Abstract
Underwater Television (UWTV) surveys provide fishery-independent stock size estimations of the Norway lobster (Nephrops norvegicus), based directly on burrow counting using the survey assumption of “one animal = one burrow”. However, stock size may be uncertain depending on true rates of burrow occupation. For the first time, 3055 video transects carried out in several Functional Units (FUs) around Ireland were used to investigate this uncertainty. This paper deals with the discrimination of burrow emergence and door-keeping diel behaviour in Nephrops norvegicus, which is one of the most commercially important fisheries in Europe. Comparisons of burrow densities with densities of visible animals engaged in door-keeping (i.e. animals waiting at the tunnel entrance) behaviour and animals in full emergence, were analysed at time windows of expected maximum population emergence. Timing of maximum emergence was determined using wave-form analysis and GAM modelling. The results showed an average level of 1 visible Nephrops individual per 10 burrow systems, depending on sampling time and depth. This calls into question the current burrow occupancy assumption which may not hold true in all FUs. This is discussed in relation to limitations of sampling methodologies and new autonomous robotic technological solutions for monitoring.
Similar content being viewed by others
Introduction
The Norway lobster, Nephrops norvegicus (L.), is one of the most commercially important fisheries in Ireland and also Europe1. The 2019 EU Total Allowable Catch (TAC) for Nephrops2 for the north east Atlantic Functional Units (FU) was close to 44,000 tonnes, and valued at approximately 360 million EUR in 20163. Traditional fishery-dependent sampling methods such as commercial trawling provide indirect biomass estimates of exploited stocks, by means of abundance indices derived from surface density data (i.e., the number of animals per haul-swept area4,5,6).
However, animals construct and inhabit burrow systems used for shelter and for territorial control7 and are not available for trawl capture when hiding in the substrate8,9. The burrow emergence rhythmicity of populations causes marked fluctuations in catch rates over the 24-h10. Peaks in trawl Catch Per Unit Effort (CPUE) shift in timing with increasing fishing depth11,12,13: from full night to dusk- dawn transitions, going from upper to middle-lower shelves, to be finally fully diurnal (i.e. at midday) on upper and middle slopes. This indicates that the species sets its timing of burrow emergence upon a maximum illumination threshold that varies on the depth axis, based on the differential penetration of light as the sun progresses through its diurnal trajectory10,14.
The diel rhythm of burrow emergence is more complex than previously thought and it can be subdivided in three different phases11,15 : Full emergence, full retraction and an intermediate period in which individuals wait at the burrow entrance (i.e. door-keeping16). To date, the proportion of animals not emerging from their burrows on a daily basis is still largely undetermined, although acoustic tagging of individuals of a philetically closely related species has offered some insight17. In addition to environmental light, other ecological reasons seem to modulate the predisposition of individuals toward emergence or retraction. For example, crustaceans are at intermediate levels of the marine food webs and their feeding activity (coinciding with burrow emergence in the case of Nephrops) is the product of a mortality risk ratio between hunger state and chances to meet visual predators18,19.
Alternative fishery-independent assessment methods as Underwater Television (UWTV) surveys using towed camera-sledges, have been developed to estimate stock abundance20,21. Those video-based surveys are carried out in several European countries and are coordinated through the International Council for the Exploitation of the Sea Working Group on Nephrops Surveys (WGNEPS)1,22,23. This more direct (i.e. image-based) method of assessment counts burrow systems, based on their characteristic structural features (i.e. large crater-like entrances4,24,25 and their characteristic arrangement individual burrow entrances20. Those systems are composed of multiple entrances, shafts and tunnels and can be readily identified by classic features and orientation of the individual burrow entrances, where the apexes of those entrances facing each other in a simple U-shaped system or converging on one central point in a more developed system (i.e. T-shape)26,27. This method is independent from the time of the day and season. The burrow system counts can be used as a relative or absolute index for determination of Nephrops’ stock status and together with catch data can provide a Harvest Rate (HR; catch in numbers/burrow numbers)28, 29.
To use UWTV burrow abundance to calculate catch and landings derived from an acceptable Harvest Rate (HR = catch in numbers/UWTV abundance) it is necessary to adjust using agreed correction factor which takes into account the bias associated with UWTV surveys. The key bias contributions for individual Nephrops Functional Unit (FU) have been documented and the cumulative correction factor considers edge effect, burrow identification, burrow detection and burrow occupancy28,29.
Given the strong territorial behavior of the species, burrow counting seems to be a good proxy for local population abundance, assuming the condition “one burrow system, one animal”30, which is the current assumption for Nephrops stock assessment28,29. The burrow system acts as the center of a strong territorial rhythmic behavior7,31,32 and two adult lobsters are rarely found in the same shelter33. No spatial segregation occurs between juveniles and adults34 and the majority of juveniles build adult-juvenile burrow complexes, which become separated as juveniles grow, and each individual develops its own section35. However, burrows systems could also be inhabited by other benthic fish and crustacean species or may remain empty and intact for an unknown period of time after the animals’ death (e.g. due to fishing or natural mortality30,36). These factors still pose uncertainties about the true numbers of animals occupying video-counted burrow systems, representing a problem when using UWTV data in stock assessment models30.
To improve knowledge of the stock assessment assumption “one burrow system, one animal” a precise temporal description of burrow emergence rhythmicity could be provided by temporally distributed transects within UWTV surveys, similar to that explored with trawl data11. The sum of animals engaged in both behaviours could then be compared with counted burrows at phases of maximum population emergence. Unfortunately, temporally scheduled UWTV operations have never been systematically performed and the analysis of rhythmic fluctuations in video-observed animals performing full emergence and door-keeping is not yet available.
Here, we used UWTV survey data reporting densities of full emergence and door-keeping animals and burrow systems from more than three thousand video transects, conducted in the past decade around Ireland, to temporally define both behaviours and their reciprocal relationship over the 24-h. Results on estimated densities of animals engaged in full emergence and door-keeping were then compared with burrow system density estimates, to provide a comparison to the stock assessment assumption “1 burrow system, one animal”.
Materials and methods
The study areas and the UWTV surveys methodology
Video footage and derived data were collected from 3055 UWTV transects conducted from 2002 to 2013 in FU areas in the seas around Ireland (Fig. 1). Footage from each transect for all survey areas had a minimum recorded duration of 10 min. The counted minutes of each transect was in line with the prevailing international counting procedure; in years 2002 to 2008 10 min were counted, and in years 2008 to 2013 7 min were counted28,29. For FU 16, 10 min were counted for all years, due to the lower densities observed and the relative scale of variation between minutes was higher than typically found in other areas. All considered data were collected in spring–summer surveys (from May to September) (Table 1), in order to avoid variations in the number of video-counted animals and based on reproductive and moulting cycles (see next section).
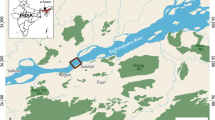
The UWTV survey areas around the Irish coast. Numbers represent the ICES Nephrops Functional Units (FUs), as defined by the ICES Nephrops Working Group. Depth (m) for the study areas (GEBCO bathymetry data). Map created using: R version 3.61 (2019–07-05) software, https://tinyurl.com/yy6rzlut.
Sampling design followed either a randomised isometric grid with a station spacing dependent on the individual survey area or a random stratified design37 (Table 1). The initial ground perimeter was established by using a combination of integrated logbook-VMS38 and habitat data (see methods described in Ligas et al.39). The final perimeter has been established using an adaptive approach where stations were located beyond the previously known perimeter of the ground, until the burrow system densities were zero or very close to zero. Once established, the survey area was not changed between years.
At each station, the UWTV sledge was deployed and once stable on the seabed, a 10 min’ tow was recorded onto DVD. The field of view of the camera (Kongsberg OE14-366) at the bottom of the screen with the sledge flat on the seabed (i.e. no sinking), was validated at 75 cm by two parallel spot lasers. Vessel position (by Differential Global Positioning System-DGPS) and sledge position (using an on-board Ultra Short Baseline-USBL, transponder) were recorded every 2 to 5 s. USBL navigational data were used to calculate the video transect distance over the ground, as required for surface density estimates (see next section). The navigational data were quality controlled using an “R” script developed by the Marine Institute28,29.
Data processing
The same footage was viewed and counted by two scientists independently of each other and burrows were identified based on key structural features from an established set of classification keys20,25,40. All scientists were trained prior to counting following the ICES recommendations28,29, in such a way the counters can be quite consistent in the recognition of a N. norvegicus burrow as compared to other species. Final burrow densities were based on an average from the two independent counts after passing quality control processes such as screening for outliers and use of Lin’s concordance CCC to evaluate counter performance20. The quality assured burrow density values were then corrected for stock specific survey cumulative bias as described in ICES29.
Video-counts of door-keeping animals, defined as those with partial cephalothorax or claws were visible across the burrow mouth entrance, and animals in full emergence which were entirely visible were available10,15. As with burrow systems, densities of animals in door-keeping and full emergence were obtained for each transect by dividing respective counts by the video-swept surface.
Each density estimate for burrow systems and animals in both behavioural categories was associated to a time stamp, represented by the time at mid transect length. All density data were grouped per depth ranges within the upper and lower shelf, based on the previous knowledge from trawl catch patterns11,41,42 nominally as: 15–50, 51–100, 101–160 and 340–570 m. No data were available for 161–339 m depth. Data for the bathymetric range 340–570 m were only available in FU 16 for the years 2012 and 2013, and this inclusion was necessary to characterize behavioural rhythms in the deepest range for comparison with shallow shelf observations (as previously done with trawling11).
The rational for those depth groupings was that Nephrops burrow emergence is an adaptive life trait under strong selection which can be temporally described as different on upper and lower shelves as well as slopes11. Moreover, the burrow emergence rhythm manifests itself similarly in all its geographic range, with coincident nocturnal, crepuscular or diurnal timings according to the depth light driven peaks not blurred by the tidal status10,43. This behaviour is constant through years, subjected only to a seasonal reproduction and growth pattern (e.g. berried females do not emerge15,44,45), the effects of which were eliminated here by selecting only summer data (see Table 1).
Statistical analysis
Firstly, we ran a waveform analysis to describe averaged full emergence and door-keeping behavioural rhythms over the 24-h within the established depth ranges (see above). Waveforms plots describe the phase of a rhythm as an averaged peak into a time series of density data for both behavioural categories. Waveform computing procedure was as follows46. A standard day was divided into 1-h intervals and all density estimates for animals in full emergence and door-keeping were pooled together from all the surveys within the same depth range and then averaged at corresponding 1-h timing.
The resulting set of averaged density estimates were then represented over the 24-h with their standard deviations, plus the Midline Estimated Statistic of a Rhythm (MESOR47). MESOR is a re-average of all waveform values to be represented onto waveform plots as a horizontal threshold line. All waveform values above the threshold delimit the duration of the peak (i.e. activity peak duration46). Waveforms for full emergence and door-keeping density estimates were plotted together to highlight their temporal relationship.
Then, we fitted Generalised Additive Models (GAM) onto full emergence and door-keeping data for spring–summer at the established depth ranges (see above), to achieve statistic formalization of observed emergence patterns beyond data variability (Appendix 1). The package ‘mgcv’48 in R49 was used with the restricted maximum log-likelihood approach. The effects of the inter-annual variability and the variability among FUs were assessed in the models. The Hour of the Day (HD), from zero to 23 h, was the covariate used to characterize behavioural rhythms. The day-length and the average location of the transects (latitude and longitude) were adopted as spatiotemporal covariates in the full model, following the form:
where E(NEP) is the Expected value of Nephrops full emergence or door-keeping, conditionally distributed according to the Gamma distribution family. g is the log link function, \({\beta }_{0}\) is the intercept. s is the smoothing function with the term bs = "cc" specifying the 24 h’ knot based (k = 24) cyclic cubic regression spline. The day-length was estimated as the difference between the sunrise and sunset times. te is the tensor smooth function for the interaction among transect locations (i.e. latitude and longitude) accounting for spatial dependence on diel activity rhythms affecting NEP. Alternatively to the te(Lat, Lon) effect, the potential effect of the station locations per FU, te(Lat, Lon, by = FU), and the interaction between the station locations with the year survey ti(Lat, Lon, year, d = c(2,1), were also tested in the models. The ti tensor product spline tested the significance of the space-year interaction effect. The 2-dimensional space and the 1-dimensional year factor were specified with the argument d = c(2,1).
The models showing significant HD term (i.e. behavioural rhythm), and other significant covariates substantially improving the model variance were selected as the final models (see Appendix 1). The different models were fitted and compared using the percentage of explained deviance and the Akaike Information Criterion (AIC) to select the best one50. The range of AIC values of models within depth ranges was generally narrow and not assumed to be critical for the final model choice. The selected final model followed the form (see Appendix 1):
where f(Cov) represented the term te(Lat, Lon) for the emergence and door keeping behaviours in the upper depth range (15–50 m). In the case of the emergence behaviour at the depth ranges between 51 and 160 m and door keeping at 101-160 m, f(Cov) represented the term s(Daylength). Because of the indistinguishable effect of the terms te(Lat, Lon) or s(Daylength), on the NEP behavioural pattern (see Appendix 1), here we show results and focus on the behaviour produced by the HD term of NEP.
We averaged over periods of 1-h, and this probably resulted in models with relatively less variable behaviour during the day, even if the general distribution of Nephrops in full emergence and door-keeping did not show appreciable changes. The depth range models allowed identifying peaks timing and duration of full emergence and door-keeping behaviours from the fitted values above the mean.
Finally, in order to identify the temporally optimum moment to count the highest number of individuals (i.e. those in full emergence plus those in door-keeping) in relation to burrow counting, hence obtaining a best estimate of burrow occupancy, a temporally integrated chart of all waveform and GAM results phases48,49 was created. Peaks were represented together as continuous horizontal lines and plotted in order to achieve an overall perspective of their temporal relationship51,52.
Results
Waveform analysis (Fig. 2) indicated that Nephrops full emergence varied from nocturnal toward midday hours with increasing depth of sampling. This pattern is particularly evident, when comparing the two extremes of the sampling depth range: upper shelf (15–50 m depth) with two peaks (hour intervals: 2 to 9 and 18 to 0) versus middle slope (340–570 m depth) with single peak (hour interval: 9 to 17). At intermediate sampling depths of the lower shelf (51–100 m depth) and shelf-break (101–160 m depth), a less clear crepuscular (dusk and dawn oriented) pattern was reported, with less distinct peaks merging toward daytime. In contrast, door-keeping behaviour had some defined pattern with crepuscular peaks coinciding with full emergence only on the upper shelf (15–50 m depth) and the shelf-break (101–160 m depth). No defined rhythms were discernible at the other depth zones.
Waveform analysis results depicting the change in burrow emergence behaviour upon depth in terms of full emergence (black) and door-keeping (red). MESORs are the threshold horizontal dashed lines (respective values are also reported with corresponding colour), which identify peak temporal limits (i.e. values above it; coloured vertical arrows). The peak duration is an indication of global averaged activity for that behavioural component in the population. Separate peaks were identified if 2 or more consecutive points were below the MESOR.
The statistical model results by GAM (Fig. 3), revealed an overall pattern of full emergence and door-keeping behaviour similar to that found from the waveform analysis (Fig. 2). In the upper continental shelf (15–50 m depth), the model shows a nocturnal bimodal (i.e. two peaks) emergence pattern (hour intervals: 2 to 7 and 17 to 23). On deeper shelf areas (from 51 to 160 m depth), the emergence pattern becomes diurnal with a plateau shape (i.e. no major crepuscular peaks). Finally, in the upper slope (340–570 m depth) the emergence shows a single peak (hour interval: 7 to 17). Consistent with the waveform analysis (see Fig. 2), door-keeping, showed a less clear temporal pattern (Fig. 3). As with emergence, door-keeping was nocturnal with weak crepuscular increases at 15–50 m depth range (hour intervals: 0–7 and 15–0). The temporal pattern was lost between 51 and 100 m to be regained with a crepuscular aspect on the shelf break (hour intervals: 4–7 and 15–21), becoming again completely arrhythmic on the upper slope.
Significant (p < 0.05) GAM modeled temporal patterns for full emergence (grey) and door-keeping (red) behaviors by depth ranges over the 24-h cycle. Shadowed areas represent 95% confidence intervals of modelled patterns. Horizontal black dashed lines are the zero-mean values, taken as a reference to estimate representative time ranges of full emergence and door-keeping activity peaks (i.e. values above the mean). Door-keeping model fits at 51–100 and 340–570 m depth ranges were not significant (p > 0.05).
The temporal comparison between the waveform analysis (see Fig. 2) and GAM model outputs (see Fig. 3) in peak timings and duration per depth stratum is presented in Fig. 4. In the upper shelf (i.e. 15–50 depth), GAM modelling indicated a slightly shorter timing of nocturnal emergence. At intermediate and lower shelf (from 51 to 160 m depth), the waveform and GAM analysis shows a slight drop of emergence at noon. On the slope (340–570 m depth), the midday timing for emergence indicated for waveform analysis (hour interval: 9–17) was modelled by GAM as taking place for a longer duration (hour interval: 7–17).
Integrated chart showing temporal relationships among peaks timings, as derived from plots of waveform and GAM analyses (i.e. phases as values above horizontal threshold lines; see Figs. 2 and 3). According to GAMs, the best timings of UWTV surveying where highest (maximum) number of animals can be observed, are indicated with the letter M and grouped by blue rectangles.
The same comparison for door-keeping behaviour (see Fig. 4) showed a nocturnal rhythmicity at depths 15–50 m with both waveform analysis and GAM, with a duration slightly larger for the latter. Although no significant temporal pattern was detected by the GAM modelling from 51 to 100 m depth and from 340 to 570 m depth, on the shelf-break some weak crepuscular temporization was detected by the two analysis approaches.
Independently of the survey time, the maximum densities of emergence and door-keeping were detected in the 51–100 m depth layer (means of 0.058 and 0.020 Ind./m2, respectively, in the FU 15, West Irish Sea), coinciding with the maximum number of burrows per area (0.908 burrows/m2) (Table 2). This corresponds to a visible occupancy of 0.086 individuals per burrow.
The timing of the maximum number of animals in full emergence varied between depth strata (Fig. 4, Table 3). The mean densities of animals ranged from 0.024 and 0.061 Ind./m2 over the continental shelf (from 15 to 160 m depth), and one order of magnitude lower on the slope (340–570 m depth) with densities between 0.0064 and 0.009 Ind./m2.
Focussing on mean density values for all visible animals (combined totals of both emergence and door-keeping behaviours) at peak timing as a proxy of total population densities (Fig. 4), the following observation can be made: the animal density increases from 0,034 to 0,075 Ind./m2 over the continental shelf, and from 0,012 to 0,013 Ind./m2 over the slope. The fraction of door-keeping animals (i.e. from the total emergence and door-keeping animals) is slightly lower on the continental shelf than on the slope (18–41% and 32–54%, respectively) (Table 3). The number of total animals visible per burrow system ranged from 0,059 to 0,119 Ind./m2 across the shelf and the slope.
Discussion
The present work describes for the first time the diel behavioural rhythms of Nephrops in terms of burrow emergence and door-keeping, based on observations in more than three thousand UWTV transects. Populations emergence patterns varied their timing from the shelf to the slope with a timing shift, which is consistent with previous observations based on trawl catch temporal rates (i.e. in capture peaks from nocturnal to crepuscular and then to fully diurnal hours as the depth increases11). In contrast, the description of the temporal variation in door-keeping behaviour is an entirely new finding for Nephrops, since individuals at the entrance of their tunnel systems are unlikely to be catchable in trawling operations53. Here, we provide evidence of arrhythmic fluctuations in counts of animals expressing this behaviour, with relevant counts sparse over the whole 24-h cycle. This points out that the arrhythmia of observations of door-keeping animals could be due to: a behaviour which is in fact arrhythmic in some individual, or that animals retract into the burrows because they perceive approaching sleds. An unknown part of the population may therefore avoid haul capture by a quick withdrawal of individuals into their burrows when trawls approach54. Consequently, the number of counted “door-keeping animals” could be dependent upon sledge towing speed (i.e. animals reacting to the approach of the sledge and retreating25), as well as the presence of the sledge light system and overall noise. In any case, for trawl gear to efficiently catch Norway lobsters, they have to be fully outside of the burrows.
Burrow emergence rhythms and the UWTV-based stock assessment assumptions
Estimated densities of visible animals engaged in both emergence and door-keeping behaviours were compared with burrow system counts and derived density estimates, to provide evidence putative biases to the standard stock assessment assumption that “1 burrow system is occupied and maintained by one animal”20. Present results suggest a visible individuals’ ratio of around 1 visible Nephrops to 10 counted burrows. This result is a general estimate considering all the FUs, with results closer to the 1:1 assumption in some areas (i.e. FU 15). Taken together, our results indicate that there may well be variations in the burrow occupancy across different FUs. In fact, this difference appears to actually be related to the latitude/longitude position rather than the FU as the GAM showed FU to be irrelevant, while sample location (or day length) was influential in the model (Appendix 1). Video-derived animal densities during the consecutive hours were variable and the minimum estimates of stock densities should be derived focusing on the hours at which maximum densities are visible at the surface. Temporal windows at which we reported maximum emergence (plus door-keeping) densities of Nephrops can be considered good time periods to compare animals and burrow systems numbers together.
This burrow occupancy assumption has been identified a major uncertainty in UWTV bases assessment approach, particularly when using the UWTV index as an absolute measure of stock abundance20. Field observations indicate a more complex behavioural situation where single individuals can inhabit a single or complex burrow system with a variable number of entrances depending on local population densities30. At the same time, laboratory studies on aggressive hierarchy show that dominant individuals attempt to evict the subordinates to conquer their burrows nearby9. Even in periods of peak emergence it is possible that not all individuals are visible at the surface. Aguzzi et al.18 suggested that the predisposition of animals toward burrow emergence depends on the hunger state (and the presence of carrion and prey) and the absence of potential predators or sympatric competitors. Furthermore, laboratory tests on large numbers of individuals indicate that shelf animals may exhibit a differently phased dusk or dawn emergence, possibly to reduce interspecific competing pressure13. This matches present results where the temporal patterns in emergence observed in Nephrops UWTV transects generally follow those described by trawl catches on the shelf. The environmental factors such as the lunar cycle, tides and bottom currents, whose strength could vary according to the different local topography in different FUs, could also impact on burrow emergence16. Depending upon the future spatiotemporal availability of environmental data (to date missing) new variables could also be modelled, to improve the model correction approach.
It is possible that the number of burrow systems are over estimated, for a variety of reasons. Despite the training systems in place to ensure consistency in Nephrops burrow identification, the accuracy of burrow identification may vary across FUs. In some areas sympatric fish and other decapod species occupy or even construct burrows with morphology similar to those of Nephrops30. It is also possible, in environmentally stable lightly trawled grounds, that unoccupied burrows may persist, and appear to be active (clearly inactive systems are not counted). However, most of the grounds in our study are heavily trawled with swept area ratios > 528,29. The number of burrow entrances per counted burrow system may also be variable in different habitat types. It is unlikely that all these factors can fully account for the discrepancy in the animals to burrow count ratios observed here between areas.
Considering our results and the previously accounted sources of uncertainty for the UWTV-based stock assessment equation, our estimation of the general value of “1 Ind./10 burrow correction factor” does call into question the use of UWTV surveys as an absolute index on Nephrops abundance. A visible Nephrops index does provide a minimum population estimate of those emerging on a diel basis, but may not account for those concealed. The Irish Sea (FU 15) is a very dynamic system with strong bottom currents and highly populated sea bed28,29 so the 1:1 assumption could likely hold in that area. At the same time, this assumption could be quite different in FU 20–21, which is less fished and has lower densities of burrow systems. The HR for FU 15 has for long periods been around 20% and that observation clearly invalidates any possibility that our ratio of 0.1 Ind./burrow can be close to the true value (since the local fishery would be catching twice the number of animals/burrows annually). Still that doesn’t mean that the ratio 1:1 is true for all other FUs.
Trawling is a traditional sampling approach for the scientific monitoring of demersal resources but it does not provide data on the behaviour of the target species nor how such a behaviour can influence catchability51. UWTV surveying has distinct advantages over trawling, being more ecologically sensitive, causing minimal physical damage to seabed habitats and allowing better behavioural characterization. However, considerable work remains to be done in order test the key assumptions used in the assessments based on UWTV survey programmes.
The methodological constrains of our study based on input data typology
We chose to group the data for depth ranges and across years rather than keeping at a higher level of granularity, since Nephrops behavior is usually highly variable55. In fact, the fitted GAMs suggested that there are no significant effects of the survey year (i.e. inter-annual variability) on the emergence or door keeping patterns. The models also suggested that the variability across FUs is not relevant (i.e. significant) in explaining the behavioral patterns.
Additional data sub-grouping based on light data (not available) could have been performed. However, the estimation of any environmental illumination index based on transect timing, geographic position and depth would result in a mere modelling exercise. Factors such as cloud cover, water column primary productivity and turbidity have a significant impact on light scattering and absorbance (i.e. extinction) coefficients56,57, and are unavailable at the high spatiotemporal frequency of UWTV transects for all the FU areas considered.
Moreover, Nephrops rhythmicity is part of burrowing behavioral life-styles under natural selection in crustacean decapods, which was shown to be expressed independently from contingent variations in background light intensity58. The species possesses a biological clock that would ensure a temporally averaged burrow emergence pattern. The biological clock activates the locomotor activity (at the base of burrow emergence), every 12-h or 24-h, depending from the shelf or slope depth stratum considered13,59. This rhythmicity is self-sustained since it keeps its period and phase based on an environmental memory of previously experienced environmental light conditions when animals are transferred to laboratory constant conditions (i.e. entrainment upon intensity and photophase duration)47,60.
Another source of data variation may be the underlying dynamics of the populations due to recruitment variations, fishing and natural mortality39. In the case of the Mediterranean Nephrops stocks, fishery overexploitation is not reducing the number of captured animals but the biomass (i.e. animals are getting smaller)61. To date, there is no evidence of a similar finding for the Irish Sea. The local stocks are not experiencing declines due to excessive fishing mortality (e.g. FU 15, has continuously yielded ~ 10,000 tonnes of catch for nearly 60 years)62. It is feasible that a behavioral mechanism modulating emergence is preserving the populations from the fishery exploitation (see all considerations above).
Toward a more technologically sustained fishery-independent stock assessment
Towing the UWTV sledge could bias counts of emerged individuals causing them to flee outside the field of view and cause door-keepers to retract inside their burrows53. To improve stock assessments, more intensive data collection efforts are needed to collect data for improved models. Data collection may include optoacoustic by multi-beam cameras that should be used in combination with High-Density (HD) imaging from Autonomous Underwater Vehicles (AUVs)63 and Internet Operated Vehicles (IOVs) such as crawlers64. A complete photomosaic of the targeted parcel describing burrow systems and their reciprocal positioning, should be undertaken as a first step. Only after that, hourly scheduled AUV and IOV’ acoustic sweeps should be continuously performed during consecutive day-night cycles, replicated in different seasons, to picture emerging and door-keeping Nephrops and associated predator and prey species under silent and non-light conditions.
In addition, different stand-alone or cabled observatories, holding several seabed and water column sensors for environmental monitoring (e.g. the OBSEA or SmartBay; respectively, https://www.obsea.es and https://www.smartbay.ie/), could be used to picture burrow emergence modulation (for an insight on monitoring network geometry and characteristics see65,66). The different observational points could be synchronously used to account for the control of oceanographic and ecological drivers on the burrowing behavior of the species67. With such a multidisciplinary demographic, behavioral and environmental approach one may finally derive more accurate stock assessment models, predicting the density of animals that could be sampled with different fishery-dependent and independent tools67.
Conclusions
Our results highlight that Nephrops is highly cryptic and has fascinating behavioural patterns that affect its availability to visual as well as capture-based surveys. The temporal treatment of UWTV video data within the chosen depth ranges showed the behavioural pattern of burrow emergence is predominantly dusk and dawn-oriented above 50 m, bimodal and tending to be diurnal between 50 and 100 m, temporally diffused between 101 and 160 m, and finally fully diurnal between 340 and 570 m, partially matching depth-dependent patterns in trawl catch rates. The door-keeping behaviour is only temporally defined above 50 m (being nocturnal) and bimodal with a nocturnal increase between 100 and 160 m. During the hours of maximum peak abundance of visible individuals (summing up the video-counted individuals in emergence and door-keeping behaviours), we have observed that on average there is about 1 visible individual per 10 burrows, at most. This represents the average peak, although there were higher peaks within individual transects. In general, considering all areas together, our ratio is well below that assumed in current stock assessments (i.e. “1 burrow system:1 animal”), suggesting that a high proportion of the population remains cryptic even during periods of peak emergence. This bias should be carefully considered since an undefined number of animals may avoid the sledge at its approach. Further technological development toward optoacoustic technologies and additional effort for calibration and modelling to integrate observations of visible individuals may further improve the utility of UWTV surveys for stock assessment. Four lines for technological based calibration should be foreseen in future stocks monitoring actions: burrow identification, as other sympatric fish and decapod species occupy or even construct burrows with morphology similar to those of Nephrops; intraspecific aggressive relationships and hierarchy, where dominant individuals may occupy different burrow systems nearby; emergence enhancement and inhibition depending on hunger state, due to predators’ presence and the quantity of emerging conspecifics at the “rush hour timing”; and finally burrow persistence after an animal’s death, depending on the density of burrow-dwelling species, local hydrographic and fishing pressure conditions.
References
ICES. Final report of the Working Group on Nephrops Surveys (WGNEPS), 2–3 November 2017. (2017).
EU. Council Regulation (EU) 2019/124 of 30 January 2019 fixing for 2019 the fishing opportunities for certain fish stocks and groups of fish stocks, applicable in Union waters and, for Union fishing vessels, in certain non-Union waters. L29, 1–166 (2019).
EUROSTAT. The collection and compilation of fish catch and landing statistics in member coutries of the European economic area. (2020).
Aguzzi, J., Bozzano, A. & Sardà, F. First observations on Nephrops norvegicus (L.) burrow densites on the continental shelf off the Catalan coast (western Mediterranean). Crustaceana 77, 299–310 (2004).
Maynou, F. X., Sardà, F. & Conan, G. Y. Assessment of the spatial structure and biomass evaluation of Nephrops norvegicus (L.) populations in the northwestern Mediterranean by geostatistics. ICES J. Mar. Sci. 55, 102–120 (1998).
Sala, A. Influence of tow duration on catch performance of trawl survey in the Mediterranean Sea. PLoS ONE 13, (2018).
Farmer, A. S. D. Synopsis of the biological data on the Norway lobster Nephrops norvegicus (Linnaeus, 1758). FAO Fish. Synopsis 112, 1–97 (1975).
Atkinson, R. J. A. & Eastman, L. B. Burrow dwelling in Crustacea. Nat. Hist. Crustac. 2, 78–117 (2015).
Sbragaglia, V. et al. Fighting over burrows: the emergence of dominance hierarchies in the Norway lobster (Nephrops norvegicus ). J. Exp. Biol. 220, 4624–4633 (2017).
Aguzzi, J. & Sardà, F. A history of recent advancements on Nephrops norvegicus behavioral and physiological rhythms. Rev. Fish Biol. Fish. 18, 235–248 (2008).
Aguzzi, J., Sardà, F., Abelló, P., Company, J. B. & Rotllant, G. Diel and seasonal patterns of Nephrops norvegicus (Decapoda: Nephropidae) catchability in the western Mediterranean. Mar. Ecol. Prog. Ser. 258, 201–211 (2003).
Bell, M. C., Redant, F. & Tuck, I. Nephrops species. In Lobsters: Biology (ed. Phillips, B.) 412–461 (Oxford Blackwell Publishing, 2006).
Sbragaglia, V. et al. Dusk but not dawn burrow emergence rhythms of Nephrops norvegicus (Crustacea: Decapoda). Sci. Mar. 77, 641–647 (2014).
Chapman, C. J., Johnstone, A. D. F. & Rice, A. L. The Behaviour and Ecology of the Norway Lobster, _Nephrops norvegicus_ (L). Barnes H Proc. 9th Eur. Mar. Biol. Symp. Aberdeen Univ. Press. Aberdeen 59–74 (1975).
Aguzzi, J., Company, J. B. & Sardà, F. The activity rhythm of berried and unberried females of Nephrops norvegicus (Decapoda, Nephropidae). Crustaceana 80, 1121–1134 (2007).
Sbragaglia, V., García, J. A., Chiesa, J. J. & Aguzzi, J. Effect of simulated tidal currents on the burrow emergence rhythms of the Norway lobster (Nephrops norvegicus). Mar. Biol. 162, 2007–2016 (2015).
Tuck, I. D., Parsons, D. M., Hartill, B. W. & Chiswell, S. M. Scampi (Metanephrops challengeri) emergence patterns and catchability. ICES J. Mar. Sci. 72, i199–i210 (2015).
Aguzzi, J., Company, J. B. & Sardà, F. Feeding activity rhythm of Nephrops norvegicus of the western Mediterranean shelf and slope grounds. Mar. Biol. 144, 463–472 (2004).
Hemmi, J. M. Predator avoidance in fiddler crabs: 1. Escape decisions in relation to the risk of predation. Anim. Behav. 69, 603–614 (2005).
Leocadio, A., Weetman, A. & Wieland, K. Using UWTV surveys to assess and advise on Nephrops stocks. ICES Cooperative Research Report No. 340. 49 (2018).
Morello, E. B., Antolini, B., Gramitto, M. E., Atkinson, R. J. A. & Froglia, C. The fishery for Nephrops norvegicus (Linnaeus, 1758) in the central Adriatic Sea (Italy): preliminary observations comparing bottom trawl and baited creels. Fish. Res. 95, 325–331 (2009).
ICES. Report of the Working Group on Nephrops Surveys (WGNEPS) 6–8 November 2018. (2018).
ICES. Working Group on Nephrops Surveys (WGNEPS; outputs from 2019). ICES Scientific Reports. 2:16. https://doi.org/10.17895/ices.pub.5968 (2020).
Campbell, N., Dobby, H. & Bailey, N. Investigating and mitigating uncertainties in the assessment of Scottish Nephrops norvegicus populations using simulated underwater television data. ICES J. Mar. Sci. 66, 646–655 (2009).
Martinelli, M. et al. Towed underwater television towards the quantification of Norway lobster, squat lobsters and sea pens in the Adriatic Sea. Acta Adriat. 54, 3–12 (2013).
ICES. Report of the Workshop and training course on Nephrops Burrow Identification (WKNEPHBID). (2008).
ICES. Report on the Workshop on Nephrops Burrow Counting (WKNEPS) 9–11 November 2016. (2016).
ICES. Report of the Study Group on Nephrops (WKNEPH), 28 February–1 March 2009. (2009).
ICES. Report of the Benchmark Workshop on Nephrops (WKNEPH), 2–6 March 2009. (2009).
Sardà, F. & Aguzzi, J. A review of burrow counting as an alternative to other typical methods of assessment of Norway lobster populations. Rev. Fish Biol. Fish. 22, 409–422 (2012).
Rice, A. L. & Chapman, C. J. Observations on the burrows and burrowing behaviour of two mud-dwelling decapod crustaceans, Nephrops norvegicus and Goneplax rhomboides. Mar. Biol. Int. J. Life Ocean. Coast. Waters 10, 330–342 (1971).
Chapman, C. J. & Rice, A. L. Some direct observations on the ecology and behaviour of the Norway lobster Nephrops norvegicus. Mar. Biol. Int. J. Life Ocean. Coast. Waters 10, 321–329 (1971).
Cobb, J. S. & Wang, D. Fisheries biology of lobsters and crayfishes. Provenzano A.D. Biol. Crustac. 10, 167–247 (1985).
Maynou, F. & Sardà, F. Nephrops norvegicus population and morphometrical characteristics in relation to substrate heterogeneity. Fish. Res. 30, 139–149 (1997).
Tuck, I. D., Atkinson, R. J. A. & Chapman, C. J. The structure and seasonal variability in the spatial distribution of nephrops norvegicus burrows. Ophelia 40, (1994).
Tuck, I. D., Chapman, C. J. & Atkinson, R. J. A. Population biology of the Norway lobster, Nephrops norvegicus (L.) in the Firth of Clyde, Scotland - I: Growth and density. ICES J. Mar. Sci. 54, 125–135 (1997).
ICES. Report of the Study Group on Nephrops Surveys (SGNEPS), 6–8 March 2012. (2012).
Gerritsen, H. & Lordan, C. Integrating vessel monitoring systems (VMS) data with daily catch data from logbooks to explore the spatial distribution of catch and effort at high resolution. ICES J. Mar. Sci. 68, 245–252 (2011).
Ligas, A., Sartor, P. & Colloca, F. Trends in population dynamics and fishery of Parapenaeus longirostris and Nephrops norvegicus in the Tyrrhenian Sea (NW Mediterranean): the relative importance of fishery and environmental variables. Mar. Ecol. 32, 25–35 (2011).
Morello, E. B., Froglia, C. & Atkinson, R. J. A. Underwater television as a fishery-independent method for stock assessment of Norway lobster (Nephrops norvegicus) in the central Adriatic Sea (Italy). ICES J. Mar. Sci. 64, 1116–1123 (2007).
Atkinson, R. J. A. & Naylor, E. An endogenous activity rhythm and the rhythmicity of catches of Nephrops norvegicus (L). J. Exp. Mar. Bio. Ecol. 25, 95–108 (1976).
Hammond, R. D. & Naylor, E. Effects of dusk and dawn on locomotor activity rhythms in the Norway lobster Nephrops norvegicus. Mar. Biol. 39, 253–260 (1977).
Katoh, E., Sbragaglia, V., Aguzzi, J. & Breithaupt, T. Sensory biology and behaviour of Nephrops norvegicus. Adv. Mar. Biol. 64, 35–106 (2013).
Aguzzi, J., Allué, R. & Sardà, F. Characterisation of seasonal and diel variations in Nephrops norvegicus (Decapoda: Nephropidae) landings off the Catalan Coasts. Fish Res. 69, 293–300 (2004).
Powell, A. & Eriksson, S. P. Reproduction: life cycle, larvae and larviculture. In Advances in Marine Biology 64, 201–245 (Elsevier, 2013).
Refinetti, R. Circadian Physiology. Fr. Taylor, New York. https://doi.org/10.1201/b19527 (2006).
Chiesa, J. J., Aguzzi, J., García, J. A., Sardà, F. & De La Iglesia, H. O. Light intensity determines temporal niche switching of behavioral activity in deep-water Nephrops norvegicus (Crustacea: Decapoda). J. Biol. Rhythms 25, 277–287 (2010).
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 73, 3–36 (2011).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org (2020).
Wood, S. N., Pya, N. & Säfken, B. Smoothing parameter and model selection for general smooth models. J. Am. Stat. Assoc. 111, 1548–1563 (2016).
Aguzzi, J. & Company, J. B. Chronobiology of deep-water decapod crustaceans on continental margins. In Advances in Marine Biology 58, 155–225 (Elsevier, 2010).
Gibson, R. N., Atkinson, R. J. A. & Gordon, J. D. M. Challenges to the assessment of benthic populations and biodiversity as a result of rhythmic behaviour: video solutions from cabled observatories. Oceanogr. Mar. Biol. An Annu. Rev. 50, 233–284 (2012).
Catchpole, T. L. & Revill, A. S. Gear technology in Nephrops trawl fisheries. Rev. Fish Biol. Fish. 18, 17–31 (2008).
Main, J. & Sangster, G. I. The Behaviour of the Norway Lobster, Nephrops norvegicus (L.), During Trawling. Scottish Fish. Res. Rep. 34, 1–23 (1985).
Ungfors, A. et al. Nephrops fisheries in European waters. Adv. Mar. Biol. 64, 247–314 (2013).
Jerlov, N. G. Optical Oceanography 194 (Elsevier, 1968).
Herring, P. The biology of the deep ocean. J. Hered. 93, (2002).
Laidre, M. E. Evolutionary ecology of burrow construction. In The Natural History of the Crustacea: Life Histories (eds. Thiel, M. & Wellborn, G.) 5, 279–302 (Oxford University Press, 2018).
Trenkel, V. M., Rochet, M. & Mahevas, S. Interactions between fishing strategies of Nephrops trawlers in the Bay of Biscay and Norway lobster diel activity patterns. Fish. Manag. Ecol. 15, 11–18 (2008).
Aguzzi, J. et al. Monochromatic blue light entrains diel activity cycles in the Norway lobster, Nephrops norvegicus (L.) as measured by automated video-image analysis. Sci. Mar. 73, 773–783 (2009).
Colloca, F., Scarcella, G. & Libralato, S. Recent trends and impacts of fisheries exploitation on Mediterranean stocks and ecosystems. Front. Mar. Sci. 4, (2017).
Marine Institute. The Stock Book 2019: Annual Review of Fish Stocks in 2019 with Management Advice for 2020. Mar. Institute, Galway, Irel. (2019).
Aguzzi, J. et al. New high-tech flexible networks for the monitoring of deep-sea ecosystems. Environ. Sci. Technol. 53, 6616–6631 (2019).
Chatzievangelou, D., Aguzzi, J., Ogston, A., Suárez, A. & Thomsen, L. Visual monitoring of key deep-sea megafauna with an Internet operated crawler as a tool for ecological status assessment. Prog. Oceanogr. 102321 (2020).
Rountree, R. A. et al. Towards an optimal design for ecosystem-level ocean observatories. Front. Mar. Sci. 1–69 (2019).
Masmitja, I. et al. Mobile robotic platforms for the acoustic tracking of deep water demersal fishery resources. Sci. Robot. 5, eabc3701 (2020).
Aguzzi, et al. Fish-stock assessment using video imagery from worldwide cabled observatory networks. ICES J. Mar. Sci. 77, 2396–2410 (2020).
Acknowledgements
This work has been lead and carried out by members of the Tecnoterra associated unit of the Scientific Research Council through the Universitat Politècnica de Catalunya, the Jaume Almera Earth Sciences Institute and the Marine Science Institute (ICM-CSIC). This work received financial support from the Spanish Ministerio de Economía y Competitividad (Contract TEC2017-87861-R Project RESBIO, RTI2018-095112-B-I00 Project SASES and CTM2017-82991-C2-1-R Project RESNEP), from the Generalitat de Catalunya “Sistemas de Adquisición Remota de datos. This work acknowledges the ‘Severo Ochoa Centre of Excellence’ accreditation (CEX2019-000928-S). We also acknowledge the project “Norway lobster (Nephrops norvegicus) population dynamics from automated video-monitoring at SmartBay cabled underwater observatory (SmartLobster)”, funded by the Multidisciplinary Seafloor and water column Observations (EMSO)-LINK Trans National Access (TNA). The research leading to these results has been conceived under the International PhD Program “Innovative Technologies and Sustainable Use of Mediterranean Sea Fishery and Biological Resources (https://www.FishMed-PhD.org).
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the analysis and writing of this work. J.D. and C.L. provided the WGNEPS field data from the UWTV surveys.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aguzzi, J., Bahamon, N., Doyle, J. et al. Burrow emergence rhythms of Nephrops norvegicus by UWTV and surveying biases. Sci Rep 11, 5797 (2021). https://doi.org/10.1038/s41598-021-85240-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85240-3
This article is cited by
-
Burrow emergence rhythms of deep-water Mediterranean Norway lobsters (Nephrops norvegicus) revealed by acoustic telemetry
Reviews in Fish Biology and Fisheries (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.