Abstract
Territoriality is costly, and the accurate identification of intruders and the decision to perform aggressive responses are key behavioral traits in social animals. We studied aggression among individuals belonging to close and distant nests of the plant-ant Azteca muelleri, which lives in stems of the pioneer tree Cecropia glaziovii. More specifically, we aim to investigate if the DE (dear-enemy effect—less aggression towards neighbors than strangers) or NN (nasty-neighbor effect—less aggression to strangers than neighbors) effects or even none of them apply for this iconic Azteca-Cecropia system. We further checked if ant aggression towards conspecifics is related to cuticular hydrocarbon profiles (CHCs), which provide chemical cues for nestmate recognition. Therefore, we sampled 46 nests of A. muelleri in three Brazilian Atlantic forest fragments and performed behavioral trials within and between sites. Consistently with the DE effect, we found higher aggression levels in ‘between sites’ versus ‘within sites’ treatments as well as a positive effect of spatial distance on ant aggressiveness. We found no effect of the overall dissimilarities on CHC blend on ant aggressiveness, but of one CHC class, the methylated alkanes. Overall, we provide key insights on nest-mate recognition in obligatory ant-plant mutualisms.
Similar content being viewed by others
Introduction
Territorial defense behavior is a remarkable feature of animal societies1,2. Territoriality is costly since it implies the use of defensive forces. The accurate identification of intruders and the decision to perform aggressive responses are key behavioral traits in social animals3. The aggression levels to intruders have received substantial research attention, and generally, two opposite outcomes are expected4. Firstly, when distant invaders are potentially more dangerous than closer ones regarding resource-threatening, territorial animals will respond less aggressively to neighbors than strangers (named as “dear enemy” effect—DE)5,6. Distant invaders can be more threatening when: (1) their colonies boundaries are not well-know, (2) their intrusions are less predictable in space and time, and (3) when they are looking for new territories7. On the other hand, if resource competition is stronger between closer conspecifics, higher aggressions should be displayed to neighbors than strangers (known as “nasty neighbor” effect—NN)8. The NN effect would be more likely when transient strangers are smaller or when resource levels are fluctuating, encouraging usurpation of available resources by neighbors4. Notably, the optimal level of territorial aggression is highly context-dependent, with the interaction outcome influenced by the life-histories of the involved organisms6,9.
Among social animals, ants represent an outstanding example of social organization, which may be a key factor explaining their ecological dominance in terrestrial ecosystems10,11,12. Importantly, ants might be aggressive and are especially efficient in chemically recognizing nestmates from non-nestmates13,14. Therefore, ants represent appropriate biological models to investigate neighbor-stranger discrimination and aggression. However, like it is also true for other animals, there are mixed pieces of evidence, with some ant behavioral studies pointing to a DE15,16,17 and others to NN effects18,19,20. Besides, there are even some cases where no clear signs of neighbor-stranger discrimination occur21,22.
Despite the increasing number of studies focusing on ant nestmate recognition and aggressiveness23,24,25, this literature is mainly focused on ground ant species26, and studies on arboreal ants are rare. However, arboreal ants are often involved in mutualistic interactions with their host-plants, and aggressiveness often play a key role in shaping these ant-plant relationships27. For example, to our knowledge, while there are studies involving neighboring colonies on ant-plants28,29,30, no published paper has specifically studied nestmate recognition for obligatory mutual relationships between ants and plants. However, it is well known that, in exchange for food and shelter, plant-ants present a highly aggressive behavior against their host plant’s natural enemies31. Both the NN and the DE are possible regarding neighbor-stranger aggression among plant-ants. The NN would more likely occur when the colonies’ borders are not well delimited, and potential invasions are harder to predict4,7. Additionally, when local nesting site availability is low, neighbors are competing for the few available nesting sites left, generating the NN. The DE would be more likely if the different colonies have well-defined borders7, generating high temporal and spatial predictability of the colony boundaries. Here, we investigated these two possible outcomes, NN or DE, and aim to understand if chemical recognition promotes intercolonial aggression.
Ants perform nestmate recognition mainly based on chemical cues, mostly involving cuticular hydrocarbon profiles (also called CHCs)32,33. Each colony has its own chemical identity (“colony odors”), which forms an odor template used to discriminate between nestmates and invaders34,35,36. Recognition cues are thought to be compared to a template that resides in the peripheral and central nervous system37. Any incompatibility between intruders and template odors may result in aggression38,39,40. Colony recognition might be particularly important in obligatory mutualisms, where the loss of the territory, i.e., the host plant, implies the death of the whole colony31,41. Despite that, there are no empirical studies investigating neighbor-stranger discrimination and aggression in the heavily studied obligatory ant-plant systems.
Based on this, we studied recognition and aggression among individuals belonging to close and distant nests of the plant-ant Azteca muelleri, which lives in hollow stems of the pioneer tree Cecropia glaziovii. Like other mutualistic Azteca species41,42, A. muelleri is strongly aggressive towards herbivores and effectively protects its host plant43. More specifically, we aim to investigate if the DE or NN effects or even none of them apply to this Azteca-Cecropia system. To take a step forward, we further checked how ant aggression towards conspecifics was related to differences in CHC profiles between ant colonies. More specifically, we designed the study to answer the following questions: (a) Is there an effect of spatial distance on ant aggressiveness? (b) Is there an effect of chemical distances on ant aggressiveness?
Methods
Study area
We sampled A. muelleri colonies in three Atlantic Forest fragments located in Viçosa town (20°48′ 07′′ S, 42°51′ 31′′ W), state of Minas Gerais, Southeastern Brazil: “Mata do Paraíso” (MP), “Mata da Biologia” (MB) and “Mata do Seu Zé” (SZ). The region has a subtropical climate, with annual precipitation of 1300 to 1400 mm and an average temperature of 19 °C44. All sites are equally comprised of secondary Seasonal Atlantic Forest vegetation45, but they have different sizes and regeneration times. The MP is a research station from the Federal University of Viçosa (UFV), with an area of 195 ha, and ~ 60 years of regeneration process following coffee plantation. MB is located within the university campus, has an area of 75 ha, and more than 90 years of regeneration, following cattle-grazed pastureland and coffee plantation. The SZ is a private area, with ~ 20 ha, and its vegetation has approximately ten years of regeneration after cattle pasturelands. We calculated the distance between sites through the online geographic calculator of the “Instituto Nacional de Pesquisas Espaciais” (INPE), where the distance in meters between the sites and between the plants in each site was estimated using GPS data (see in Fig. 1). All parts of this work comply with the current research laws of Brazil. Besides, we had all the specific permits from the SZ owner and the UFV environmental managers to sampling in our three sites.
Schematic representation of behavioral trials showing the number of ant nests (trees) and trials within and between the three sampling sites. The number within circles represent the average spatial distance between ant nests (trees) that were engaged in behavioral trials from the six possible combinations of sites. Within sites—MP versus MP; SZ versus SZ; MB versus MB and between sites: MP versus MB; MP versus SZ; MB versus SZ. SZ—“Seu zé”; MB—“Mata da Biologia” and MP—“Mata do paraíso”.
Biological system
Cecropia glaziovii Snethl. (Cecropiaceae), is a fast-growing tree usually found in Forest regeneration fragments, reaching up to 20 m in height and restricted to an altitudinal range between 600 and 1500 m46. Cecropia glaziovii is usually involved in mutualistic associations with the ant Azteca muelleri (Dolichoderinae)47,48, an aggressive ant species that is an effective protector of its host plant against herbivores and other ants49. Indeed, there is often fierce intra- and interspecific competition between Azteca colonies for nesting sites within their Cecropia hosts50,51,52. The density of Azteca colonies inhabiting Cecropias is strictly related to the density of their plant host41,52. While we have no data concerning nest density and dispersal of A. muelleri in our focal region, prior studies on other Azteca species showed that queens could disperse to long distances53,54. While A. muelleri can colonize different species of Cecropia55, we found only C. glaziovii as a host plant of A. muelleri in our three sampling areas.
Ant-plant sampling
We sampled 46 A. muelleri nests located inside 46 C. glaziovii individuals’ plants, being 14 on MP, 12 in MB, and 20 in SZ, between February–April 2017 and August–November 2018. In this way, we could carry out experiments with plants from all locations in different seasons (dry and rainy). On each plant, we scanned A. muelleri’s workers’ presence on the plant surface by beating the trunk. If there were active workers, then we measured the plant height. As prior studies have found a strong relationship between the host-plant size and colony size and age for ant-plants56,57, we further used tree height as a proxy of colony size and age. After detecting active workers, we cut the plant and collected the alive queen and around 50 ant workers per plant and stored it in perforated plastic pots together with a small piece of the upper part of each tree trunk. We considered a colony unit all the ants within a single plant, where we found only one queen. Plants with no queens or with two or more queens were not considered. Therefore, our sampling size was constrained by the availability of whole individual trees in the studied area (in total, 125 trees were collected, including those used for pilot sampling and final tests). The plastic pots were stored in a breeding room with controlled humidity and temperature (45% and 25 °C, respectively) at the “Laboratório de Ecologia de Formigas” from “Universidade Federal de Viçosa”. After the trials (which will be detailed in the next section), we collected ant individuals for each colony, which had their identity assessed morphologically by ant taxonomists. Alternatively, we also sequenced the mitochondrial gene partial region that encodes cytochrome oxidase subunit I (COI). We used the COI data as a ‘genetic barcode’ to confirm that ants collected from our three sites belong to the same species, i.e. Azteca muelleri (see details in Online Resource S1, Fig. S1).
Behavioral trials
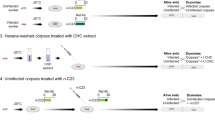
For the ant behavioral tests, we performed 23 trials using 46 nests. Each colony was engaged in only one behavioral trial. We performed one trial per nest pair since even collecting a few individuals could bring more stress to the whole colony (which was already under stress in laboratory conditions). The trials were divided into two distinct groups. For the first group (hereafter ‘within sites’), we ran 13 trials and used workers from different colonies but the same site. For the second group (hereafter ‘between sites’), we ran ten trials and also used workers from different colonies, but now from different sites (Fig. 1). For each trial, we placed ten A. muelleri individuals from two colonies (five individuals per colony) in a 25 cm2 plastic Gerbox arena and observed their interactions for 5 min. We spread odorless talc powder only in the Gerbox upper border to prevent ants from escaping the arena. Before each behavioral trial, we divided our arena into two parts using a 5 cm plastic ruler barrier, and after placing the ants in the arena (each colony on one side of the barrier), we waited for 5 min for ant acclimation. After that, we removed the plastic rule and let the ants interact freely inside the arena for 5 min. Azteca ants belonging to the same species can be highly variable morphologically57 as it is common for ants in general58,59. Noticeable differences in size and color can be related to the colony's maturity stage and aspects of the host-plant57. Additionally, before any behavioral trial, we carefully observed the colony to obtain cues over their morphological differences. Therefore, we were able to distinguish the ant individuals from the two different colonies. Finally, we did the last assessment after performing the trials by assuring that there were no aggressive interactions between the ants considered from the 'same colony,' as it is well known that ants belonging to the same colony do not attack each other. During these 5 min, we classified all the behaviors of each pair of interacting ants following a modified version of the ‘protocol of aggressiveness’ proposed by Holway et al. (1998)60 and adapted by Giraud et al. (2002)61. Thus, every time one ant from a different nest approaches each other, we classified the behavioral interactions between then into six levels on a scale from 0 to 5 as follows: 0—ignore, has no physical contact and shows no interest; 1—antennation, repeated antenna taps on the other ant body; 2—avoidance, retract to the opposite direction after contact; 3—dorsal flexion of the gaster and mandible opening; 4—aggression, bites or pulls the head, legs or other parts of the body and 5—fight, prolonged aggression, locking the jaw in one body part of the other ant, not releasing until the end of the trial. Based on this scale, we consider the scores 0–2 as “non-aggressive” and 3–5 as “aggressive” behaviors. After that, we calculated a behavioral index by summing the number of times (frequency) that each behavior was scored and dividing this number by the total number of interactions displayed within 5 min. For example, in a trial where we observed two interactions scored as level 2, and one as level 3, our behavioral index would be 2.5 = (2 * 2 + 1 * 3)/3.
Chemical analyses
After the aggression tests, we weighted the ants and stored them at − 22 °C until the extraction of the cuticular hydrocarbons (CHCs) by hexane. The extraction was performed by immersing five workers per nest in 50 μl of hexane for 2 min, followed by the removal of the supernatant. On each sample, we added 50 µL−1 of E, E-Farnesol (Sigma-Aldrich, St. Louis, MO, USA), with a concentration of 50 ng µL−1 to the extracts as an internal standard62. This procedure was repeated three times in each nest in order to obtain triplicates of samples from each colony. For that, ants of similar weight were placed in each of the three samples. CHCs were quantified by GC-FID (Shimadzu GC-17A equipped with a Restek RTX-5 capillary column: 30 m × 0.25 mm × 0.25 µm), with a temperature program starting at 100 °C (1 min), with a maximum temperature of 280 °C (maintained for 10 min), after a heating ramp of 10 °C per minute62. Injector and FID temperatures were set at 250 °C. Samples were injected (1 µl) on splitless mode. Helium was used as a carrier gas, flowing at 1 ml per minute. Quantification data was used to measure the difference in hydrocarbon profiles between A. muelleri colonies. To avoid potential false-positive errors from GC-FID, we did not consider extracted compounds with a concentration lower than 1 ng µL−1. We also took out from our statistical analysis the chemical compounds with abnormally high standard deviation (i.e., greater than the mean) calculated from the triplicates of the same ant colony in order to avoid imprecise quantification. It is important to state that after performing these two compound exclusion methods above cited, we only discarded 7.15% (1467 out of 20,529 ng µL−1) of the total concentration of all compounds (9 out of 26) detected from our 46 ant nests.
Qualitative analysis was performed by GC–MS analyses (Shimadzu QP-2010 Plus equipped with a Restek RTX-5 capillary column: 30 m × 0.25 mm × 0.25 µm). Temperature and flow conditions were identical to the described for GC-FID analyses. Structural elucidation was performed based on retention indexes63, mass spectra interpretation, and NIST library. Retention indices (RIs) were determined using an n-alkane standard mixture (C7–C40, Supelco, Bellefonte, PA, USA). Retention indices and mass spectra were used to compare and identify CHCs already described in the literature63,64,65.
Double bond positions of unsaturated compounds were determined based on the analysis of mass spectra obtained after the derivatization of natural extracts with DMDS66. The position of methyl groups on branched hydrocarbons was determined based on retention index patterns and the relative intensity of fragments on MS spectra67.
Statistical analyses
To investigate the effect of spatial distance on ant aggressiveness, we used a general linear model (GLM), with spatial distance as the explanatory variable and the ant aggression index as the response variable. To test if ants presented higher aggression levels in ‘between sites’ versus ‘within sites’, we carried out a factorial GLM similar to a one-way ANOVA. For this, we used the site pairs (all the six possible combinations: within sites—MP vs. MP; SZ vs. SZ; MB vs. MB and between sites—MP vs. MB; MP vs. SZ; MB vs. SZ) as an explanatory variable (factor) and the aggression index as a response variable. Tukey HSD pairwise comparisons were performed after running the factorial GLM. After performing residual analyses, we discover that the models mentioned above followed Gaussian error distributions.
To test for the possible effect of chemical distances on ant aggressiveness, we first calculated the chemical distance between ant nests using two approaches. First, we calculated the Bray–Curtis index of dissimilarity among the overall chemical profiles (the concentration of all hydrocarbon compounds securely detected by IGC) between each pair of ant nests placed in behavioral trials (n = 23). Second, we calculated the same Bray–Curtis index of dissimilarity but now using separately the compounds belonging to the three most common classes of CHC as follows: linear alkenes, linear alkanes, and methylated alkanes. After that, we performed four GLM models using chemical distance (using the overall, linear alkenes, linear alkanes, and methylated alkanes CHCs separately) as the explanatory variables and ant aggression index as the response variable. Again, all the models described above followed Gaussian error distributions.
We used the R software68 for all statistical analyses and performed residual analyses by checking error distributions suitability for all models.
Results
Behavioral tests
We found that across the 23 pairwise behavioral tests, considering “within-sites” encounters, most interactions were non-aggressive (54.05%), while 45.9% were aggressive (Table 1). On the other hand, for the “between-sites” encounters, almost all interactions (97.5%) were aggressive (Table 1). Concordantly, when we compared the aggression index between the site pairs, we found that ant aggression was significantly higher in “between-sites” than “within-sites” trials (Fig. 2, F5,17 = 15.907, P < 0.001) (Fig. 2). Despite the higher aggression in “between-sites” trials, the comparisons between MB versus MB and MP versus MB did not differ from each other (Fig. 2).
Mean (± SE) ant behavioral index per pair of sites comparing the six possible combinations of sites. Within sites—MP versus MP; SZ versus SZ; MB versus MB and between sites: MP versus MB; MP versus SZ; MB versus SZ. SZ—“Seu zé”; MB—“Mata da Biologia” and MP—“Mata do paraíso. Inside each bar is the number of trials within and between the three sampling sites.
We ruled out ant’s weight and plant’s height as having a significant influence on ant aggressiveness, as we failed to find significant relationships (ant weight: F1,21 = 0.301, P = 0.59; plant height: F1,21 = 0.9472, P = 0.33). Therefore, these features of the colony structure did not explain the aggressive behavior between ants.
Aggression versus spatial distance
We found a positive influence of spatial distance on ant aggressiveness (Fig. 3, F1,21 = 30.098, P < 0.001) (Fig. 3). These results indicate a DE effect in our system once the aggressive behavior increase with the distance between nests.
Relationship between the spatial distance and the ants’ behavioral index. Each point represents a pair of ant nests (five individuals per nest submitted to behavioral trials, N = 23). The solid line represents the linear regression fit to the data. The doted horizontal line indicates the threshold between non-aggressive (bellow the line) and aggressive (above de line) behaviors.
Aggression versus chemical distance
We detected a total of 26 CHCs in the A. muelleri chemical profile. Among these compounds, we observed three main classes: linear alkenes, linear alkanes, and methylated alkanes (Online Resource Table S1). After the compound selection analysis (see “Methods” section), a total of 15 CHCs were considered for the ant aggression analysis. There was no relationship between ant aggression and the overall chemical dissimilarity (Fig. 4A, F1,18 = 0.556, P = 0.46) (Fig. 4). When looking specifically for each of the three most important CHC classes, we found a positive relationship between methylated alkanes dissimilarity and the ant aggressiveness (Fig. 4D, F1,21 = 4.391, P = 0.048), whereas linear alkanes and alkenes were not significantly related with aggressiveness in our tests (Fig. 4B, F1,21 = 0.428, P = 0.52; Fig. 4C, F1,21 = 0.076, P = 0.78) (Fig. 4).
Relationship between ants’ CHC profile dissimilarity and the spatial distance between nests. (A) chemical dissimilarity using total CHC blend; (B) chemical dissimilarity using Alkanes only; (C) chemical dissimilarity using Alkenes only; (D) chemical dissimilarity using Methyl alkanes only. The solid line represents the linear regression fit to the data. Each point represents a pair of ant nests (the same ones submitted to behavioral trials, N = 23).
Discussion
This study investigated neighbor–stranger aggressiveness in plant-ants, and by using an experimental approach, we found support for the “dear enemy” rather than “nasty neighbor” effects. Thus, we found that A. muelleri ants were more aggressive towards colonies located far away from their nests when compared to closer ones. We also checked for the importance of chemical recognition in leading the DE effect. We found no effect of the overall chemical blend but a significantly positive effect of one CHC class dissimilarity, the methylated alkanes. Our findings bring new light into the understanding of aggressive territorial defense in ants, and more specifically, in intricated ant-plant mutualisms31,57,69.
The differences in aggressive responses from neighbors or strangers are highly context-dependent, and factors such as resource predictability and colony delimitation can be essential to define the direction of the aggressive behaviors70,71. In ant-plant systems involving obligatory mutualisms, the ants rely on their host plants as shelter, and therefore there should be much competition for available nesting sites, i.e., whole plants50,51,52. After the colonization stage, the colonies of A. muelleri are well delimited, consisting of a single tree individual7. Thus, the borders between colonies are clear, which is an essential factor for the DE effect19. Well defined borders allow for a higher spatial and temporal predictability of the movement between nearby colonies4. More specifically, each neighboring colony of A. muelleri have already a delimited nest (one C. glaziovii tree) in their possession and so far poses less threat to another, especially because their status is generally known and they have less to gain from conflict. On the other hand, A. muelleri individuals coming from distant areas could be then recognized as bigger treats by the simple fact of being strangers.
The DE effect is widespread in ant communities7,72, and here we provide evidence that this effect is also important in obligatory ant-plant interactions. The main reason is that neighboring ant colonies already have an established resource (i.e., the whole tree), which is often restricted18,73. Moreover, in an obligatory ant-plant system, the host plant offers most of the necessary resources for the colony development and maintenance, and being dislodged from a tree means almost certainty colony death74,75,76. Thus, the host tree in an ant-plant mutualistic system might be ecologically interpreted as a typical example of an absolute territory77,78,79. As aggressive behavior is highly energy costly, the mutualistic ants might be able to recognize the enemies which present at the same time the highest threats for both the ants and the plants. As mutualistic ants normally present a high foraging activity on the tree and in its vicinities, they would have an increased probability of recognizing strangers as a greater threat than neighbors, ultimately increasing the DE effect.
The description of territorial defense behavior is much more common for vertebrates than other groups6,80,81. However, there is an increasing number of studies involving invertebrates, especially social organisms, such as ants16,82. Vertebrates can use distinct tactics for enemy recognition, including visual, olfactory, and vocal cues6. For ants, aggressiveness towards enemies is based primarily on chemical compounds33,83,84. Each ant colony has its unique odor composed of cuticular hydrocarbon compounds (CHCs), which form a chemical template that can guide the ant's behavior85,86. If intruder ants present an odor that does not fit the colony's chemical template, the resident ants often show an aggressive response38,87. However, our study could not find strong evidence that the entire blend of CHCs cuticle profiles is involved in eliciting aggression in Azteca ants living in our focal Cecropia trees. We have two non-exclusive lines of arguments to explain this, which will be presented in the next paragraph.
Firstly, insect cuticular lipids typically contain more than CHCs (e.g., fatty acids and esters), and these other chemical compounds may also have a role on nestmate recognition83,88,89,90. For example, for other ant species, like leaf-cutters89,90,91 and cuckoo ants88, the fatty acids are more important in nestmate recognition and aggressive behavior than CHCs. It could also be important for ants foraging on trees as the plant environment, i.e., its surface, contains fatty acids, which may be eaten by ants influencing cuticle chemical formation. Secondly, when analyzing the entire hydrocarbon template, we might be dealing with multiple functions at the same time that could be different from aggressiveness (e.g., mating attraction, food, and nest location)92,93,94,95. Specific hydrocarbons classes such as methyl-alkanes and alkenes have been related to aggressive behavior or conspecific recognition39,40,96, while alkanes can function as cuticle lubrication or even act as chemical barriers against microbes97. Here, we found evidence for the role of the methylated alkanes in guiding ant’s aggression, supporting the findings of other studies showing an important role for methyl-branched alkanes in nestmate recognition95,98,99. However, as the relationship between methyl-alkanes dissimilarity and ant aggression was not strong, these results should be interpreted with care, and potentially other factors might be of greater importance than CHC in eliciting aggression in A. mulleri colonies.
Conclusion
Despite the “nasty neighbor” effect might occur more frequently in social insects19,20,100, we find here that the plant-ant A. muelleri is more aggressive to strangers than to neighbors, following the “dear enemy” effect101. We finally suggest that the DE effect might be related to mutualistic strength between partners102,103. Surprisingly, there is still a dearth of studies investigating territorial defense behavior in obligatory mutualistic systems. Therefore, we suggest that future studies directly investigate the relationship between neighbor-stranger conspecific aggression and mutualistic protection effectiveness.
Data availability
The authors intend to deposit the dataset at Dryad after the manuscript acceptance.
References
Wilson, E. O. Sociobiology (Harvard Press, 1975).
Hölldobler, B. & Lumsden, C. J. Territorial strategies in ants. Science 210, 732–739 (1980).
Baker, R. R. Insect territoriality. Annu. Rev. Entomol. 28, 65–89 (1983).
Christensen, C. & Radford, A. N. Dear enemies or nasty neighbors? Causes and consequences of variation in the responses of group-living species to territorial intrusions. Behav. Ecol. 29, 1004–1013 (2018).
Fisher, J. B. Evolution and bird sociality. In Evolution as a process (eds. Huxley, J., Hardy, A. C. & Ford, E. B.) 71–83. (Allen & Unwin, Australia, 1954).
Temeles, E. J. The role of neighbours in territorial systems: when are they “dear enemies”?. Anim. Behav. 47, 339–350 (1994).
Adams, E. S. Territoriality in ants (Hymenoptera: Formicidae): a review. Myrmecol. News 23, 101–118 (2016).
Müller, C. A. & Manser, M. B. “Nasty neighbours” rather than “dear enemies” in a social carnivore. Proc. R Soc. B Biol. Sci. 274, 959–965 (2007).
Tanner, C. J. & Adler, F. R. To fight or not to fight: context-dependent interspecific aggression in competing ants. Anim. Behav. 77, 297–305 (2009).
Mabelis, A. A. Wood ant wars. Neth. J. Zool. 29, 451–620 (1979).
Hölldobler, B. Recruitment behavior, home range orientation and territoriality in harvester ants, Pogonomyrmex. Behav. Ecol. Sociobiol. 1, 3–44 (1976).
Hölldobler, B. Tournaments and slavery in a desert ant. Science 80(192), 912–914 (1976).
Carlin, N. F. & Hölldobler, B. The kin recognition system of carpenter ants (Camponotus spp.) - I. Hierarchical cues in small colonies. Behav. Ecol. Sociobiol. 19, 123–134 (1986).
Carlin, N. F. & Hölldobler, B. The kin recognition system of carpenter ants (Camponotus spp.)—II. Larger colonies. Behav. Ecol. Sociobiol. 20, 209–217 (1987).
Langen, T. A., Tripet, F. & Nonacs, P. The red and the black: habituation and the dear-enemy phenomenon in two desert Pheidole ants. Behav. Ecol. Sociobiol. 48, 285–292 (2000).
Dimarco, R. D., Farji-Brener, A. G. & Premoli, A. C. Dear enemy phenomenon in the leaf-cutting ant Acromyrmex lobicornis: behavioral and genetic evidence. Behav. Ecol. 21, 304–310 (2010).
Yagound, B., Crowet, M., Leroy, C., Poteaux, C. & Châline, N. Interspecific variation in neighbour–stranger discrimination in ants of the Neoponera apicalis complex. Ecol. Entomol. 42, 125–136 (2017).
Benedek, K. & Kóbori, O. T. “Nasty neighbour” effect in Formica pratensis retz. (Hymenoptera: Formicidae). N. West J. Zool. 10, 245–250 (2014).
Newey, P. S., Robson, S. K. A. & Crozier, R. H. Know thine enemy: why some weaver ants do but others do not. Behav. Ecol. 21, 381–386 (2010).
Sanada-Morimura, S. et al. Encounter-induced hostility to neighbors in the ant Pristomyrmex pungens. Behav. Ecol. 14, 713–718 (2003).
Boulay, R., Cerdá, X., Simon, T., Roldan, M. & Hefetz, A. Intraspecific competition in the ant Camponotus cruentatus: should we expect the “dear enemy” effect?. Anim. Behav. 74, 985–993 (2007).
Frizzi, F. et al. The rules of aggression: How genetic, chemical and spatial factors affect intercolony fights in a dominant species, the mediterranean acrobat ant Crematogaster scutellaris. PLoS ONE 10, 1–16 (2015).
Crosland, M. W. Kin recognition in the ant Rhytidoponera confusa I. Environmental odour. Anim. Behav. 37, 912–919 (1989).
Beye, M., Neumann, P. & Moritz, R. F. A. Nestmate recognition and the genetic gestalt in the mound-building ant Formica polyctena. Insectes Soc. 44, 49–58 (1997).
Beye, M., Neumann, P., Chapuisat, M., Pamilo, P. & Moritz, R. F. A. Nestmate recognition and the genetic relatedness of nests in the ant Formica pratensis. Behav. Ecol. Soc. 43, 67–72 (1998).
Martin, S. & Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 35, 1151–1161 (2009).
Rico-Gray, V., Oliveira, P. S. & Oliveira, P. S. The Ecology and Evolution of Ant-plant Interactions (University of Chicago Press, 2007).
Adams, E. S. Boundary disputes in the territorial ant Azteca trigona: effects of asymmetries in colony size. Anim. Behav. 39, 321–328 (1990).
Adams, E. S. Territory defense by the ant Azteca trigona: maintenance of an arboreal ant mosaic. Oecologia 97, 202–208 (1994).
Frederickson, M. E. & Gordon, D. M. The intertwined population biology of two Amazonian myrmecophytes and their symbiotic ants. Ecology 90, 1595–1607 (2009).
Heil, M. & McKey, D. Protective and in ecological model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 34, 425–453 (2003).
Hölldobler, B. The chemistry of social regulation: Multicomponent signals in ant societies. Proc. Natl. Acad. Sci. USA 92, 19–22 (1995).
Howard, R. W. & Blomquist, G. J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393 (2005).
Boulay, R., Hefetz, A., Soroker, V. & Lenoir, A. Camponotus fellah colony integration: worker individuality necessitates frequent hydrocarbon exchanges. Anim. Behav. 59, 1127–1133 (2000).
Errard, C., Hefetz, A. & Jaisson, P. Social discrimination tuning in ants: template formation and chemical similarity. Behav. Ecol. Sociobiol. 59, 353–363 (2006).
Brandstaetter, A. S., Rössler, W. & Kleineidam, C. J. Friends and foes from an ant brain’s point of view—neuronal correlates of Colony Odors in a social insect. PLoS ONE 6, e21383 (2011).
Leonhardt, S. D., Brandstaetter, A. S. & Kleineidam, C. J. Reformation process of the neuronal template for nestmate-recognition cues in the carpenter ant Camponotus floridanus. J. Comp. Physiol. 193, 993–1000 (2007).
Guerrieri, F. J. et al. Ants recognize foes and not friends. Proc. R Soc. B Biol. Sci. 276, 2461–2468 (2009).
Newey, P. Not one odour but two: a new model for nestmate recognition. J. Theor. Biol. 270, 7–12 (2011).
Martin, S. J., Vitikainen, E., Drijfhout, F. P. & Jackson, D. Conspecific ant aggression is correlated with chemical distance, but not with genetic or spatial distance. Behav. Gen. 42, 323–331 (2012).
Longino, J. T. Azteca ants in Cecropia trees: taxonomy, colony structure, and behavior. In Ant-Plant Interactions (eds Huxley, C. R. & Cutler, D. F.) 271–288 (Oxford University Press, 1991).
Schupp, E. W. Azteca protection of Cecropia: ant occupation benefits juvenile trees. Oecologia 70, 379–385 (1986).
Oliveira, K. N. et al. The effect of symbiotic ant colonies on plant growth: a test using an Azteca-Cecropia system. PLoS ONE 10, 1–13 (2015).
Silva, C. A., Vieira, M. F. & Amaral, C. H. Floral attributes, ornithophily and reproductive success of Palicourea longepedunculata (Rubiaceae), a distylous shrub in southeastern Brazil. Rev. Bras. Bot. 33, 207–210 (2010).
Veloso, H. P., Rangel Filho, A. L. R. & Lima, J. C. A. Classificação da Vegetação Brasileira Adaptada a um Sistema Universal (Ibge, 1991).
Berg, C. C., Rosselli, P. F. & Davidson, D. W. Cecropia. Flora Neotropica. 94, 1–230 (2005). Retrieved April 22, 2020, from www.jstor.org/stable/4393938
Emery, C. & de Voyage, M. M. Bedot et Pictel dans l’Archipel Malais. Formicides de l’Archipel Malais [Travel of MM. Bedot and Pictel in the Malaysian Archipelago. Formicides from the Malaysian Archipelago]. Rev. Suisse. Zool. 1, 187–229 (1893).
Davidson, D. W. & Fisher, B. L. Symbiosis of ants with Cecropia as a function of light regime. In Ant-Plant Interactions (eds. Huxley, C. R. & Cutler, D. F.) 289–309 (Oxford University Press, UK, 1991).
Davidson, D. W. & McKey, D. Ant-plant symbioses: stalking the chuyachaqui. Trends Ecol. Evol. 8, 326–332 (1993).
Fonseca, C. R. & Ganade, G. Asymmetries, compartments and null interactions in an Amazonian ant-plant community. J. Anim. Ecol. 65, 339–347 (1996).
Fonseca, C. R. Amazonian ant-plant interactions and the nesting space limitation hypothesis. J. Trop. Ecol. 15, 807–825 (1999).
Longino, J. T. Geographic variation and community structure in an ant-plant mutualism: Azteca and Cecropia in Costa Rica. Biotropica 21, 126–132 (1989).
Bruna, E. M., Izzo, T. J., Inouye, B. D., Uriarte, M. & Vasconcelos, H. L. Asymmetric dispersal and colonization success of Amazonian plant-ants queens. PLoS ONE 6, e22937 (2011).
Yu, D. W. et al. Experimental demonstration of species coexistence enabled by dispersal limitation. J. Anim. Ecol. 73, 1102–1114 (2004).
Rocha, C. F. D. & Bergallo, H. G. Bigger ant colonies reduce herbivory and herbivore residence time on leaves of an ant-plant: Azteca muelleri vs. Coelomera ruficornis on Cecropia pachystachya. Oecologia 91, 249–252 (1992).
Campbell, H., Fellowes, M. D. E. & Cook, J. M. Arboreal thorn-dwelling ants coexisting on the savannah ant-plant, Vachellia erioloba, use domatia morphology to select nest sites. Insectes Soc. 60, 373–382 (2013).
Marting, P. R., Wcislo, W. T. & Pratt, S. C. Colony personality and plant health in the Azteca-Cecropia mutualism. Behav. Ecol. 29, 264–271 (2018).
Tschinkel, W. R. Sociometry and sociogenesis of colonies of the fire ant Solenopsis invicta during one annual cycle: ecological archives M063–002. Ecol. Monogr. 63, 425–457 (1993).
Wills, B. D., Powell, S., Rivera, M. D. & Suarez, A. V. Correlates and consequences of worker polymorphism in ants. Ann. Rev. Entomol. 63, 575–598 (2018).
Holway, D. A., Suarez, A. V. & Case, T. J. Loss of intraspecific aggression in the success of a widespread invasive social insect. Science 282, 949–952 (1998).
Giraud, T., Pedersen, J. S. & Keller, L. Evolution of supercolonies: the Argentine ants of southern Europe. PNAS 99, 6075–6079 (2002).
Fischer, D. C. Fundamentos de cromatografia. Rev. Bras. Cienc. Farm. 42, 308–308 (2006).
Koo, I., Shi, X., Kim, S. & Zhang, X. IMatch2: Compound identification using retention index for analysis of gas chromatography-mass spectrometry data. J. Chromatogr. A 1337, 202–210 (2014).
El-Sayed, A. M. The Pherobase: Database of Pheromones and Semiochemicals. https://www.pherobase.com. Accessed 11 July 2020 (2020).
NIST Livro de Química na Web. Base de dados de Referência padrão do NIST número 69. http://webbook.nist.gov/chemistry/. Accessed 13 July 2020 (2016).
Vidal, D. M., Fávaro, C. F., Guimaraes, M. M. & Zarbin, P. H. Identification and synthesis of the male-produced sex pheromone of the soldier beetle Chauliognathus fallax (Coleoptera: Cantharidae). J. Brazil. Chem. Soc. 27, 1506–1511 (2016).
Carlson, D. A., Bernier, U. R. & Sutton, B. D. Elution patterns from capillary GC for methyl-branched alkanes. J. Chem. Ecol. 24, 1845–1865 (1998).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org. Accessed 16 June 2020 (2017).
Lanan, M. C. & Bronstein, J. L. An ant’s-eye view of an ant-plant protection mutualism. Oecologia 172, 779–790 (2013).
Briefer, E., Rybak, F. & Aubin, T. When to be a dear enemy: flexible acoustic relationships of neighbouring skylarks Alauda arvensis. Anim. Behav. 76, 1319–1325 (2008).
Hyman, J. Seasonal variation in response to neighbors and strangers by a territorial songbird. Ethology 111, 951–961 (2005).
Sturgis, S. J. & Gordon, D. M. Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol. News 16, 101–110 (2012).
Matthews, R. W. & Matthews, J. R. Insect Behavior (Springer, 2009).
Boucher, D. H., James, S. & Keeler, K. H. The ecology of mutualism. Annu. Rev. Ecol. Evol. Syst. 13, 315–347 (1982).
Connor, R. C. The benefits of mutualism: a conceptual framework. Biol. Rev. 70, 427–457 (1995).
Bronstein, J. L. The costs of mutualism. Am. Zool. 41, 825–839 (2001).
Hölldobler, B. & Wilson, E. O. The Ants (Harvard University Press, 1990).
Dejean, A., Corbara, B., Orivel, J. & Leponce, M. Rainforest canopy ants: the implications of territoriality and predatory behavior. Funct. Ecol. Commun. 1, 105–120 (2007).
Dejean, A., Grangier, J., Leroy, C. & Orivel, J. Predation and aggressiveness in host plant protection: a generalization using ants from the genus Azteca. Naturwissenschaften 96, 57–63 (2009).
Tripovich, J. S., Charrier, I., Rogers, T. L., Canfield, R. & Arnould, J. P. Acoustic features involved in the neighbour-stranger vocal recognition process in male Australian fur seals. Behav. Process. 79, 74–80 (2008).
Favaro, L., Gamba, M., Gili, C. & Pessani, D. Acoustic correlates of body size and individual identity in banded penguins. PLoS ONE 12, e0170001 (2017).
Heinze, J., Foitzik, S., Hippert, A. & Hölldobler, B. Apparent dear-enemy phenomenon and environment-based recognition cues in the ant Leptothorax nylanderi. Ethology 102, 510–522 (1996).
Vander Meer, R. K. & Morel, L. Nestmate Recognition in Ants. 79–103 (Pheromone communication in Soc. Insects, 1998).
Provost, E., Blight, O., Tirard, A. & Renucci, M. Hydrocarbons and insects’ social physiology. Insect Physiology: New Research 19–72 (2008).
Crozier, R. H. & Dix, M. W. Analysis of two genetic models for the innate components of colony odor in social Hymenoptera. Behav. Ecol. Sociobiol. 4, 217–224 (1979).
Ozaki, M. et al. Behavior: ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309, 311–314 (2005).
Starks, P. T. Recognition systems: from components to conservation. Ann. Zool. Fennici. 41, 689–690 (2004).
Franks, N., Blum, M., Smith, R. K. & Allies, A. B. Behavior and chemical disguise of cuckoo ant Leptothorax kutteri in relation to its host Leptothorax acervorum. J. Chem. Ecol. 16, 1431–1444 (1990).
Hernández, J. V. et al. Leaf-cutter ant species (Hymenoptera: Atta) differ in the types of cues used to differentiate between self and others. Anim. Behav. 71, 945–952 (2006).
Nehring, V. et al. Chemical disguise of myrmecophilous cockroaches and its implications for understanding nestmate recognition mechanisms in leaf-cutting ants. BMC Ecol. 16, 1–11 (2016).
Hernández, J. V., López, H. & Jaffe, K. Nestmate recognition signals of the leaf-cutting ant Atta laevigata. J. Insect. Physiol. 48, 287–295 (2002).
Howard, R. W. & Blomquist, G. J. Chemical ecology and biochemistry of insect hydrocarbons. Ann. Rev. Entomol. 27, 149–172 (1982).
Sturgis, S. J., Greene, M. J. & Gordon, D. M. Hydrocarbons on harvester ant (Pogonomyrmex barbatus) Middens Guide Foragers to the Nest. J. Chem. Ecol. 37, 514–524 (2011).
Greene, M. J. & Gordon, D. M. Cuticular hydrocarbons inform task decisions. Nature 423, 32–32 (2003).
Sano, K., Bannon, N. & Greene, M. J. Pavement ant workers (Tetramorium caespitum) assess cues coded in cuticular hydrocarbons to recognize conspecific and heterospecific non-nestmate ants. J. Insect. Behav. 31, 186–199 (2018).
Guillem, R. M., Drijfhout, F. P. & Martin, S. J. Species-specific cuticular hydrocarbon stability within European Myrmica Ants. J. Chem. Ecol. 42, 1052–1062 (2016).
Sprenger, P. P. & Menzel, F. Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: how and why they differ among individuals, colonies, and species. Myrmec. News 30, 1–26 (2020).
Dahbi, A., Cerdá, X., Hefetz, A. & Lenoir, A. Social closure, aggressive behavior, and cuticular hydrocarbon profiles in the polydomous ant Cataglyphis iberica (Hymenoptera, Formicidae). J. Chem. Ecol. 22, 2173–2186 (1996).
Boulay, R., Katzav-Gozansky, T., Hefetz, A. & Lenoir, A. Odour convergence and tolerance between nestmates through trophallaxis and grooming in the ant Camponotus fellah (Dalla Torre). Insectes Soc. 51, 55–61 (2004).
Dunn, R. R. & Messier, S. H. Evidence for the opposite of the dear enemy phenomenon in termites. J. Insect. Behav. 12, 461–464 (1999).
Temeles, E. J., Muir, A. B., Slutsky, E. B. & Vitousek, M. N. Effect of food reductions on territorial behavior of purple-throated caribs. Condor 106, 691 (2004).
Pacheco, P. S. M. & Del-Claro, K. Pseudomyrmex concolor Smith (Formicidae: Pseudomyrmecinae) as induced biotic defence for host plant Tachigali myrmecophila Ducke (Fabaceae: Caesalpinioideae). Ecol. Entomol. 43, 782–793 (2018).
Hager, F. A. & Krausa, K. Acacia ants respond to plant-borne vibrations caused by mammalian browsers. Curr. Biol. 29, 717-725.e3 (2019).
Acknowledgements
This article was part of GZ master thesis that was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and by the Programa de Pós-graduação em Ecologia from the Universidade Federal de Viçosa (UFV). We thank Ricardo Solar for helping with data analyses, Pedro Rocha, Carolina Castro, Caio Paz, Júlio Chaul, Laura Machuca Mesa, Suelen Speridião, and Laila Rezende for the assistance in the field and the laboratory analyses.
Funding
This study was funded by the Project APQ-01424–15/FAPEMIG coordinated by RIC.
Author information
Authors and Affiliations
Contributions
G.Z. and R.I.C. conceived and designed the experiments. G.Z. led the field sampling and performed the behavioral trials with assistance from R.I.C. and M.D. G.Z., M.D., D.M.V., and E.L. performed the chemical analyses. A.F. did the COI analysis. G.Z., F.C., and R.I.C. analyzed the data. G.Z., R.I.C., and F.C. led the manuscript writing. M.D., D.M.V., A.F., and E.L. provided insightful revisions in the early version of the manuscript. Finally, as the first author, I declare that all authors have made a crucial scientific contribution to the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zorzal, G., Camarota, F., Dias, M. et al. The dear enemy effect drives conspecific aggressiveness in an Azteca-Cecropia system. Sci Rep 11, 6158 (2021). https://doi.org/10.1038/s41598-021-85070-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85070-3
This article is cited by
-
When neighbors become family: the dear-enemy effect of swimming crab and the verification of the formation hypothesis
Behavioral Ecology and Sociobiology (2024)
-
Dynamic changes to signal allocation rules in response to variable social environments in house mice
Communications Biology (2023)
-
Habitat as a conditionality factor of ant-plant mutualistic interaction in the Cecropia-Azteca system
Arthropod-Plant Interactions (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.