor to the soil diazotroph community, a finding consistent with reports by Yang53 and Zou54. Moreover, soil NO3− content was identified as an important predictor of 16S rDNA gene abundance and the α-diversity of the diazotroph community (Supplementary Fig. S1). Neutral or slightly acidic soil conditions are conducive to biological N fixation18, while the strong acidity in high N and P may be a severe problem for N fixation, because in such environments legume nodules fail to form, and some rhizobia become inactive18. Seminal work by Wang16 highlighted the importance of soil pH as a fundamental driver of the distribution of the diazotrophic community, and we reached the same conclusion. In this study, soil acidification in black soil in northeast China, caused by high levels of N fertilization, usually leads to problematic nutrient deficiency or mineral toxicity during N fixation55. These findings may aid in predicting the response and feedback of the diazotroph community in farmland ecosystems to high levels of N fertilization.
More recently, researchers have shown that diazotroph diversity and richness are mainly influenced by soil available P53 and available K16; our results again validated these conclusions. Our findings indicated that soil nutrient availability, which was highly responsive to fertilizer input, was crucial for the establishment of the soil diazotrophic community structure56 in black soil in Northeast China.
Conclusion
Our work provided solid evidence, after 34 and 35 years of experiments, that N fertilization largely influenced diazotroph communities in the soil in two successive crop seasons in northeast China. N is likely to have a greater influence than P on diazotrophic bacteria. The community structure of N-fixing bacteria and rhizobia was clearly associated with the level of N fertilizer. The lower diazotrophic abundance under N fertilizer treatments may have diminished the capacity for biological N fixation in the two seasons. N had greater effects on diazotrophic diversity and the relative abundance of the dominant genus Bradyrhizobium in the wheat season than in the soybean season. The different response patterns of diazotrophic abundance, community composition, and diversity to the soil properties revealed a complicated mechanism underlying the diazotrophic population's adaptation to long-term N and P fertilization in two crop seasons. However, we only conducted research at the DNA level, and future research will determine the impact of N fertilization on the functional diversity of diazotrophs in two seasons based on mRNA profiling of nifH genes.
Materials and methods
Experimental design and sample collection
The experimental site was set up in 1979 in Harbin city, Heilongjiang Province, China (45° 40ʹ N, 126° 35ʹ E and altitude 151 m), which is in the secondary terrace of the Songhua River. The soil type is black soil, and the parent material is flooded loess-like clay. The tillage method is a combination of shallow tillage and deep rotation. The annual crop rotation of wheat, soybean and maize was repeated every 3 years in the field with five fertilization treatments in a completely randomized block design with three replicates: CK (without fertilizer), N1 (low N), N2 (high N), N1P1 (low N plus low P) and N2P2 (high N plus high P). Taking into account the difference in N needs for wheat and soybean, we chose different amounts of low N (75 and 150 kg urea ha−1 year−1 for wheat and soybean, respectively) and high N (150 and 300 kg urea ha−1 year−1 for wheat and soybean, respectively) treatments. The detailed types and amounts of fertilizer were shown in Supplementary Table S1. N, P, and K fertilizers are all applied after harvest of the previous crop in autumn (September). We collected soil samples in September 2013 and 2014 after wheat and soybean harvests, respectively before the next fertilization. The annual average soil temperature, 10 cm below the surface of the soil was 7.4 and 5.9 °C, and the annual precipitation was 2262 and 296 mm in 2013 and 2014, respectively44. The experimental design and sample collection are shown in Fig. 6 [The map was produced using ‘R (i386 3.1.2, https://www.R-project.org, The R Core Team, 2019)’, with the open source packages ‘maps’, ‘mapdata’, and ‘maptools’57]. Soil samples were randomly collected from the plow layer of soil (5–20 cm) and stored as described in our previous research44.
Soil chemical properties
Soil pH was measured at a 1:5 ratio of soil to distilled water (weight/volume). Nitrate (NO3−–N) and ammonium (NH4+–N) were extracted from 5 g of air- dried soil with 2 M KCl, steam distillation and titration. The available P (Avail P) was extracted with 0.5 M NaHCO3 and determined with the molybdenum blue method. Available potassium (Avail K) was extracted with 1 M ammonium acetate and determined by flame photometry. The organic matter (OM) and total N (TN) were determined according to Strickland and Sollins (1987).
High-throughput sequencing and bioinformatics analysis
Fast DNA SPIN Kit (MP Biomedicals, Santa Ana, CA, USA) was used to extract DNA from 0.5 g of fresh soil. The nifH gene was amplified using the primer pairs nifH f and nifH r58. Primer sets and PCR reactions were as detailed in Supplementary Table S2, and amplification reactions were sequenced on the Illumina MiSeq PE300 platform. The raw reads have been deposited in the National Center for Biotechnology Information Database (SRX 1034826). The nifH nucleotide sequences were analysed with the QIIME-1.9.1 pipeline. Briefly, the low quality sequences were discarded, and the remaining sequences were converted to amino acid sequences using the FunGene Pipeline of the Ribosomal Database Project according to Ref.11. The sequences encoding proteins that contained termination codons or that did not match the nifH protein sequence were removed. Operable classification units (OTUs) were classified with a similarity of 95%. The phylogenetic tree for diazotrophic phylotypes at the OTU level in the ecological clusters was built and visualized with GraPhlAn11 based on a logarithmic scale. Hierarchical clustering analysis was performed at the genus and species levels with PAST software (version 3.01, folk.uio.no/ohammer/past/)59.
Quantitative PCR analysis
The abundance of bacterial 16S rDNA and the nifH gene were analysed with an ABI 7500 Real-Time PCR detection system with primers 515F-806R3 and nifH f-nifH r. Primer sets, the qPCR amplification system and the thermal programme are detailed in Supplementary Table S2. Plasmid DNA containing 16S rDNA and nifH fragments were used for quantitative PCR standards. The specificity was determined by melting curve analysis and agarose gel electrophoresis60. The ratio of N-fixing microorganisms to bacteria was calculated according to the nifH gene and 16S rDNA copy numbers.
Statistical analysis
Analysis of variance was performed with a randomized complete block design in IBM SPSS Statistics 21. Linear regression analysis was performed to test for statistical significance and the strength of associations between soil chemical properties and α-diversity and gene copy numbers (16S rDNA and nifH) in Origin 2020. Phylogenetic tree was visualized using GraPhlAn261 with the data of OTU representive sequences and OTU abundant table. On the basis of Bray–Curtis similarity distance, nonmetric multidimensional scaling (NMDS) was used to analyse the nifH community structure at the OTU level. A multivariate regression tree (MRT) analysis was performed with the package “mvpart” in the “R” statistical program to interpret the main relationships between the biological data (at the class level) and environmental factors [soil chemical properties and climate status (temperature and precipitation)]62. The correlations between the N-fixing communities (at the genus level) and environmental factors were determined with redundancy analysis (RDA), by using CANOCO 5.0. A logarithmic transformation was performed to normalize the data and the significance (P-value) for the first two canonical axes was evaluated by means of Monte Carlo tests based on 999 permutations.
References
Berthrong, S. T. et al. Nitrogen fertilization has a stronger effect on soil nitrogen-fixing bacterial communities than elevated atmospheric CO2. Appl. Environ. Microb. 80, 3103–3112. https://doi.org/10.1128/AEM.04034-13 (2014).
Millar, N., Robertson, G. P., Grace, P. R., Gehl, R. J. & Hoben, J. P. Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (Maize) production: An emissions reduction protocol for US Midwest agriculture. Mitig. Adapt. Strat. Gl. 15, 185–204. https://doi.org/10.1007/s11027-010-9212-7 (2010).
Zhou, J. et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 90, 42–51. https://doi.org/10.1016/j.soilbio.2015.07.005 (2015).
Ding, J. et al. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl. Soil Ecol. 111, 114–122. https://doi.org/10.1016/j.apsoil.2016.12.003 (2017).
Zhou, J. et al. Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. Biochem. 95, 135–143. https://doi.org/10.1016/j.soilbio.2015.12.012 (2016).
Liu, J. et al. Diversity and distribution patterns of acidobacterial communities in the black soil zone of northeast China. Soil Biol. Biochem. 95, 212–222. https://doi.org/10.1016/j.soilbio.2015.12.021 (2016).
Pan, H. et al. Organic and inorganic fertilizers respectively drive bacterial and fungal community compositions in a fluvo-aquic soil in northern China. Soil Till. Res. 198, 104540. https://doi.org/10.1016/j.still.2019.104540 (2020).
Ma, M. et al. Chronic fertilization of 37 years alters the phylogenetic structure of soil arbuscular mycorrhizal fungi in Chinese Mollisols. AMB Express 8, 57. https://doi.org/10.1186/s13568-018-0587-2 (2018).
Hu, X. et al. Long-term manure addition reduces diversity and changes community structure of diazotrophs in a neutral black soil of northeast China. J. Soils Sediments 18, 2053–2062. https://doi.org/10.1007/s11368-018-1975-6 (2018).
Liu, J. et al. Ammonia-oxidizing archaea show more distinct biogeographic distribution patterns than ammonia-oxidizing bacteria across the black soil zone of northeast China. Front. Microbial. 9, 171. https://doi.org/10.3389/fmicb.2019.00023 (2018).
Fan, K., Delgado-Baquerizo, M., Guo, X., Wang, D. & Chu, H. Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 7, 143. https://doi.org/10.1186/s40168-019-0757-8 (2019).
Kumar, U. et al. Long-term aromatic rice cultivation effect on frequency and diversity of diazotrophs in its rhizosphere. Ecol. Eng. 101, 227–236. https://doi.org/10.1016/j.ecoleng.2017.02.010 (2017).
Gaby, J. C., Rishishwar, L., Valderrama-Aguirre, L. C., Green, S. J. & Kostka, J. E. Diazotroph community characterization via a high-throughput nifH amplicon sequencing and analysis pipeline. Appl. Environ. Microbiol. 84, 01512–01517. https://doi.org/10.1128/AEM.01512-17 (2018).
Wang, J. et al. Temporal variation of diazotrophic community abundance and structure in surface and subsoil under four fertilization regimes during a wheat growing season. Agric. Ecosyst. Environ. 216, 116–124. https://doi.org/10.1016/j.agee.2015.09.039 (2016).
Van Kessel, C. & Hartley, C. Agricultural management of grain legumes: Has it led to an increase in nitrogen fixation?. Field Crops Res. 65, 165–181. https://doi.org/10.1016/S0378-4290(99)00085-4 (2000).
Wang, C. et al. Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol. Biochem. 113, 240–249. https://doi.org/10.1016/j.soilbio.2017.06.019 (2017).
Feng, M. et al. Long-term fertilization influences community assembly processes of soil diazotrophs. Soil Biol. Biochem. 126, 151–158. https://doi.org/10.1016/j.soilbio.2018.08.021 (2018).
Fan, L. Response of diazotrophic microbial community to nitrogen input and glyphosate application in soils cropped to soybean. (2013).
Cheng, F. et al. Isolation and application of effective nitrogen fixation rhizobial strains on low-phosphorus acid soils in South China. Chin. Sci. Bull. 54, 412–420. https://doi.org/10.1007/s11434-008-0521-0 (2009).
Qiao, Y. et al. The effect of fertilizer practices on N balance and global warming potential of maize–soybean–wheat rotations in Northeastern China. Field Crops Res. 161, 98–106. https://doi.org/10.1016/j.fcr.2014.03.005 (2014).
Hsu, S. F. & Buckley, D. H. Evidence for the functional significance of diazotroph community structure in soil. ISME J. 3, 124–136. https://doi.org/10.1038/ismej.2008.82 (2009).
Chen, J., Shen, W., Xu, H., Li, Y. & Luo, T. The composition of nitrogen-fixing microorganisms correlates with soil nitrogen content during reforestation: A comparison between legume and non-legume plantations. Front. Microbiol. 10, 508. https://doi.org/10.3389/fmicb.2019.00508 (2019).
Saleem, M., Law, A. D., Sahib, M. R., Pervaiz, Z. H. & Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 6, 47–51. https://doi.org/10.1016/j.rhisph.2018.02.003 (2018).
Zhang, X. et al. Response of the abundance of key soil microbial nitrogen-cycling genes to multi-factorial global changes. PLoS ONE 8, e76500. https://doi.org/10.1371/journal.pone.0076500 (2013).
Coelho, M. et al. Molecular detection and quantification of nifH gene sequences in the rhizosphere of sorghum (Sorghum bicolor) sown with two levels of nitrogen fertilizer. Appl. Soil Ecol. 42, 48–53. https://doi.org/10.1016/j.apsoil.2009.01.010 (2009).
Wakelin, S. A. et al. The effects of stubble retention and nitrogen application on soil microbial community structure and functional gene abundance under irrigated maize. Fems Microbiol. Ecol. 59, 661–670. https://doi.org/10.1111/j.1574-6941.2006.00235.x (2006).
Shirani, H., Hajabbasi, M. A., Afyuni, M. & Hemmat, A. Effects of farmyard manure and tillage systems on soil physical properties and corn yield in central Iran. Soil Till. Res. 68, 101–108. https://doi.org/10.1016/S0167-1987(02)00110-1 (2002).
Sheffer, E., Batterman, S. A., Levin, S. A. & Hedin, L. O. Biome-scale nitrogen fixation strategies selected by climatic constraints on nitrogen cycle. Nat. Plants 1, 15182. https://doi.org/10.1038/nplants.2015.182 (2015).
Guo, J. H. et al. Significant acidification in major Chinese croplands. Science 327, 1008–1010. https://doi.org/10.1126/science.1182570 (2010).
Ding, J. et al. Effect of 35 years inorganic fertilizer and manure amendment on structure of bacterial and archaeal communities in black soil of northeast China. Appl. Soil Ecol. 105, 187–195. https://doi.org/10.1016/j.apsoil.2016.04.010 (2016).
Soman, C., Keymer, D. P. & Kent, A. D. Edaphic correlates of feedstock-associated diazotroph communities. GCB Bioenergy 10, 343–352. https://doi.org/10.1111/gcbb.12502 (2018).
He, D. et al. Evolvement of structure and abundance of soil nitrogen-fixing bacterial community in Phyllostachys edulis plantations with age of time. Acta Pedol. Sin. 52, 934–942. https://doi.org/10.11766/trxb201408070397 (2015).
Ning, Q. et al. Effects of nitrogen deposition rates and frequencies on the abundance of soil nitrogen-related functional genes in temperate grassland of northern China. J. Soils Sediments 15, 694–704. https://doi.org/10.1007/s11368-015-1061-2 (2015).
Huang, J. et al. Responses of soil nitrogen fixation to Spartina alterniflora invasion and nitrogen addition in a Chinese salt marsh. Sci. Rep. 6, 20384. https://doi.org/10.1038/srep20384 (2016).
Zhu, C. et al. N-fertilizer-driven association between the arbuscular mycorrhizal fungal community and diazotrophic community impacts wheat yield. Agric. Ecosyst. Environ. 254, 191–201. https://doi.org/10.1016/j.agee.2017.11.029 (2018).
Coelho, M. et al. Diversity of nifH gene pools in the rhizosphere of two cultivars of sorghum (Sorghum bicolor) treated with contrasting levels of nitrogen fertilizer. FEMS Microbiol. Lett. 111, 114–122. https://doi.org/10.1111/j.1574-6968.2007.00975.x (2007).
Velagaleti, R. R. & Marsh, S. Influence of host cultivars and Bradyrhizobium strains on the growth and symbiotic performance of soybean under salt stress. Plant Soil 119, 133–138. https://doi.org/10.1007/BF02370277 (1989).
Appunu, C. & Dhar, B. Symbiotic effectiveness of acid-tolerant Bradyrhizobium strains with soybean in low pH soil. Afr. J. Biotechnol. https://doi.org/10.5897/AJB06.131 (2006).
Kunert, K. J. et al. Drought stress responses in soybean roots and nodules. Front. Plant Sci. 7, 1015. https://doi.org/10.3389/fpls.2016.01015 (2016).
Ahemad, M. & Khan, M. S. Insecticide-tolerant and plant growth promoting Bradyrhizobium sp. (vigna) improves the growth and yield of greengram [Vigna radiata (L.) Wilczek] in insecticide-stressed soils. Symbiosis 54, 17–27. https://doi.org/10.1007/s13199-011-0122-6 (2011).
Chen, J., Zhou, Z. & Gu, J. Occurrence and diversity of nitrite-dependent anaerobic methane oxidation bacteria in the sediments of the South China Sea revealed by amplification of both 16S rRNA and pmoAgenes. Appl. Microbiol. Biotechnol. 98, 5685–5696. https://doi.org/10.1007/s00253-014-5733-4 (2014).
Santoscaton, I. R., Caton, T. M. & Schneegurt, M. A. Nitrogen-fixation activity and the abundance and taxonomy of nifH genes in agricultural, pristine, and urban prairie stream sediments chronically exposed to different levels of nitrogen loading. Arch. Microbiol. https://doi.org/10.1007/s00203-018-1475-5 (2018).
Zhou, J. et al. Effects of long term application of urea on ammonia oxidizing archaea community in black soil in Northeast China. Sci. Agric. Sin. 49, 294–304. https://doi.org/10.3864/j.issn.0578-1752.2016.02.010 (2016).
Zhou, J. et al. Consistent effects of nitrogen fertilization on soil bacterial communities in black soils for two crop seasons in China. Sci. Rep. 7, 3267. https://doi.org/10.1038/s41598-017-03539-6 (2017).
Diedrick, K. A. Field Investigations of Nitrogen Fertility on Corn and Soybeans and Foliar Manganese-Glyphosate Interactions on Glyphosate-Tolerant Soybeans in Ohio (The Ohio State University, 2010).
Salamone, I., Bereiner, J., Urquiaga, S. & Boddey, R. Biological nitrogen fixation in Azospirillumstrain-maize genotype associations as evaluated by the 15N isotope dilution technique. Biol. Fertil. Soils 23, 249–256. https://doi.org/10.1007/BF00335952 (1996).
Carelli, M. et al. Genetic diversity and dynamics of sinorhizobium meliloti populations nodulating different alfalfa cultivars in Italiansoils. Appl. Environ. Microbiol. 66, 4785–4789. https://doi.org/10.1128/AEM.66.11.4785-4789.2000 (2000).
Coelho, M. R. et al. Diversity of Paenibacillus spp. in the rhizosphere of four sorghum (Sorghum bicolor) cultivars sown with two contrasting levels of nitrogen fertilizer assessed by rpoB-based PCR-DGGE and sequencing analysis. J. Microbiol. Biotechnol. 17, 753–760. https://doi.org/10.1007/s10295-007-0209-5 (2007).
Cao, Y., Wang, E., Zhao, L., Chen, W. & Wei, G. Diversity and distribution of rhizobia nodulated with Phaseolus vulgaris in two ecoregions of China. Soil Biol. Biochem. 78, 128–137. https://doi.org/10.1016/j.soilbio.2014.07.026 (2014).
Ahmed, I. H., Francina, L. B., Isabella, H. R. & Galaletsang, S. Nodulation efficacy of Bradyrhizobium japonicum inoculant strain WB74 on soybean (Glycine max L. Merrill) is affected by several limiting factors. Afr. J. Microbiol. Res. 8, 2069–2076. https://doi.org/10.5897/ajmr2014.6709 (2014).
Yan, J. et al. Effects of long-term fertilization strategies on soil productivity and rhizobial diversity in Chinese mollisol. Pedosphere 29, 784–793. https://doi.org/10.1016/S1002-0160(17)60470-3 (2019).
Riffkin, P. A., Quigley, P. E., Kearney, G. A., Cameron, F. J. & Thies, J. E. Factors associated with biological nitrogen fixation in dairy pastures in south-western Victoria. Aust. J. Agric. Res. 50, 261–272. https://doi.org/10.1071/a98035 (1999).
Yang, L. et al. Diazotroph abundance and community structure are reshaped by straw return and mineral fertilizer in rice-rice-green manure rotation. Appl. Soil Ecol. 136, 11–20. https://doi.org/10.1016/j.apsoil.2018.12.015 (2019).
Zou, Y. et al. Effects of different land use patterns on nifH genetic diversity of soil nitrogen-fixing microbial communities in Leymus Chinensis steppe. Acta Ecol. Sin. 31, 150–156 (2011).
Zahran, H. H. Rhizobium-Legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. R 63, 968–989. https://doi.org/10.1016/j.chnaes.2011.03.004 (1999).
Tang, Y. et al. Impact of fertilization regimes on diazotroph community compositions and N2-fixation activity in paddy soil. Agriculture, Ecosystems & Environment: An International Journal for Scientific Research on the Relationship of Agriculture and Food Production to the Biosphere (2017).
Gao, P., Li, Y., Tan, L., Guo, F. & Ma, T. Composition of bacterial and archaeal communities in an alkali-surfactant-polyacrylamide-flooded oil reservoir and the responses of microcosms to nutrients. Front. Microbiol. 10, 2197. https://doi.org/10.3389/fmicb.2019.02197 (2019).
Rösch, C., Mergel, A. & Bothe, H. Biodiversity of denitrifying and dinitrogen-fixing bacteria in an acid forest soil. Appl. Enviro. Microbiol. 68, 3818–3829. https://doi.org/10.1128/AEM.68.8.3818-3829.2002 (2002).
Wei, G. et al. Similar drivers but different effects lead to distinct ecological patterns of soil bacterial and archaeal communities. Soil Biol. Biochem. 144, 107759. https://doi.org/10.1016/j.soilbio.2020.107759 (2020).
Sun, R., Guo, X., Wang, D. & Chu, H. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. 95, 171–178. https://doi.org/10.1016/j.apsoil.2015.06.010 (2015).
Asnicar, F., Weingart, G., Tickle, T. L., Huttenhower, C. & Segata, N. Compact graphical representation of phylogenetic data and metadata with GraPhlAn. PeerJ 3, 1029. https://doi.org/10.7717/peerj.1029 (2015).
Gao, P. et al. Spatial isolation and environmental factors drive distinct bacterial and archaeal communities in different types of petroleum reservoirs in China. Sci. Rep. 6, 20174. https://doi.org/10.1038/srep20174 (2016).
Acknowledgements
This work was funded by the National Natural Science Foundation of China for Young Scholars [No. 41807053] the National Natural Science Foundation of China [No. 31672255], the Agricultural Science and Technology Innovation Program of CAAS (No. CAAS-ZDRW202009), and the Special Fund for Establishment of Modern Agricultural R&D System, Ministry of Finance and Ministry of Agriculture, China (CARS-04). We wish to thank Dr. Wei-Qun Li and Dr. Baoku Zhou of the Heilongjiang Academy of Agricultural Sciences for their fieldwork assistance.
Author information
Authors and Affiliations
Contributions
J.Z., M.M., and J.L. conceived the experiments, D.G., X.J., N.Z., and F.S. conceived and conducted the experiments, Y.K. analysed the results, and Z.J. wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, J., Ma, M., Guan, D. et al. Nitrogen has a greater influence than phosphorus on the diazotrophic community in two successive crop seasons in Northeast China. Sci Rep 11, 6303 (2021). https://doi.org/10.1038/s41598-021-85829-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85829-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.