Abstract
The design of highly active and cost-effective photoelectrocatalysts for effective hydrogen generation becomes a mandatory issue due to the demands on sustainable solar fuels. Herein a novel ternary Co–Cd–Fe LDH/PbI2 nanocomposite (T-LDH/PbI2NC) was fabricated by combining strategies of doping and in-situ loading of ternary Co–Cd–Fe LDH. The morphological, structural, and optical properties of PbI2, T-LDH, and T-LDH/PbI2 NC were studied by different techniques. LDH narrows the bandgap of the nanocomposite to 2.53 eV which prolongs the lifetime of the photo-induced electrons. Subsequently, the use of T-LDH/PbI2 NC improves the photoelectrocatalytic (PEC) H2 production rate. T-LDH/PbI2 NC shows a catalytic H2 production rate of 107.53 mmol h−1 cm−2 with IPCE% of 83.8% for 307 nm and 67.3% for 508 nm. The ABPE% reaches its supreme of 4.24% for − 0.58 V and 5.41% for − 0.97 V, these values are the highest values yet for LDH-based photocatalysts. The influences of the operating temperature and monochromatic illumination on the PEC performance were studied. Also, the electrochemical surface area, thermodynamic parameters, and Tafe slopes are calculated to label the hydrogen evolution mechanism. Moreover, the stability and reusability of the T-LDH/PbI2 NC photoelectrode were investigated. This work not only illustrated a simplistic and accessible way to produce a new category of highly efficient photocatalysts compared to the previously reported LDH-based PEC catalysts but also demonstrates a new point of view for improving PEC performance towards industrial water splitting under sunlight irradiation.
Similar content being viewed by others
Introduction
Increasing demands for energy and elevating the environmental crisis have inspired researchers to develop low-cost, environmentally friendly, and reasonable sources of energy. Water splitting through the photoelectrochemical (PEC) route is considered as one of the promising approaches to produce hydrogen as chemical fuel1,2. The PEC water splitting process needs semiconductor photocatalysts to convert sunlight photons directly to hydrogen molecules as clean fuels. The semiconducting materials require a remarkable performance in sunlight absorption, electrons-holes separation, and electrons/holes mobility. But still, the electron–hole recombination is the main challenge in the choice of photocatalyst for PEC3,4. Layered-metal halides (for example; PbI2 and CdI2) are concerned with increasing interest in electrocatalysis because of their uses in the design of perovskite halides. These perovskite structures offered noticeable photoelectrocatalytic performances5 PbI2 offers high photoelectrocatalytic performances among the different SC materials however it necessitates illumination with photons of wavelengths less than ∼ 350 nm (absorption band onset). It has a bandgap wider than 3.10 eV. Then, its performance under visible light is limited. As a result, the modification of PbI2 using co-catalysts with suitable bandgaps for the visible light photons is considered the most common method to improve its photocatalytic hydrogen evolution (PHE) efficiency6,7.
Layered double hydroxides (LDH) have attracted much attention because of their high layer charge density along with two-dimensional interlayer spaces of height, which are available for generating a rational path for charge conveying8. NiFe, ZnCr, CoAl-LDH, and NiAl-LDH were used as co-catalysts in the field of water splitting to generate O2 and H29,10,11,12,13,14. Zhang et al. has designed a modular catalyst of Ni–MgO–Al2O3 via the template of NiMgAl-LDH. He showed excellent coke- and wintering-resistance in the drying of methane reaction15. Kulamani et al.16 has reported that NiFe-LDH/g-C3N4 photocatalyst shows excellent photoelectrocatalytic performances for water splitting reactions. These remarkable photocatalytic performances on materials based on LDH render them a rational platform for exploring novel and efficient photocatalysts17,18,19,20,21.
In this work, we report for the first time a novel photoelectrocatalyst containing (Co–Cd–Fe) LDH and PbI2 to form T-LDH/PbI2 nanocomposite. Different characterization instruments have been used to investigate the properties (structures, morphologies, compositions, and optical and photoelectrocatalytic properties) of the prepared photoelectrodes. Co–Cd–Fe LDH, PbI2, and T-LDH/PbI2 NC photoelectrodes are deposited on the surface of a graphite substrate and used for H2 generation through PEC water splitting. The PEC performance was evaluated in terms of electrode stability, reusability, optical filter effect, temperature effect, conversion efficiencies, Tafel slopes, and electrochemical surface area (ECSA). Finally, the number of evaluated hydrogen moles are calculated.
Results and discussion
Samples characterizations
Structural properties
XRD charts of (Co–Cd–Fe) LDH, PbI2 and T-LDH/PbI2 NC are displayed in Fig. 1A–D. The XRD chart of the (Co–Cd–Fe)LDH appears highly similar to the hexagonal phase of the hydrotalcite LDH (Fig. 1A). The observed XRD peaks referred to as the diffractions from (003), (006), (101), (009), (107), (018), (110), and (113) planes of a usual LDH22. The high reflection intensity of these peaks shows the high crystallinity of the studied LDH. However, the diffraction peaks of the lead iodide appear at 2 theta = 12.6°, 25.9°, 34.2°, 39.5°, 45.2°, 47.5°, 53.2°, 63.7° and 67.5° (Fig. 1B). These peaks can be respectively indexed to (001), (011), (012), (110), (013), (021), (022), (121), and (114) of PbI2 (ICDD Card No. 04-007-3845). The observed chart of PbI2 agreed well with previously observed charts in literature23.
The mean crystallites sizes (DC) of PbI2 were estimated utilizing Scherrer's relation. The calculated mean crystallites size was ~ 70 nm24. The mean value of the microstrain for PbI2 was ~ 0.2%. The dislocations density (δd = N/DC2, N is constant) was also estimated to evaluate the density of defects and the quality of the crystal. The smallest δd for PbI2 was calculated when N = 125. The obtained value of δd is 2.05 × 10−4 that refers to the high quality of the synthesized PbI2 crystal.
The XRD chart of T-LDH/PbI2 illustrates the main XRD peaks of the (Co–Cd–Fe)LDH but with observed shifts in the position (Fig. 1C). Also, a mixed-phase between PbI2 and LDH appears in this pattern. The observed XRD peaks are referred to diffractions from (003), (006), (101), (012), (009), (107), (018), and (113) planes. Moreover, the significant rise in the XRD peaks intensities refers to the distribution of PbI2 on LDH layers and the good distribution of PbI2 between the layers of LDH.
Morphologies of the samples
The morphologies of (Co–Cd–Fe)LDH and T-LDH/PbI2 NC were investigated using FE-SEM and TEM (Fig. 2). The prepared (Co–Cd–Fe)LDH looks like agglomerated nanoparticles stacked together, Fig. 2A. These particles have non-uniform shapes with different sizes. These particles are subsequently folded as our brains. A close examination of the sample using a high magnification SEM image reveals the existence of many small nano protrusions on the surface of LDH particles. After the formation of PbI2 on Co–Cd–Fe LDH, PbI2 distributes between the layers of LDH particles and increases the porosity of LDH. As a result, the surface area has increased and the particle size distribution was found to be 70 ± 10 nm, Fig. 2B. A TEM micrograph of T-LDH/PbI2 NC, Fig. 2C, illustrates the presence of PbI2 particles on Co–Cd–Fe LDH particles. It is seen that the layered structure of the LDH prevents the agglomeration of the PbI2 particles. This is useful for the separation of photogenerated electrons and holes. Hence, it is highly expected that this photocatalyst can be applied efficiently for photoelectrochemical hydrogen generation. The nanoporous features of the nanocomposite are shown in the magnified images of Fig. 2C.
Figure 3A–C shows high resolution-transmission electron microscopy (HR-TEM) images of T-LDH with PbI2. The distributions of PbI2 particles on the Co–Cd–Fe-LDH platelets are clearly observed. The highly magnified HR-TEM images were shown in Fig. 3B,C are used to confirm the fine structure of T-LDH/PbI2 NC, which showed the stacking of the layered nanosheets. The Co–Cd–Fe LDH component showed a plate-like morphology. The selected area electron diffraction (SAED) pattern illustrates the existence of the diffraction rings, inset of Fig. 3A, these rings confirmed the polycrystalline state and homogeneous distribution of PbI2 on the Co–Cd–Fe LDH layers. These results may enhance the ECSA-value and improve the separation of interfacial charge transfer between Co–Cd–Fe-LDH and PbI2 particles.
The EDX spectrum, Fig. 3D, of T-LDH/PbI2, and the inserted quantitative analysis Table in Fig. 3 clearly indicate the presence of cobalt, iron, and cadmium signal within the walls. The molar ratios of Co:Cd:Fe were found to be approximately 1:1:1. These ratios are in good agreement with the ratios used during the preparation of T-LDH.
Function groups identification
The FTIR charts of (Co–Cd–Fe)LDH, PbI2, and T-LDH/PbI2 NC are displayed in Fig. 4A–C. For (Co–Cd–Fe)LDH, the identified band at 3424 cm−1 is familiar to the OH-stretching of LDH and the H2O interlayer25. The observed peak close to 1630 cm−1 refers to the O–H bending and 1350 cm−1 refers to the bending mode of the H2O molecule. While the peak that appears at 1430 cm−1 is ascribed to the NO3−-stretching mode26. The noticed modes below 1000 cm−1 are assigned to the M–O vibrations of LDH Fig. 4A. The FTIR spectral modes of PbI2, Fig. 4B, are ascribed to vibrations of inorganic clusters. The mode at 1490 cm−1 is credited to the H2O molecule's absorption27. Figure 4B confirms the presence of strong interactions between Pb–I clusters. Symmetric and asymmetric modes are observed at 3055 and 3700 cm−1 of the Pb–I28. The peak at 2400 cm−1 was ascribed to the water stretching region. After the combination, the FTIR spectrum of T-LDH/PbI2 NC exhibits a shift to lower wavenumbers (redshift) which may be ascribed to the distribution of Pb–I2 into layers of LDH was shown at Fig. 4C.
Optical properties of Co–Cd–Fe LDH, PbI2, and T-LDH/PbI2 NC
The light absorption property of all samples is explored by UV–Vis absorption spectra as displayed in Fig. 5. The absorption edge of pure PbI2 appears at 300 nm. No important features are observed in the Vis/IR regions. This was due to its intrinsic bandgap absorption Fig. 5. After the growth of T-LDH/PbI2 NC, the absorption edge of Co–Cd–Fe LDH redshifts to the visible-region. So, a noticeable improvement in absorption can be observed in Fig. 5. Also, the layers of LDH facilitate the motion of the photo-produced electrons29. As well, the T-LDH/PbI2 NC showed a wider absorption band in the visible-light-region. These enhanced absorption capabilities result from the extension of the band to cover a broad region of the incident photons (300–800 nm). This range represents > 43% of sunlight at the Earth's surface. Therefore, T-LDH/PbI2 NC can efficiently be used as a key material for different solar energy applications including the PEC hydrogen generation. The light absorption by T-LDH/PbI2 NC around 500 nm is assigned to the metal–metal charges transfer that contributes efficiently to the PEC H2O splitting Fig. 5 i.e., the enhanced absorption capabilities toward the visible photons is accredited to electron transfer from the PbI2 conduction band to the LDH surface. Therefore, the absorption edge of T-LDH/PbI2 NC is observed in the visible-light-region, which leads to the reduction of the bandgap energy, Fig. 5.
Based on the UV–visible absorbance spectra (Fig. 6), the optical band gap of PbI2, Co–Cd–Fe LDH, and T-LDH/PbI2 NC can be obtained utilizing the absorption values (A) and absorption coefficient (αA) according to Eq. (1) (Tauc relation)1;
wherever Eph = hν and Eg are for the photon energy and bandgap energy. The values of αA are obtained from Eq. (2)30,31:
wherever \(\beta\), \(\ell\), and Cp are the material density, quartz cell width (1 cm), and suspended material concentration.
The bandgap energies were determined by inferring the linear portion of (αA Eph)2–Eph plot with the Eph-axis, Fig. 6.
The bandgap energy is estimated to be 3.04 eV for PbI2, which agreed to the previously stated bandgap values for nanostructure PbI2 (> 2 eV). This bandgap is originated from Pb s to Pb p interband transitions was shown in Fig. 6A. Also, there are two other bands at 2.31 and 2.51 eV due to the existence of two discrete absorption plasmons as a result of the quantum confinement effects and the orbital hybridizations between the Ip-orbitals and Pbs-orbitals. On the other hand, the bandgap values are estimated from to be 2.28 and 2.53 eV for Co–Cd–Fe LDH, and T-LDH/PbI2 NC; respectively Fig. 6B,C. Figures 5 and 6 refer to the improvement of the optical absorption and the redshift of the optical band gap of LDH due to the incorporation of PbI2 to form T-LDH/PbI2 NC. So it is highly expected that T-LDH/PbI2 NC can be more effective than its constituents for applying in PEC H2 generation. In Fig. 6B, the Co–Cd–Fe LDH showed the existence of two band gaps at 2.28 and 3.79 eV. Whereas the T-LDH/PbI2 NC band gaps are shifted to 2.53 and 4.22 eV, Fig. 6C. The blue shift of the band gap and the absorption edge is ascribed to the Burstein–Moss shift; whereas the electronic transitions from oxygen 2p to metal ns or np levels enhance the possibility of filling the bottom of the conduction band with electrons. Based on the exclusion principle this leads to blue shifts in the optical absorption band position. Based on that, the bandgap broadening and the intensive absorbance of the visible-light photons of T-LDH/PbI2 NC as compared to the pure LDH will facilitate the electronic transitions and offer more electron/hole pairs under sunlight illumination. All of these encourage us to apply the designed T-LDH/PbI2 NC for photoelectrochemical H2O-splitting under sunlight32.
PEC properties of T-LDH/PbI2 NC
Effect of photocatalyst and white light illumination
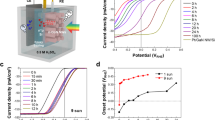
The efficiency of PEC depends on the photo responsive of the catalyst. So we measured the PEC characteristics for the three electrodes; PbI2, Co–Cd–Fe LDH, and T-LDH/PbI2 NC for determining the different current densities (Jph) for photo electrodes. The photocurrent density–voltage (Jph–V) curves are performed with increment 1 mVs−1 in 0.3 M KOH (100 ml) solution at 25 °C was shown at Fig. 7. The surface area of two electrodes (working and counter) electrodes are 1 cm2. A light power is adjusted to be 100 mW cm−2 with aid of 500 W Meitrcury-Xenon light source (Newport, MODEL: 66926-500HX-R07). The measured current densities for PbI2, Co–Cd–Fe -LDH and T-LDH/PbI2 NC photoelectrodes were 1.22, 4.86, and 53.27 mA cm−2 in − 1 V, respectively. The current density using the T-LDH/PbI2 NC photoelectrode generated the greater no of electrons than others under white light exposure. This is ascribed to its highly electrical surface charges and the suitable optical bandgap which in turn to increase in the absorbance in the Vis/IR range.
In pristine PbI2, there are decline in kinetics of water oxidation on the surface thus in valence band, the accumulation of positive holes will occur and stimulate electron/hole recombination in the conduction band, which displays low photo response33.
While Co–Cd–Fe LDH (TLDH) represented as an effective co-catalyst. It distinguishes with highly conductivity and faster carrier transfer which enhances the kinetic of water oxidation so helps in initiation of the removal of the photogenerated holes accumulated at the surface of system34.
While after introduction of Co–Cd–Fe LDH with PbI2, the performance of ternary T-LDH/PbI2 towards PEC is higher than others. Its outstanding efficiency is attributed to the synergistic effect of TLDH and PbI2 in the ternary T-LDH/PbI2.
Generally, the white light illumination has a crucial effect on PEC technique.the light stimulates the electrons of photocatalyst for hydrogen generation than dark conditions. The photocurrent was Jph = 53.27 and 3.12 eV mA cm−2 for dark condition as shown in Fig. 6. This amazing increase is assigned to the photoexcitation of electrode and generation of charged carrier (e−/hole+ pair) which helps in water splitting and hydrogen generation35.
Tafel slopes and ECSAs of the photoelectrodes
Tafel curves, Voltage–log(Jph), are presented in Fig. 8A–C from the Jph–Voltage characteristics of Fig. 8A to mark the hydrogen evaluation reaction (HER) mechanism36. The Tafel slopes of the straight lines in Fig. 8B,C are represented by β1 and β2 at low and high HER potential36. β1 and β2 values are reported in Table 1 with their standard deviations and the correlation R2-coefficients. Tafel slopes of 30, 40 and 120 mV dec−1 apply to Volmer–Tafel (recombination is rate-limiting) mechanism, Volmer–Heyrovsky (PEC desorption is rate-limiting) mechanism, and the dependency on different reaction paths of surface coverage by adsorbed H2. β1 and β2 remind us of the over-potentials required to increase the HER rate by 10 folds36. Thus, the calculated β1 and β2, Table 1, of the T-LDH/PbI2 NC electrode (49.91 and 79.61 mV dec−1) evidenced its improved PEC characteristic in HER.
The values of ECSAs for the three electrodes are obtained using the Randles–Sevcik equation, ECSA = I(R T/v D)1/2/[0.446 (C n F)3/2], where n = 1 refers to one electron contribution in the redox reaction, F and R denote to the Faraday and gas-molar constants37. Also, C signifies the analytes concentration, T signifies the reaction temperature, and D represents the analyte-diffusion constant37. Using J–V curves, Fig. 8, the ECSAs values for the three electrodes are calculated using ECSA = Q·(m·C)−1. Whereas Q, m, and C refer to the hydrogen-adsorption charges in the negative-scan after double-layered charges correction, photocatalyst mass, and the complete monolayer charges of hydrogen atoms, respectively, covering the electrode38,39. At a scanning-rate of 10 mV, the Q values are estimated using the J–V curves integrations/10 mV. The values of ECSAs of the three electrodes are 4.39, 18.98, 60.22 m2 g−1, respectively. The high value of ECSA for the T-LDH/PbI2 NC electrode compared to the LDH electrode and PbI2 electrode explains its high photocatalytic performance.
Reusability and stability of photoelectrode
The photostability of T-LDH/PbI2 NC for PEC behaviors was studied for many runs in 0.3 M KOH as a sacrificial agent, Fig. 9A. our findings were showed that The performance of this photoelectrode is stable with a long time. From this figure, the Jph of the T-LDH/PbI2 approximately not changed. The stability of the T-LDH/PbI2 photoelectrode during the hydrogen evolution reaction was investigated in 0.3 M KOH through studying the variation of the Jph vs. the exposure time at constant − 0.9 V, Fig. 9B displayed the behavior of Jph in a very short period (10 s) to achieve nearly a stable value of 5.5 mA cm−2. The dramatic decrease in this time is caused by unnoticeable corrosion that takes place in the beginning of electrolyte reaction40,41.
For using the faraday laws of electrolysis, The amount of produced H2-during a time (t) may be estimated (Eq. 3)42:
Based on the Jph–t characteristic, Fig. 9B, the produced number of H2-moles per unit area was 107.53 mmol h−1 cm−2 for T-LDH/PbI2 NC.
The stability of TLDH/PbI2NC was further confirmed using the EDX analysis after ten runs at Fig. 10. The chemical composition of T-LDH/PbI2 did not change which confirms the stability of photocatalyst after many runs for H2 generation. But the oxygen content in the catalyst matrix was decreased from 12.55 to 10.92, which may be referred to as the generation of oxygen vacancies due to the applied potential in the presence of KOH electrolyte. Oxygen vacancies (OVs) are considered one of the defects formed in the semiconductors. These defects are generated by the removal of an oxygen atom from the catalyst matrix while it is still charged with extra electrons. OVs which diffuse at the interfaces layer in the LDH and form an interlayer of a different crystal phase due to their influence on the phase stability40.
The XRD analysis after ten runs can be considered as another proof of the stability of T-LDH/PbI2NC was shown at Fig. 1D. The structural properties of T-LDH/PbI2 are almost the same after many runs. The phase of the catalyst did not change which was attributed to the stability of photocatalyst after many runs for H2 generation. But the diffraction peak (003) disappears after ten runs for H2 generation which confirms the formation of oxygen vacancies. In these studies, the enhanced performance of PEC was systematically correlated with a higher density of OVs, which cause a higher Incident Photon to Current Efficiency (IPCE)40.
Effect of optical filters and calculation of conversion efficiencies
Different wave length filters (307–636 nm) were applied for determination of the most suitable wave length for photoelectrode in PEC system, was represented at Fig. 11A. The PEC behaviors in 0.3 M KOH (100 ml) solution at 25 °C and increment 1 mV/at different optical filters were studied. We noticed The alteration of the monochromatic light changes the Jph value. The behavior of the T-LDH/PbI2NC photoelectrode under the monochromatic illumination can be significant related to its absorbance response for different wave lengths light and its ability to absorb a large part of visible sunlight.
Effect of optical filters wavelength on Jph-voltage characteristics (A); IPCE% vs. wavelength in − 1 V (B), ABPE% vs. the applied voltage at different wavelengths (C), and color fill contours of the two ABPE% maximum values in the optimized voltage at different wavelengths utilizing T-LDH/PbI2 NC electrode in 0.3 M KOH (D, E).
Generally, IPCE (incident-photon-to-current conversion efficacy) and ABPE (applied bias-photon-to-current efficacy) are main factors for qualifying the PEC solar hydrogen generation. IPCE value was measured at different wave length filters for determining the actual number of charge-carriers that related to the generated photocurrent per incident photon. Using the power density (P (mW cm−2)) and the wavelength (λ (nm)) of the monochromatic light as shown Fig. 11B, the IPCE is given using Eq. (4)43
IPCE values in − 1 V in different wave lengths is presented in Fig. 11B. The maximum IPCE for T-LDH/PbI2 NC photoelectrode was ~ 83% for 307 nm. Another maximum of ~ 67% was observed at 508 nm. The positional wavelengths of the two maxima are matched well with the absorption edges observed in the optical analysis of T-LDH/PbI2 NC, Figs. 5 and 6.
However, ABPE value describes the photo-response efficiency of a T-LDH/PbI2 NC electrode under an applied voltage44,45. ABPE can be calculated using the following equation:
For T-LDH/PbI2 NC photoelectrode in 0.3 M KOH, the ABPE% is estimated vs. the Vapp at various wavelengths and presented in Fig. 11C. As shown in this figure, there are two maximum values of ABPE; 4.24% centered at − 0.58 V and 5.41% centered at − 0.97 V. This result may be due to the appearance of two band gaps and can be described based on the well-known photoelectric effect. This photoelectrode has a higher efficiency than the previously reported photoelectrodes46. The full 3D data of ABPE vs. the applied potentials and the incident wavelengths for the two maxima are presented in the color fill contours, Fig. 11D,E. The noticeable electrode response at lower potential can be advantageous for PEC cells.
Electrochemical impedance spectroscopy (EIS)
Charge carrier dynamics play a vital role in the photocatalytic water-splitting process in deciding the photocatalytic performance of photoelectrode. To investigate the charge carrier dynamics of the T-LDH/PbI2 NC electrode, EIS data have been measured by an electrochemical workstation (CHI660E) at room temperature. The photoelectrode was immersed in a 0.3 M KOH electrolyte and the EIS measurements were carried out under illumination at 0 V (vs Ag/AgCl) for a frequency range of 0.01–100,000 Hz. For this photoelectrode, the Nyquist plot is shown in Fig. 12A. This plot exhibited a semicircle at high frequencies due to charge transfer processes in electrode/electrolyte boundaries (charge transfer resistance) and two straight line segments observed at low frequencies with slopes ~ 44° and ~ 69° due to diffusion-controlled processes (Warburg impedance) and additional minimal capacitive activity (double-layer capacitance) as shown in the insets of Fig. 12A. That is to say, mixed diffusion and kinetic controlled routes are illustrated by the EIS data. The results obtained are fitted to a simple equivalent circuit in order to explain the EIS measurements through the hydrogen evolution process. Figure 12B inset displays the suggested Randle equivalent circuit for the simulation of EIS results using the ZSimpWin software (version 3.2; https://echem-software-zsimpwin.software.informer.com/3.2/). This circuit contains the electrolyte resistance (Rs = 22 Ω with Fitting error = 0.04965) that can be obtained from Nyquist plot intercept at high frequency, charge transfer resistance (Rct = 4.3 Ω) equals to the semicircle diameter in the Nyquist plot, double-layer capacitance (CdI = 1.472 μF) and Warburg impedance (W = 9.525 × 10–5). The reported Rs and Rct values are much smaller than any literature values for LDH-based electrodes, which promoting the PEC hydrogen production47,48,49.
Figure 12B,C presents Bode plots for the T-LDH/PbI2 NC electrode, measured at room temperature using 0.3 M KOH electrolyte at 0 V (vs Ag/AgCl). Figure 12B illustrates the total impedance (Z) vs. the frequency, whilst Fig. 12 displays the behavior of the phase vs. the logarithm of the frequency and shows a resistive regime related to the Rct at low frequency as well as capacitive contributions related to the Cdl of the electrode at high frequencies50. From Fig. 12C, the maximum phase shift (Өmax in degree), and the frequency at the maximum phase (fmax in Hz), are estimated to be 40.9° in 0.022 Hz. The lifetime of the charge carriers can be estimated from Fig. 12 via the relationship \({\tau }_{n}\) = 1/2π ƒmax50. The value of the obtained lifetime of the charge carriers for the T-LDH/PbI2 NC electrode is estimated to be 7.23 s. The obtained parameters indicate a great reduction in the charge recombination at the electrolyte/electrode interfaces. This also refers to a kinetically facile PEC system, improved ionic conductivity, and electrolytes diffusion through the T-LDH/PbI2 NC electrode. Therefore, this photoelectrode showed the highest photocatalytic performance to produce large amounts of H2 compared to the previously reported LDH-based electrodes.
Effect of applied temperature and calculation of thermodynamic parameters
The operating temperature is considered a vital parameter that can affect the photoelectrode performance. Figure 13A shows the influence of the applied temperature from 298 to 358 K on the performance of the T-LDH/PbI2 NC photoelectrode. The Jph increases with increasing the applied temperature to reach its maximum value (73.8 mA cm−2) at 253 K. Then, the hydrogen generation rate is increased sharply (1.5 fold) by increasing temperature. i.e., a high reaction temperature will improve dehydrogenation kinetics and release hydrogen at elevated temperature. This increase is due to the decline of the photoelectrode bandgap energy and the increase of the charge transfer rate. According to the Arrhenius plots50 (Fig. 13B), the apparent activation energy (Ea) of the HER using T-LDH/PbI2 NC photoelectrode was calculated to be 9.09 kJ mol−1. This value is lower than most of the previously reported values for other LDH-based catalysts51. Also, the other thermodynamic parameters such as enthalpy (ΔH*) and entropy (ΔS*) were estimated using the Eyring equation, Fig. 13C. The ΔH* of T-LDH/PbI2 NC is deliberated from the slope to be 12.99 kJ mol−1. While from the intercept, the ΔS* for T-LDH/PbI2 NC is 78.97 kJ mol−1. Table 2 illustrates the PEC performance of our T-LDH/PbI2 NC photoelectrode comparing with the previously studied LDH-based PEC catalysts52,53,54,55,56,57,58. As shown in this table, the presented PEC parameters are much higher than the reported performances for the LDH-based catalysts in literatures. As an example, the IPCE% of Ni–Fe LDH/ZnO nanostructures was 82% at 380 nm52. Also, ABPE% was 1.24% at 0.62 V for BiVO4/CdS/NiCo-LDH56. Moreover, Jph of 2.72 mA cm−2/1.23 V was reported for BiVO4/CdS/NiCo-LDH57.
Finally, a simple hydrogen generation mechanism is illustrated in Fig. 14 and presented as follows. The incorporation of PbI2 nano-semiconductor with a second nano-semiconductor of lower bandgap (Co–Cd–Fe LDH) form a promising photocatalyst to harvest visible light. Some photocatalytic composites can enhance the PEC properties because of the overlapping between the band gaps of two different photocatalysts, which could favor the charge carrier transfer and separation. During applying the external potential bias, photons excite electrons and holes’ separation. The excited electrons migrate from the valence-band (VB) to the conduction-band (CB) of LDH. Then they are transferred to the PbI2 catalyst. After that, the transferred electron reacts with the adsorbed H+ ion-producing H2 molecule. Simultaneously, the residual holes are combined with and removed by the sacrificial reagents of the KOH solution, whereas PbI2 avoids the e–h+ recombination52. Finally, PbI2 facilities the transfer of the additional CB electrons to Pt and hurries the generation of H2 at the active Pt surfaces as shown in Fig. 14.
Experimental details
Preparation of photocatalysts
Preparation of PbI2 electrode
Precipitate PbI2 by dissolving both lead (II) nitrate and potassium iodide in distilled water then add a few drops of potassium iodide to the lead (II). As soon as the solutions touch, bright yellow lead iodide is produced. Finally, add the rest of the potassium iodide solution to the lead (II) nitrate. A bright yellow precipitate (PbI2) is produced from two clear solutions. PbI2 crystals were synthesized by taking the flask containing the PbI2 and heat it to near boiling until all of the yellow crystals dissolved and the solution is clear. Then, cool it to room temperature by placing the flask in the lab fridge overnight. After that, Filter is used to isolate the PbI2. Carefully remove the tiny crystals on the filter paper to obtain a very beautiful sheet of golden-colored PbI2.
Preparation of Co–Cd–Fe LDH
NaOH (5 M) was dissolved in 200 mL of distilled water. Another 200 mL aqueous solution of Fe(NO3)3·9H2O (0.1 M), Co (NO3)2·6H2O (0.1 M), and Cd (NO3)2·4H2O (0.1 M) was prepared. This later solution was stirred for 24 h. A pH 10 of the reaction is adjusted by using sodium hydroxide solution. At pH 10, the solution was divided into two solutions; one of them is stirred for 24 h and the second one is put in the autoclave for 3 h. A washing process using DI water is carried out for the resulting precipitate to reduce the pH to 7. Finally, the product is dried at 80 °C for one day.
Fabrication of T-LDH/PbI2 NC
In a general synthesis technique, in-situ growth of the metal cations, typically, NaOH (5 M) in 200 ml of distilled H2O is prepared. Another solution of Fe (NO3)3·9H2O (0.1 M), Co (NO3)21·6H2O (0.1 M), Cd (NO3)2·4H2O (0.1 M), and 2.5 g PbI2 was prepared. This later solution was stirred for 24 h. A pH 10 of the reaction is adjusted by using the sodium hydroxide solution. After reaching pH 10, the solution was remained under continuous stirring for 24 h. A washing process using DI water is carried out for the resulting precipitate to reduce the pH to 7. After washing, a drying process is carried out at 80 °C for one day.
Fabrication of the PEC photoelectrodes
Three different photoelectrodes are fabricated to be used for the PEC hydrogen production. 3% of each photocatalyst (PbI2, Co–Cd–Fe LDH, and T-LDH/PbI2 NC) is mixed with 3% of C6H4 (CO2C4H9)2 plasticizer (DBP, 99.8%) and 3% of (C2H3Cl)n (PVC, 99.8%) in the least amount of (CH2)4O (THF, 99.9%). DBP, PVC, and THF were obtained from the Egyptian Middle East company. The three products of the mixing process are moved to three 5 cm-Petri dishes. The mass of each batch is 0.35 g. Then, the three Petri dishes are left to dry and sealed off with three filter papers. By fixing the amount of THF and carrying out the drying process for one day, the thickness of each photoelectrode is fixed to be 200 μm.
Characterization of different photocatalysts
The XRD patterns of PbI2, Co–Cd–Fe LDH, and T-LDH/PbI2 NC were obtained by Philips X’Pert1-MRD X-ray diffraction (λCuKa = 0.15418 nm). Samples morphology is investigated using a field-emission scanning electron microscope (FESEM,HRTEM, Zeiss SUPRA/55VP with GEMINI/column). (Fourier Transform Infrared Spectroscopy (FTIR) was performed by A Shimadzu-FTIR-340-Jasco spectrometer to obtain the important functional groups of the samples. Finally, the optical absorbance behaviors of the products are investigated by Lambda 900-UV/Vis/IR Perkin Elmer spectrophotometer up to 1200 nm.
Conclusion
In summary, a novel technique for loading Cd–Co–Fe-LDH/PbI2 has been introduced to fabricate an efficient nanocomposite photocatalyst. For comparison, the different properties of PbI2, Co–Cd–Fe LDH, and T-LDH/PbI2 NC were investigated using various instruments; XRD, FTIR, HR-TEM, FE-SEM, and UV–Vis–IR spectrophotometer. The growth of LDH on PbI2 prevents the agglomeration of LDH nanoparticles and allows the distribution of the particles to increase the surface area and decrease the particle size. Loading of LDH narrows the bandgap of PbI2 from 3.04 to 2.53 eV for T-LDH/PbI2 NC, which prolongs the lifetime of the photo-induced electrons. Consequently, the application of T-LDH/PbI2 NC improves the PEC H2 production rate to reach 107.53 mmol h−1 cm−2 and IPCE% to reach 83.8% in 307 nm and 67.3% in 508 nm. The ABPE% reach its maximum value (4.24%) at − 0.58 V and (5.41%) at − 0.97 V. To the best of our knowledge, the performance of T-LDH@PbI2 NC as a PEC catalyst is higher than any previously reported LDH-based photocatalysts. The effects of the operating temperature and monochromatic illumination on the PEC performance were studied. Also, the electrochemical surface area, thermodynamic parameters, and Tafel slopes are calculated to label the hydrogen evolution mechanism. The T-LDH/PbI2 NC photoelectrode displayed lower Tafel slopes and a much higher electrochemical surface area compared to T-LDH and PbI2 electrodes. Moreover, the activation energy of T-LDH/PbI2 NC was 9.09 kJ mol−1, which was lower than any previously reported value for LDH catalysts. This study has provided a new viewpoint to design highly active photocatalysts for solar light-driven H2 production.
References
Zhu, Y. P., Guo, C., Zheng, Y. & Qiao, S. Z. Engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc. Chem. Res. 50, 915–923 (2017).
Luo, J. et al. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 345, 1593–1596 (2014).
Zhou, H. et al. Highly efficient hydrogen evolution from edge-oriented WS2(1–x)Se2x particles on three-dimensional porous NiSe2 foam. Nano Lett. 16, 7604–7609 (2016).
Li, J., Li, H., Zhan, G. & Zhang, L. Solar water splitting and nitrogen fixation with layered bismuth oxyhalides. Acc. Chem. Res. 50, 112–121 (2017).
Zhang, L. W., Fu, H. B. & Zhu, Y. F. Efficient TiO2 photocatalysts from surface hybridization of TiO2 particles with graphite like carbon. Adv. Funct. Mater. 18, 2180–2189 (2008).
Pirkarami, A., Rasouli, S. & Ghasemi, E. 3-D CdS@NiCo layered double hydroxide core-shell photoelectrocatalyst used for efficient overall water splitting. Appl. Catal. B. 241, 28–40 (2019).
Shi, R. et al. Interstitial P-doped CdS with long-lived photogenerated electrons for photocatalytic water splitting without sacrificial agents. Adv. Mater. 30, 1705941 (2018).
Sun, X. & Dey, S. K. Insights into the synthesis of layered double hydroxide (LDH) Formation mechanisms of LDH. J. Colloid Interface Sci. 458, 160–168 (2015).
Boppella, R., Choi, C. H., Moon, J. & Kim, D. H. Spatial charge separation on strongly coupled 2D-hybrid of rGO/La2Ti2O7/NiFe-LDH heterostructures for highly efficient noble metal free photocatalytic hydrogen generation. Appl. Catal. B. 239, 178–186 (2018).
Luo, B., Song, R. & Jing, D. ZnCr LDH nanosheets modified graphitic carbon nitride for enhanced photocatalytic hydrogen production. Int. J. Hydrogen Energy. 42, 23427–23436 (2017).
Cai, P., Ci, S., Wu, N., Hong, Y. & Wen, Z. GaN/Al0.1Ga0.9N‐based visible-blind double heterojunction phototransistor with a collector-up structure. Physica Status Solidi A Appl. Res. 214, 1600910 (2017).
Tonda, S., Kumar, S., Bhardwaj, M., Yadav, P. & Ogale, S. g-C3N4/NiAl-LDH 2D/2D hybrid heterojunction for high-performance photocatalytic reduction of CO2 into renewable fuels. ACS Appl. Mater. Interfaces. 10, 2667–2678 (2018).
Zhao, Y. et al. Highly dispersed TiO6 units in a layer double hydroxide for water splitting. Chem. Eur. J. 18, 11949–11958 (2012).
Silva, C. G., Bouizi, Y., Fornés, V. & García, H. Layered double hydroxides as highly efficient photocatalysts for visible light oxygen generation from water. J. Am. Chem. Soc. 131, 13833–13839 (2009).
Du, X. et al. Design of modular catalysts derived from NiMgAl-LDH@m-SiO2 with dual confinement effects for dry reforming of methane. Chem. Commun. 49, 6770–6772 (2013).
Nayak, S., Mohapatra, L. & Parida, K. Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction. J. Mater. Chem. A 3, 18622–18635 (2015).
Teramura, K. et al. Photocatalytic conversion of CO2 in water over layered double hydroxides. Angew. Chemie Int. 51, 8008–8011 (2012).
Alberius, P. C. A. et al. Enhanced mesostructural order and changes to optical and electrochemical properties induced by the addition of cerium(III) to mesoporous titania thin films. Chem. Mater. 14, 3284–3294 (2002).
Hwang, D. W., Kim, J., Park, T. J. & Lee, J. S. Mg-doped WO3 as a novel photocatalyst for visible light-induced water splitting. Catal. Lett. 80, 53–57 (2002).
Mohapatra, L., Patra, D., Parida, K. & Zaidi, S. J. Enhanced photocatalytic activity of a molybdate-intercalated iron-based layered double hydroxide. Eur. J. Inorg. Chem. 3, 723–733 (2016).
Hall, D. S., Lockwood, D. J., Bock, C. & MacDougall, B. R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. Math. Phys. Eng. Sci. 471, 20140792–20140792 (2015).
Lu, H., Zhu, Z., Zhang, H., Zhu, J. & Qiu, Y. Simultaneous removal of arsenate and antimonate in simulated and practical water samples by adsorption onto Zn/Fe layered double hydroxide. Chem. Eng. J. 276, 365–375 (2015).
Song, H. B. et al. Lead iodide nanosheets for piezoelectric energy conversion and strain sensing. Nano Energy 49, 7–13 (2018).
Mohamed, F., Mostafa, R. A. & Shaban, M. Removal of safranin dye from water using polypyrrole nanofiber/Zn–Fe layered double hydroxide nanocomposite (Ppy NF/Zn–Fe LDH) of enhanced adsorption and photocatalytic properties. Sci. Total Environ. 640–641, 352–363 (2018).
Shaban, M., Mohamed, F. & Abdallah, S. Production and characterization of superhydrophobic and antibacterial coated fabrics utilizing ZnO nanocatalyst. Sci. Rep. 8, 3925 (2018).
Parida, K. M. & Mohapatra, L. Carbonate intercalated Zn/Fe layered double hydroxide: A novel photocatalyst for the enhanced photodegradation of azo dyes. Chem. Eng. J. 179, 131–139 (2012).
Aviyarasu, K., Sajan, D., Selvakumar, M. S., Thomas, S. A. & Anand, D. P. A facile hydrothermal route to synthesize novel PbI2 nanorods. J. Phys. Chem. Solids. 3(11), 71396–71400 (2012).
Zhengwe, J. Preparation of PbI27PbBr 27KCl ternary heavy-metal halide glasses. J. Mater. Sci. Lett. 16, 1656–1657 (1997).
Li, Y., Zhang, L., Xiang, X., Yan, D. & Li, F. Engineering of ZnCo-layered double hydroxide nanowalls toward high-efficiency electrochemical water oxidation. J. Mater. Chem. A. 2, 13250–13258 (2014).
Chen, C., Yu, W., Liu, T., Cao, S. & Tsang, Y. Graphene oxide/WS2/Mg-doped ZnO nanocomposites for solar-light catalytic and anti-bacterial applications. Sol. Energy. Mat. Sol. C 160, 43–53 (2017).
Ko, H., Yang, G., Wang, M. & Zhao, X. Isothermal crystallization kinetics and effect of crystallinity on the optical properties of nanosized CeO2 powder. Ceram. Int. 40, 6663–6667 (2014).
Wang, J. et al. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces. 4, 4024–4030 (2012).
Chai, B., Peng, T., Zhang, X. & Zhang, X. Synthesis of C60-decorated SWCNTs (C60-d-CNTs) and its TiO2-based nanocomposite with enhanced photocatalytic activity for hydrogen production. Dalton Trans. 42(10), 3402–3409 (2013).
Ai, G., Mo, R., Li, H. & Zhong, J. Cobalt phosphate modified TiO2 nanowire arrays as co-catalysts for solar water splitting. Nanoscale 7, 6722–6728 (2015).
Huang, Y. et al. Promoting charge carrier utilization by integrating layered double hydroxide nanosheet arrays with porous BiVO4 photoanode for efficient photoelectrochemical water splitting. ACS Sustain. Chem. Eng. 8, 4076–4084 (2020).
Shaban, M., Kholidy, I., Ahmed, G. M., Negem, M. & Abd El-Salam, H. M. Cyclic voltammetry growth and characterization of Sn–Ag alloys of different nanomorphologies and compositions for efficient hydrogen evolution in alkaline solutions. RSC. Adv. 9(39), 22389–22400 (2019).
Özgür, Ü. et al. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 98, 041301 (2005).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications 2nd edn. (Wiley-Interscience, 2001).
Shao, M.-H., Odell, J. H., Choi, S. & Xia, Y. Electrochemical surface area measurements of platinum- and palladium-based nanoparticles. Electrochem. Commun. 31, 46–48 (2013).
Fernández-Climent, R., Giménez, S. & García-Tecedor, M. The role of oxygen vacancies in water splitting photoanodes. Sustain. Energy Fuels 4, 5916 (2020).
Liu, Y. et al. Cactus-like hierarchical nanorod-nanosheet mixed dimensional photoanode for efficient and stable water splitting. Nano Energy 35, 189–198 (2017).
Laidler, K. J. Chemical Kinetics (Benjamin-Cummings, 1997).
Guo, X. Z. Study on the effect of measuring methods on incident photon-to-electron conversion efficiency of dye-sensitized solar cells by home-made setup. Rev. Sci. Instrum. 81(10), 103106 (2010).
Zhang, J. et al. Nanostructured WO3 photoanodes for efficient water splitting via anodisation in citric acid. RSC Adv. 7, 35221–35227 (2017).
Kim, T. W. & Choi, K. S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 28, 343 (2014).
Arrhenius, S. A. Über die Dissociationswärme und den Einfluß der Temperature auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 96, 116 (1889).
Tedim, J., Zheludkevich, M. L., Salak, A. N., Lisenkov, A. & Ferreira, M. G. S. Nanostructured LDH-container layer with active protection functionality. J. Mater. Chem. 21, 15464–15470 (2011).
Wang, J., Zhang, Q. & Zhang, K. Nonferrous Nanomaterials & Composites for Energy Storage and Conversion (Frontiers Media SA, 2019).
Tian, H. et al. Hierarchical (0 0 1) facet anatase/rutile TiO2 heterojunction photoanode with enhanced photoelectrocatalytic performance. Electrochim. Acta 96, 199–205 (2013).
Zhou, Y. H., Wang, S., Zhang, Z. & Gu, J. Hollow NiCo layered double hydroxide supported Pd catalysts for superior hydrogen evolution activity for hydrolysis of ammonia borane. ChemCatChem 10(15), 3206–3213 (2018).
Breckenridge, R. G. & Hosler, W. R. Electrical properties of titanium dioxide semiconductors. Phys. Rev. 91(4), 793 (1953).
Mustafa, E. et al. Efficient Ni–Fe layered double hydroxides/ZnO nanostructures for photochemical water splitting. J. Solid State Chem. 273, 186–191 (2019).
Zheng, S. et al. An inexpensive co-intercalated layered double hydroxide composite with electron donor-acceptor character for photoelectrochemical water splitting. Sci. Rep. 5, 12170 (2015).
Zhang, G. et al. Highly efficient photocatalytic hydrogen generation by incorporating CdS into ZnCr-layered double hydroxide interlayer. RSC Adv. 5, 5823 (2015).
Jian, B. J. et al. The Co Mo-LDH ultrathin nanosheet as a highly active and bifunctional electrocatalyst for overall water splitting. Inorg. Chem. Front. 5, 2964–2970 (2018).
Zhang, X. et al. Enhancing photoelectrochemical water oxidation efficiency of BiVO4 photoanodes by a hybrid structure of layered double hydroxide and graphene. Ind. Eng. Chem. Res. 56(38), 10711–10719 (2017).
Shouli, B. et al. Photoanode of LDH catalyst decorated semiconductor heterojunction of BiVO4/CdS to enhance PEC water splitting efficiency. Int. J. Hydrogen Energy. 45, 24642–24652 (2019).
Zhu, Y. et al. Interface engineering of 3D BiVO4/Fe-based layered double hydroxide core/shell nanostructures for boosting photoelectrochemical water oxidation. J. Mater. Chem. A. 5, 9952–9959 (2017).
Author information
Authors and Affiliations
Contributions
F.M.: conceptualization, methodology, formal analysis, writing manuscript. N.-B.: formal analysis, methodology. M.S.: Conceptualization, Methodology, formal analysis, conceptualization, supervision, writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, F., Bhnsawy, N. & Shaban, M. Reusability and stability of a novel ternary (Co–Cd–Fe)-LDH/PbI2 photoelectrocatalytst for solar hydrogen production. Sci Rep 11, 5618 (2021). https://doi.org/10.1038/s41598-021-85005-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85005-y
This article is cited by
-
Preparation and evaluation of the antimicrobial activity of sodium alginate-grafted diphenylamine embedded with silver nanoparticles
Polymer Bulletin (2023)
-
Mechanism of action and toxicological evaluation of engineered layered double hydroxide nanomaterials in Biomphalaria alexandrina snails
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.