Abstract
Early and accurate diagnosis is critical in reducing the morbidity and mortality associated with malaria. Microscopy (MI) is the current diagnostic gold standard in the field; however, it requires expert personnel, is time-consuming, and has limited sensitivity. Although rapid diagnostic tests for antigen detection (RDTs) are an alternative to diagnosis, they also have limited sensitivity and produce false positive results in detecting recent past infection. The automated hematology analyzer XN-31 prototype (XN-31p) (Sysmex Corporation, Kobe, Japan) is able to identify plasmodium-infected erythrocytes, count parasitemia and perform complete blood-cell counts within one minute. The performance of the XN-31p in diagnosing malaria was evaluated and compared with real-time polymerase chain reaction (qPCR), MI and RDT in an endemic area of Colombia where Plasmodium falciparum and Plasmodium vivax are present. Acute febrile patients were enrolled from July 2018 to April 2019 in Quibdó, Colombia. Malaria diagnoses were obtained from MI and RDT in the field and later confirmed by qPCR. Venous blood samples in EDTA were processed with an XN-31p in the field. Sensitivity, specificity, positive/negative predictive values, and the likelihood ratios of positive and negative tests were calculated with respect to the results from qPCR, MI and RDT. The intraclass correlation coefficient (ICC) and Bland–Altman plot were used to evaluate the concordance in the parasitemia with respect to MI. A total of 1,754 subjects were enrolled. The mean age was 27.0 years (IQR 14–44); 89.6% were Afro-Colombians, 94.3% lived in urban areas and 0.91% were pregnant. With respect to qPCR, the XN-31p showed a sensitivity of 90% (95% CI 87.24–92.34) and a specificity of 99.83% (95% CI 99.38–99.98) in detecting Plasmodium spp.; both parameters were equivalent to those for MI and RDT. Using MI as the reference, the XN-31p showed a sensitivity of 98.09% (95% CI 96.51–99.08), a specificity of 99.83% (95% CI 99.4–99.98), an ICC of 0.85 (95% CI 0.83–0.87) and an average difference of − 3096 parasites/µL when compared with thick-smear MI and an ICC of 0.98 (95% CI 0.97–0.98) and an average difference of − 0.0013% when compared with thin-smear MI. The XN-31p offers a rapid and accurate alternative method for diagnosing malaria in clinical laboratories in areas where P. falciparum and P. vivax cocirculate.
Similar content being viewed by others
Introduction
Malaria infection remains a significant public health problem worldwide. In 2018, an estimated 228 million cases of malaria infection and 405,000 associated deaths were reported. Africa has the largest burden of malaria morbidity (93%), followed by South-East Asia (3.4%) and the Eastern Mediterranean region (2.1%). Five species of the genus Plasmodium cause human infection: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae and the zoonotic parasite Plasmodium knowlesi. The most prevalent and fatal species worldwide is P. falciparum, accounting for 99.7% of estimated malaria cases in Africa. Globally, P. vivax causes 3.3% of estimated cases, with 53% of the P. vivax burden being in the South-East Asia region. P. vivax is the predominant parasite in the Americas, causing 75% of malaria cases1.
Although there has been a decrease in malaria morbidity and mortality rates since 2010, significant efforts are needed to approach the WHO global targets by 20302. Early and accurate diagnosis is one of the most important strategies for malaria control and elimination; therefore, the WHO recommends that all patients with suspected malaria have their diagnoses confirmed by microscopy (MI) or a rapid diagnostic test (RDT) before treatment2.
Malaria diagnosis is performed routinely by morphologic identification of the parasite through MI, which is considered the gold standard test for diagnosis in clinical settings. This diagnostic method is low cost, allows quantification of parasitemia, is useful during follow-up of parasitemia clearance and allows the possible identification of other pathogens. However, MI is time consuming, requests trained personal and has an operator-dependent limit of detection, as expert microscopists can identify up to 10–50 parasites/μL, while nonexperts have detection levels in the range of 100–500 parasites/μL3.
Alternatives to MI include rapid diagnostic tests (RDTs), which are point-of-care tests that can be performed quickly, are easy to use and do not require trained personnel. Although RDTs have improved access to malaria diagnoses outside health facilities, they have important limitations, such as the inability to quantify parasitemia and differentiate parasite stages and a high limit of detection (100–200 parasites/μL)3,4. In addition, HRP2-based RDTs are not useful for following up patients on treatment, since this antigen can circulate for weeks after parasite clearance5.
Molecular methods such as nucleic acid amplification techniques (NAATs), including polymerase chain reaction (PCR), provide superior sensitivity over other methods, with reported detection thresholds < 5 parasites/μL and the highest specificity6,7,8. Parasite quantification is possible, but it is not available in clinical setting. They present disadvantages for implementation in the field since they are expensive and time-consuming, require trained personnel, yield results that have little use during follow-up after treatment, and do not allow differentiation of parasite stages. However, PCR is very useful in reference laboratories3.
The search for alternative diagnostic methods for the detection and quantification of Plasmodium infections is ongoing. Automated hematology analyzers can offer fast, sensitive and cost-effective assessments of all suspected malaria infections, as they reduce analytical time and improve accuracy9,10,11. The automated hematology analyzer XN-31 prototype (XN-31p) (Sysmex, Japan) is a novel analyzer model that has been optimized to detect Plasmodium-infected red blood cells (iRBCs) circulating in human blood using a combination of optical fluorescence techniques, flow cytometry and laser-optical recognition by a violet semiconductor 405 nm laser beam. In this study, an XN-31p was used that can perform a complete blood count (CBC) and classify different blood cell populations, as well as identify iRBCs and count parasitemia within one minute with a limit of quantification of 20 iRBCs/μL10.
While the XN-31p was designed for in vitro diagnosis use, the XN-30, which had been developed for research use, had been previously evaluated in several studies. Both analyzers have the same physical characteristics, hardware configuration, and reagent composition12.
Tougan and colleagues reported the use of the XN-30 in recognizing P. falciparum parasites cultured in vitro, which permitted the identification of parasite stages and the sensitive and reproducible calculation of parasitemia13. Later, the XN-30 was applied in the screening new antiplasmodial compounds14. In addition, the XN-30 system permitted the simultaneous measurement of iRBCs in mouse blood samples infected with rodent malarial parasites and hematological evaluation15.
Two previous studies evaluated the performance of the XN-30 in a clinical setting10,11. Pillay and colleagues validated the parameters of the XN-30, such as the limit of blank (LoB), limit of detection (LoD), limit of quantitation (LoQ), carryover, precision and stability; in addition, they used 191 samples with malaria diagnoses previously confirmed by MI and RDT to assess the identification of iRBCs by the XN-3011. Post and colleagues evaluated the performance of the XN-30 in diagnosing malaria and compared it to the performance of MI and qPCR in 908 clinical samples from an endemic region of Burkina Faso, which is hyperendemic for P. falciparum; the sensitivity and specificity of XN-30 were, respectively, 98.7% and 99.4% relative to MI and 70.9% and 99.6% relative to qPCR10. Previous studies have shown the potential application of the XN-30 for malaria diagnosis in the clinical laboratories of endemic regions; however, no evaluation has been performed for the identification of Plasmodium species by the XN-30 in a clinical setting with cocirculating P. falciparum and P. vivax. In addition, the concordance in the parasite count with respect to MI has not been evaluated.
The primary aim of this study was to evaluate the performance of the XN-31p in diagnosing malaria using venous blood and compare it with that of qPCR, MI and RDT in an endemic region of Colombia. Additionally, a pilot study was performed to evaluate the concordance in the qualitative and quantitative detection of Plasmodium infection between the XN-31p with finger-prick blood and with venous blood.
Methods
Study design
An observational cross-sectional study of diagnostic accuracy was carried out to evaluate the performance of the XN-31p in the detection of Plasmodium infections in a clinical setting. Sociodemographic and clinical data were collected from each subject before conducting the diagnostic test (XN-31p, MI, RDT). Samples were tested by the XN-31p, RDT and MI simultaneously at a field site, and a reference standard test (qPCR) was performed later in the reference laboratory at the University of Antioquia, Medellin city. The Standards for Reporting Diagnostic Accuracy Studies (STARD) guidelines were used in this study16.
Study site
The study was conducted in the municipality of Quibdó, Chocó, Colombia (5.6956° N, 76.6498° W), which is located in the Pacific coast region (Supplementary Fig. 1). Malaria transmission is present in the rural and periurban settings in Chocó, and the main economic activity is gold, silver, and platinum mining. Chocó accounted for 27.3% of the total malaria cases in Colombia in 2018, with an annual parasite index (API) (number of cases/1,000 habitants) of 31.6 (high risk); uncomplicated malaria cases were attributed mainly to P. falciparum (57.5%), P. vivax (38.3%) and mixed infection (P. falciparum/P. vivax) (4.1%)17. Quibdó is the capital of Chocó state, and it reported 6.7% of all malaria cases in Colombia in 2018 from periurban areas18. The participants of this study were enrolled at the Local Hospital Ismael Roldán Valencia (HLIRV), which is the reference hospital for patients with suspected uncomplicated malaria from Quibdó and other nearby municipalities.
Sample size estimation
The sample size was estimated assuming an expected sensitivity and specificity for the XN-31p of 80% and 90%, respectively, relative to qPCR (gold standard test) for the diagnosis of malaria. The sample size was calculated assuming an expected malaria prevalence of 15% in patients from Quibdó with acute febrile illness, with a 95% confidence interval and 5% error, corresponding to 1,640 participants. A pilot study for evaluating the performance of the XN-31p with finger-prick samples included a convenience sample of 60 participants, 30 with Plasmodium infection (P. falciparum n = 15 and P. vivax n = 15) and 30 uninfected, according to the MI diagnosis made in the field. These subjects were selected among those enrolled in the framework for assessing the performance of the XN-31p using venous blood.
Participants
Participants with acute febrile syndrome were enrolled according to the following eligibility criteria: > 2 years old, body weight > 12 kg, fever at enrollment (axillary temperature > 37.5 °C) or history of fever 72 h prior to admission, less than 7 days of fever evolution, no other apparent localized cause of fever, and residence in any malaria transmission area. The participants were enrolled consecutively as they were admitted to the emergency and outpatient services of the HLIRV from July 2018 to April 2019.
Data and sample collection
All eligible participants were invited to participate and signed informed consent forms. At enrollment, a clinical evaluation was performed by a physician; information including demographic characteristics, current disease, history of malaria episodes, and use of preventive measures for malaria was recorded. A finger prick blood sample (20 µL) was obtained to determine the malaria diagnosis by MI. Venous blood samples (4 mL) were collected from each subject in EDTA tubes and analyzed by the XN-31p; the same sample was used for the malaria RDT test and analyzed by an XN-450 Sysmex analyzer, which had been approved for clinical use, to obtain subject hematological parameters. An aliquot of this sample (500 µL) was frozen in liquid nitrogen and sent to the reference laboratory at University of Antioquia in Medellín for later confirmation of the malaria diagnosis by qPCR (within the next month). When a participant was selected for the pilot study using finger-prick blood, an additional capillary sample (100 µL) was obtained using a high-blood volume lancet and microtubes containing EDTA.
Diagnostic test methods
XN-31 prototype
This study included an XN-31p automated hematology analyzer (Sysmex Corporation, Kobe, Japan). The EDTA venous blood samples were processed directly by the analyzer within 4 h after venipuncture. Briefly, 60 µL of the venous blood sample was used for analysis by the XN-31p in Low Malaria (LM) mode. The EDTA capillary sample (20 μL) was diluted with CELLPACK DCL reagent (120 μL) within 30 min after the finger prick and run by the analyzer immediately after dilution in Pre-Dilution (PD) mode.
The samples were aspirated and diluted automatically by the analyzer, and staining for nucleic acids was performed. Subsequently, infected red blood cells (iRBCs) and white blood cells (WBCs) were detected by a violet semiconductor 405 nm laser beam in the M channel. The data were automatically determined by analyzing the plotted scattergrams with forward-side scattered light and side fluorescent light, which allowed visualization of the iRBC population separate from the RBC population (Supplementary Fig. 2). The XN-31p output data separately report the following: 1) a complete blood count (CBC); 2) the presence of iRBCs: index test positive if “MI-RBC present” was displayed (iRBC ≥ LOQ; LoQ for LM mode: 20 iRBCs/μL, LoQ for PD mode: 40 iRBCs/μL), and index test negative or inconclusive otherwise (MI-RBC Abn Scattergram); 3) parasite count, both as a percentage of infected red blood cells (“MI-RBC%”) and absolute parasite density (“MI-RBC#”), expressed as %iRBC and iRBC/μL, respectively; and 4) Plasmodium species determined by flagging mechanisms using the M channel (applicable only for samples with MI-RBC# ≥ 100/μL): supplementally, it shows information on P. falciparum if the “Malaria?(P.f.)” flag is displayed, on other non-P. falciparum species if the “Malaria?(others)” flag is displayed, and on unclassified species if the “Malaria?(UNC)” flag is present. Mixed infections according to the index test were considered when both P. falciparum and other non-P. falciparum species were flagged simultaneously in a sample. All the results were automatically downloaded from the instrument as an Excel file that was exported and sent weekly to the data manager at the University of Antioquia.
Microscopy
Two thick blood films and one thin film were prepared from capillary samples from all participants and stained with Giemsa according to international guidelines19. A malaria microscopist read the thick- and thin-film slides at the field site to establish the malaria diagnosis. Later, a second expert malaria microscopist read all the slides at the reference laboratory in a blind manner. A third blind reader resolved any discrepancies (positive vs negative and differences in detected species). The microscopist in the field had 2 years of demonstrated expertise in malaria diagnosis and the two microscopists at the reference laboratory had 8 years and were classified as level expertise 1 according to WHO19.
A stained, thick blood smear was used to establish positive and negative judgment; the smear was considered negative if, after completing examination of 500 fields at 100 × magnification, no parasites (asexual or sexual) were found. A second thick smear was used as a backup for cases where there was any problem with staining or doubts with judgment. Mixed infection was determined according to WHO criteria20. For positive slides, the stained thin smear was used to confirm species diagnosis, and parasite density was calculated from both thick and thin films as previously described19. The thick films were used to count the number of asexual and sexual parasites per 500 leukocytes (when count was > 500 parasites/µL) or 1,500 leukocytes (when count was < 500 parasites/µL); parasite density was estimated using WBC count obtained by the XN-450 for each participant. The parasitemia from the thin film was calculated by establishing the percentage of infected red blood cells (iRBCs) among 10,000 counted erythrocytes. The parasitemia obtained by the second microscopist was used for comparison with the iRBC% and iRBC# calculated by the XN-31p.
Rapid diagnostic test (RDT)
Rapid diagnostic tests (SD BIOLINE Pf/Pv (HRP2/LDH), Ref 05KF80) were performed on EDTA-anticoagulated venous blood within 30 min after venipuncture, according to recommendations from the manufacturer. The RDTs were performed simultaneously with the sample processing by the Sysmex analyzers and before the MI. The test line reactivity of Plasmodium falciparum histidine-rich protein-2 (HRP2) and Plasmodium vivax parasite-lactate dehydrogenase (LDH) was scored. Any visible test line was considered positive, and the results were reported as P. falciparum, P. vivax, mixed infection (Pf/Pv) or negative.
Real-time polymerase chain reaction (qPCR)
Plasmodium genus detection: Molecular diagnosis was carried out in the reference laboratory in a blinded manner. DNA was extracted from a 160 µL aliquot of EDTA-venous blood after defrosting using a Qiagen QIAamp DNA Mini Kit (Catalog no 51106). An aliquot of 5 µL of DNA was used to amplify a region of the multicopy 18S rRNA gene from the genus Plasmodium by qPCR, as described by Rougemont et al., using Platinum Quantitative PCR superMix-UDG TaqMan Universal Master Mix (Invitrogen Thermo Scientific), primers and probes as previously reported21. Reactions were run on an Agilent AriaMx Real-Time PCR System. A positive result was determined for samples with a cycle threshold (Ct) value > 35.1, which corresponds to a parasitemia of 0.5 parasites/µL (limit of quantification (LoQ)). A negative result was reported when the Ct value > 39.5. Samples with Ct values ≥ 35.1 and ≤ 39.5 were classified with a final diagnosis of inconclusive; DNA was detected, but it was under the LoQ (very low parasitemia); qPCR genus detection was subsequently repeated for these samples to confirm the final classification. Qualitative results were reported since quantitative results do not represent the count of iRBCs.
Plasmodium species detection: All samples with a final diagnosis of positive and inconclusive were processed with P. falciparum and P. vivax species amplification protocols using two independent qPCR assays with primers described by Sing et al.22 and 5 × Hot FIREPol EvaGreen qPCR Supermix. Only samples that had not been amplified by P. falciparum or P. vivax protocols were additionally processed by qPCR for P. malariae using primers described by Sing et al.22 and detected by EvaGreen. If the Plasmodium species result persisted as inconclusive, nested PCR (nPCR) was carried out for P. ovale detection using a protocol reported by Sing et al.22. A positive result for P. falciparum, P. vivax or P. malariae species was defined according to the Ct, last relative fluorescence unit (ΔR last) and temperature melting (Tm) values, and a positive result for P. ovale was defined by the presence of bands on the agarose gel. Mixed infection was determined by the presence of two or more positive results from independent species amplification protocols. Samples with positive and inconclusive results from the genus protocol but negative results from the four species protocols were classified as Plasmodium spp.
Hematological parameters
The EDTA venous blood samples (25 μL) were processed by the automated hematology analyzer XN-450 (Sysmex Corporation, Kobe, Japan) in WB mode. The samples were processed within 4 h after venipuncture, with a maximum interval of 30 min for processing between the XN-31p and XN-450. The data for the parameters white blood cell count (WBC), red blood cell count (RBC), hemoglobin (Hb), hematocrit and platelet count (PLT) were collected from the XN-450 analyzer to describe the study participants. Anemia was defined as Hb < 11 g/dL, thrombocytopenia as PLT < 150*103/μL and severe thrombocytopenia as PLT < 50*103/μL23,24.
Data management and statistical analysis
Demographic, clinical, and epidemiological information was registered in a Microsoft Access 2010 database in the field. The database was sent weekly to the data manager at the University of Antioquia, who completed it with reference laboratory results (MI and qPCR) as well as results from the XN-31p.
Descriptive analysis was carried out for the participant characteristics. Independent analysis of the diagnostic accuracy of the XN-31p was performed for genus Plasmodium, P. falciparum and P. vivax species. For the last item, detection was assumed when the flagging mechanism for non-P. falciparum species was positive.
For the performance of the XN-31p (index test) with venous blood samples, sensitivity (SE), specificity (SP), positive/negative predictive values (PPV, NPV) and likelihood ratio of positive and negative tests (LRP and LRN) were calculated in reference to qPCR (gold standard test). In addition, the performance of the XN-31p was compared with that of MI and RDT because these are the currently available tests in the field. The McNemar test was applied to compare the SE and SP of the XN-31p, MI and RDT, all in reference to qPCR. Inconclusive results from genus qPCR were considered positive since these samples had Plasmodium DNA at very low concentrations (sometimes the result was not reproducible), and the strictest result was assumed to evaluate the performance of the analyzer. Independent analysis for genus detection was carried out assuming three scenarios for the MI-RBC Abn Scattergrams (index test inconclusive): positive, negative or excluded from analysis. The performance of the XN-31p for P. falciparum and P. vivax detection was assessed after excluding mixed infections from the results of the reference method. The intraclass correlation coefficient (ICC)25 and Bland–Altman plot26 were used to evaluate the concordance in the parasitemia between the XN-31p and MI among positive samples for both methods. The 95% confidence interval (95% CI) was estimated for each parameter.
For the performance of the XN-31p with finger-prick blood samples, SE, SP, PPV, NPV, LRP and LRN and their 95% CIs were calculated in reference to the qPCR results. Concordance in the malaria diagnosis and parasitemia between venous blood and finger-prick samples was evaluated by Cohen's kappa coefficient and the ICC/Bland–Altman plot method, respectively.
Descriptive analysis was carried out using IBM SPSS statistics program version 24 licensed by University of Antioquia, and performance analysis of the diagnostic tests was carried out using R version 3.6.3 and RStudio cloud programs with the following packages: epiR version 1.0–1427 for calculating SE, SP, PPV, NPV, LRP and LRN; DTComPair version 1.0.328 for comparing SE and SP with the McNemar test; blandr29 for generating the Bland–Altman plot; irr30 for calculating the intraclass coefficient; and psych31 for calculating Cohen's kappa coefficient.
Ethics approval and consent to participate
The study was reviewed and approved by the Facultad de Medicina Ethics Committee at the Universidad de Antioquia, Medellín, Colombia (Record 007; 11th May 2017), and Sysmex Corporation received approval from the Ethics Review Committee of the Japanese Association for the Promotion of State of the Art in Medicine (Approval date: 17th May 2017). This study complied with the principles stated in the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects), the Belmont report (respect for persons, beneficence, and justice) and resolution 8430/1993 of the Ministry of National Health of Colombia (Chapter I: Of the ethical aspects of research in human beings). Before starting any study procedure, written informed consent was obtained from all participants. For participants < 18 years old, additional informed consent from their parents or legal guardian was also obtained. All the methods were carried out in accordance with the approved guidelines. Recorded data from participants were anonymized using a code. Participants were diagnosed by microscopy and treated according to the malaria guidelines from the Health Ministry of Colombia. The results from the XN-31p were not used to manage patients since this was the exact instrument under evaluation in this study. The information obtained from the questionnaires and clinical evaluations was stored and guarded in the Malaria Group of the University of Antioquia in Medellín.
Results
Description of participants and malaria frequency
A total of 1,967 eligible participants were enrolled, and 1754 blood samples were analyzed by the XN-31p. No samples were excluded from analysis due to inadequate sample storage, sample mismatch, hemolysis or a shortage of sampled blood. Only one sample had missing MI data.
Baseline demographic and clinical characteristics and data on malaria history are presented in Table 1 according to inclusion or exclusion based on the results of the index test (XN-31p). Although the goal of this study was not to address the clinical aspects of the malaria patients, the data show that the majority had signs and symptoms of uncomplicated malaria. Based on the clinical evaluation, hemoglobin levels and platelet counts, the frequency of warning signs or severe malaria was less than 2.0%.
The results of the malaria diagnosis test for the enrolled febrile participants are presented in Table 2. The percentage of samples diagnosed with malaria according to MI was 30.3% (531/1754), with parasite densities ranging from 11 to 198,736 (median 3536; IQR 928–9984). A total of 33% (579/1754) of participants had a positive qPCR result, and submicroscopic infection (positive qPCR with negative MI) was detected in 2.7% (48/1754) of participants. All MI-positive samples were detected by qPCR. The RDT was positive for 29.2% (513/1754) of participants, and the XN-31p reported 29.4% (515/1754) positive samples, with parasite densities ranging from 22 to 291,303 (median 3508; IQR 886–10,613).
Diagnostic accuracy of the XN-31 prototype for iRBC detection
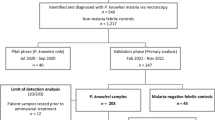
A total of 1,754 samples from febrile participants were included for the evaluation of the performance of the XN-31p with respect to the qPCR results in a clinical setting from a malaria-endemic region in Colombia (Fig. 1).
Flow chart of participants enrolled for the study of the diagnostic accuracy of the XN-31 prototype. €36 samples with Ct levels lower than the LoQ but with DNA detected. £2 samples with Ct levels lower than the LoQ but with DNA detected. ¥1 sample with Ct levels less than the LoQ but with DNA detected.
The percentage of inconclusive results from the XN-31p was 1.6% (28/1754); the demographic characteristics and history of malaria episodes of these participants were similar to those with valid results (positive or negative). However, some differences were observed among a number of hematological parameters (WBC, RBC, hematocrit and anemia) (Supplementary Table 1). Excluding those samples with inconclusive index tests, the percentage of false positive and false negative samples analyzed by the XN-31p was 0.12% (2/1726) and 3.3% (57/1726), respectively.
The parasite densities of the two false positive samples detected by the XN-31p were 30 and 40 parasites/µL. Here, the XN-31p detected iRBCs, but the flag mechanisms for detecting species were not indicated; in addition, both samples had high WBC counts (22.7*103/µL and 25.8*103/µL, respectively). On the other hand, 82.5% (47/57) of the false negative samples detected by the XN-31p had submicroscopic infections, and 76.6% (36/47) of them had a Ct level less than the LoQ from qPCR. The false-negative samples with microscopic infections (17.5%; 10/57) had parasite densities ranging from 16 to 261 parasites/µL; only three of these samples had parasitemia higher than the LoQ of the XN-31p (20 iRBCs/µL). In addition, the percentages by species among these samples were 57.9% (33/57) P. falciparum, 15.8% (9/57) P. vivax, 1.7% (1/57) mixed Pf/Pv and 24.6% (14/57) Plasmodium spp.
Table 3 presents the performance of the XN-31p in comparison with qPCR, MI and RDT for Plasmodium detection. The SE and SP of the XN-31p with respect to qPCR were 90.0% and 99.83%, respectively. The SE and SP of XN-31p with respect to MI were 98.09% and 99.83%, respectively, and similar results were found with respect to RDT.
Supplementary Table 2 shows the performance of MI and RDT with respect to qPCR, with SEs of, respectively, 91.71 and 87.39, and SPs of 100 and 99.4, which were similar to the SEs and SPs of the XN-31p. The performance results from the XN-31p were similar when samples with an inconclusive index test (MI-RBC Abn Scattergram) were considered positive or negative (data not shown).
Diagnostic accuracy of the XN-31 prototype for Plasmodium species detection
The performance of the XN-31p with flagging information for the detection of Plasmodium species was evaluated for the detection of P. falciparum and P. vivax, the most common species that cause malaria in humans. Table 4 shows the performance of the XN-31p with respect to MI, qPCR and RDT.
The SE and SP of the XN-31p with respect to qPCR in the detection of P. falciparum infections were 84.4 and 99.54, respectively. With respect to qPCR, the SE of MI and RDT for P. falciparum were higher than that of XN-31p (93.89% and 90.61%), while the SP was similar for the three methods (Supplementary Table 3). A total of 0.36% (6/1686) samples were falsely positive for P. falciparum, according to qPCR species detection, of which five had a P. vivax and one a Plasmodium spp. infection; all false positive samples were positive according to MI as well (four had a P. vivax, one had a P. falciparum and one had a mixed (Pf/Pv) infection). The false negative rate for P. falciparum was 3.6% (61/1,686); of these samples, 83.6% (51/61) had iRBCs with < 100/μl, and iRBCs were detected (> 20 iRBCs/µL) among only 45.9% (28/61).
The SE and SP of the XN-31p with respect to qPCR in the detection of P. vivax infections were 91.94 and 98.91, respectively. Both values were similar to those for MI (SE 92.31% and SP 100%), and the SE was better than that for RDT (85.94) in detecting P. vivax (Supplementary Table 3). A total of 1.01% (17/1,686) false positive samples were detected for P. vivax. According to qPCR species detection, all of them had a P. falciparum infection and were positive according to MI as well (16 had a P. falciparum and one a mixed Pf/Pv infection). The false negative rate for P. vivax was 0.59% (10/1686); all samples had iRBCs with < 100/μL, but iRBCs could not be detected among 90.0% (9/10) of the samples (< 20 iRBCs/µL).
Concordance in the parasitemia quantification in venous blood between the XN-31 prototype and MI
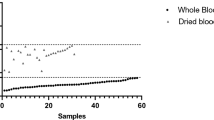
The concordance in the parasitemia between the XN-31p (MI-RBC#/µL) and the thick blood smear was good, with an ICC of 0.85 and an average difference of − 3096 parasites/µL. In addition, there was excellent concordance in the parasitemia between the thin blood smear and the XN-31p (MI-RBC%), with an ICC 0.98 and an average difference of − 0.0013% iRBCs (Table 5 and Fig. 2). Only 1.6% (8/512) and 2.5% (13/513) of samples fell outside of the concordance interval for comparison between thick and thin blood smears, respectively. These samples presented higher values for parasitemia and and lower hemoglobin levels than those within the concordance interval (data not shown). When samples outside of the concordance interval were excluded, the concordance of parasitemia between the XN-31p (MI-RBC#/µL) and the thick smears improved (ICC 0.942, 95% CI 0.931–0.951; average difference 2,084 parasites/µL, SD 3277 parasites/µL). In contrast, the comparison with the thin blood smear was relatively unchanged (ICC 0.983, 95% CI 0.980–0.986; average difference − 0.004%, SD 0.055%).
Bland–Altman plot for concordance in the parasitemia between the XN-31 prototype and microscopy. The figure shows the concordance in the parasitemia between the XN-31 prototype and microscopy from thick smears (parasites/µL) (n = 512) (a) and thin smears (iRBC%) (n = 513) (b), according to the Bland–Altman method. The difference in the parasitemia was calculated as (parasitemia according to the XN-31 prototype minus parasitemia according to MI).
Concordance in the quantification of the parasitemia by the XN-31 prototype between venous blood and finger prick samples
The results of the malaria diagnosis test for the capillary samples are presented in Table 6. Excluding samples with inconclusive index tests, the sensitivity and specificity of the XN-31p in Plasmodium detection from finger-prick samples with respect to qPCR were 90.91% (95% CI 75.67–98.08) and 100% (95% CI 86.28–100), respectively.
The concordance in the parasitemia between the XN-31p with finger-prick samples (PD mode) and with venous samples (LM mode) for Plasmodium detection was very good (kappa coefficient 0.97; 95% CI 0.90–1.00). On the other hand, the concordance in the parasitemia according to MI-RBC #/µL and MI-RBC% was excellent, with an ICC of 0.995 (95% CI 0.989–0.997), an average difference of 1,085.3 parasites/µL (SD 4,829.93 parasites/µL) an ICC of 0.957 (95% CI 0.913–0.980) and an average difference of − 0.05% (SD 0.15%), respectively (Supplementary Table 4 and Supplementary Fig. 3).
Discussion
Although Colombia is classified as a low and unstable malaria transmission region1,32,33, the area of Quibdó, where this study was conducted, reported a high risk of malaria in 2018 and 2019, with annual parasitic indexes (APIs) of 34.1 and 49.4 per 1000, respectively17,18. This condition could have favored the high percentage of malaria among our febrile participants (approximately 30%) and makes this study similar to others in high-transmission areas10,34. This study evaluated the diagnostic accuracy of the XN-31p in a prospective cohort of acute febrile patients who consulted a physician for medical care and were tested for malaria. This was an advantageous scenario for testing the performance of the XN-31p, since there was a chance to enroll patients with a wide range of clinical presentations. However, the clinical spectrum of participants in this study was ultimately very homogenous and typical of those with uncomplicated malaria, reflecting the expected percentage of severe malaria in Colombia of approximately 2%18.
The XN-31p showed good performance with respect to qPCR in diagnosing malaria (Plasmodium genus), comparable to that MI and RDT, conventional methods used in the field. Using a previously defined cutoff of 20 iRBCs/μL11, the SE and SP were high (90.0% and 99.8%, respectively), and the false positive rate was 0.12%, less than the limit of the WHO criteria for recommending the procurement of an RDT procurement (< 10%)35. The XN-31p requires mains electricity and is not portable; therefore, it is not considered a point of care test. However, it could be very useful for malaria screening among acute febrile individuals who seek care at health centers in malaria endemic areas, offering a malaria diagnosis and automated blood count simultaneously.
A false negative rate of 3.3% was found for the XN-31p, which may not be of concern for febrile individuals informed about potential warning signs and the need for serial diagnostic tests if their symptoms persist. On the other hand, in settings of active surveillance in low-transmission areas where low parasitemia is common (< 100 parasites/µL)36, the XN-31p may not be the best option for diagnosis, as a higher false positive rate is expected.
Approximately 2% of samples tested by the XN-31p had inconclusive results (MI-RBC Abn Scattergram); therefore, they would require another test. Hematological alterations in these participants could contribute to the difficulty of the analyzer in accurately classifying these samples. Pillay et al. 2019 reported a MI-RBC Abn Scattergram flag from an XN-31p in LM mode in 36% of samples with hematological alterations (low hemoglobin levels, low platelet counts, hemoglobinopathies and elevated reticulocyte counts)11. It is necessary to evaluate the performance of the XN-31p for participants with severe diseases to confirm the possible MI-RBC Abn Scattergrams caused by hematological alterations.
Current study employed expert microscopists to read all slides (Level 1)19, explaining the excellent performance of MI with respect to qPCR, in contrast to other studies37. It is possible that the performance of the XN-31p could be different than that reported here if compared with MI performed in field conditions.
When the results were evaluated by species, the performance of the XN-31p was variable; while the detection of P. vivax was equivalent to that of expert MI and RDT, P. falciparum detection was possible but with a lower performance than MI and RDT. The critical point for discriminating Plasmodium species based on the flagging mechanisms in this study was the level of parasitemia. The XN-31p can discriminate species when the parasitemia is higher than 100 iRBCs/µL; in the current study, the parasitemia for P. falciparum infections were lower than those for P. vivax infections. On the other hand, some P. vivax stages are larger in size than P. falciparum, which also favors the detection of P. vivax by the XN-31p. Additional effort is necessary to study the performance of the XN-31p with samples with ultralow parasitemia, mainly with P. falciparum infections. Moreover, these flagging mechanisms have not yet been fully validated for differentiating every species by the manufacturer due to a lack of data on rare species such as P. malarie, P. ovale or P. knowlesi. It is important to differentiate these species to determine appropriate treatment; therefore, further evaluation including these less frequent species should be performed to improve the species flagging mechanism.
The performance of the XN-31p in this epidemiological setting in Colombia is better than that reported by Post et al. 2019 in Burkina Faso (SE 90.% vs 70.9%)10, which may be because the Post et al. study was carried out in a setting with a higher percentage of complicated malaria (approximately 35%), a predominance of P. falciparum infections (99%) and a higher reporting of antimalarial uptake in the past two weeks; all conditions offer disadvantages for diagnosing malaria with a hematological analyzer.
The concordance in the parasitemia between the XN-31p and MI was very good, showing an important strength of the former because MI requires a longer reading time for the accurate estimation of parasitemia. It is important to consider that thin smear parasitemia estimates can be closer to XN-31p values since both methods count complete erythrocytes.
On the other hand, the XN-31p had malaria detection capabilities equivalent to those of qPCR and MI, even with finger-prick blood. At present, the XN-31p is intended to be used with venous blood, and it is important to accumulate positive results such as those obtained here to expand its use with finger-prick blood as well.
Limitations
The fact that there were virtually no severe malaria cases represented in our cohort constitutes a limitation of this study since the XN-31p results cannot be generalized to this group of patients. It was not possible to determine the performance of the XN-31p for mixed infections, as the percentage of coinfections was low in this study and the number of samples with this condition was insufficient to guarantee the accuracy of the results. The pilot study for evaluating the concordance in the malaria diagnosis between venous blood and finger-prick blood from the XN-31p included a limited number of samples, which were selected according to different levels of parasitemia; this could represent a potential source bias.
Conclusions
The XN-31p analyzer offers a rapid and accurate alternative method for malaria diagnosis in clinical laboratories located in endemic areas, although an economic evaluation study should be carried out. This analyzer is comparable to expert MI in the detection of Plasmodium infections and the quantification of parasitemia; in addition, it performs a complete blood-cell count and produces a results report in one minute. The XN-31p is able to discriminate parasite species; however, it shows some room for improvement in detecting P. falciparum. The performance of the XN-31p in the discrimination of coinfections needs to be evaluated in future studies.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- Hb:
-
Hemoglobin
- IQR:
-
Interquartile range
- iRBC:
-
Infected red blood cell
- LoQ:
-
Limit of quantification
- LRP:
-
Likelihood ratio of positive test
- LRN:
-
Likelihood ratio of negative test
- MI:
-
Microscopy
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- qPCR:
-
Real-time polymerase chain reaction
- RBC:
-
Red blood cell
- RDT:
-
Rapid diagnostic test
- SE:
-
Sensitivity
- SP:
-
Specificity
- WBC:
-
White blood cell
References
World Health Organization. World malaria report 2019. https://www.who.int/publications/i/item/9789241565721 (2019).
World Health Organization. Global Technical Strategy for Malaria 2016–2030. https://apps.who.int/iris/bitstream/handle/10665/176712/9789241564991_eng.pdf?sequence=1 (2015).
Mathison, B. A. & Pritt, B. S. Update on malaria diagnostics and test utilization. J Clin. Microbiol. https://doi.org/10.1128/JCM.02562-16 (2017).
Jimenez, A. et al. Analytical sensitivity of current best-in-class malaria rapid diagnostic tests. Malar. J. 16, 128. https://doi.org/10.1186/s12936-017-1780-5 (2017).
Plucinski, M. M. et al. Clearance dynamics of lactate dehydrogenase and aldolase following antimalarial treatment for Plasmodium falciparum infection. Parasit. Vectors 12, 293. https://doi.org/10.1186/s13071-019-3549-x (2019).
Zimmerman, P. A. & Howes, R. E. Malaria diagnosis for malaria elimination. Curr. Opin. Infect. Dis. 28, 446–454 (2015).
Hofmann, N. E. et al. Assessment of ultra-sensitive malaria diagnosis versus standard molecular diagnostics for malaria elimination: an in-depth molecular community cross-sectional study. Lancet Infect. Dis. 18, 1108–1116 (2018).
Wang, B. et al. Comparison of microscopy, nested-PCR, and real-time-PCR assays using high-throughput screening of pooled samples for diagnosis of malaria in asymptomatic carriers from areas of endemicity in Myanmar. J.Clin. Microbiol. 52, 1838–1845. https://doi.org/10.1128/JCM.03615-13 (2014).
Campuzano-Zuluaga, G., Hänscheid, T. & Grobusch, M. P. Automated haematology analysis to diagnose malaria. Malar. J. 30, 346. https://doi.org/10.1186/1475-2875-9-346 (2010).
Post, A. et al. The XN-30 hematology analyzer for rapid sensitive detection of malaria: a diagnostic accuracy study. BMC Med. 17, 103. https://doi.org/10.1186/s12916-019-1334-5 (2019).
Pillay, E., Khodaiji, S., Bezuidenhout, B. C., Litshie, M. & Coetzer, T. L. Evaluation of automated malaria diagnosis using the Sysmex XN-30 analyser in a clinical setting. Malar. J. 18, 15. https://doi.org/10.1186/s12936-019-2655-8 (2019).
Toya, Y., Tougan, T., Horii, T. & Uchihashi, K. Lysercell M enhances the detection of stage-specific Plasmodium-infected red blood cells in the automated hematology analyzer XN-31 prototype. Parasitol. Int. 80, 102206. https://doi.org/10.1016/j.parint.2020.102206 (2021).
Tougan, T. et al. An automated haematology analyzer XN-30 distinguishes developmental stages of falciparum malaria parasite cultured in vitro. Malar. J. 17, 59. https://doi.org/10.1186/s12936-018-2208-6 (2018).
Tougan, T., Toya, Y., Uchihashi, K. & Horii, T. Application of the automated haematology analyzer XN-30 for discovery and development of anti-malarial drugs. Malar. J. 18, 8. https://doi.org/10.1186/s12936-019-2642-0 (2019).
Tougan, T. et al. Application of the automated haematology analyzer XN-30 in an experimental rodent model of malaria. Malar. J. 17, 165. https://doi.org/10.1186/s12936-018-2313-6 (2018).
Bossuyt, P. M. et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 351, 5527. https://doi.org/10.1136/bmj.h5527 (2015).
Instituto Nacional de Salud. Boletin Epidemiologico Semanal. https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2018Boletínepidemiológicosemana52.pdf (2018).
Salas, D. Informe de evento malaria, Colombia, 2018. https://www.ins.gov.co/buscador-eventos/Informesdeevento/MALARIA_2018.pdf (2018).
World Health Organization & UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Microscopy for the detection, identification and quantification of malaria parasites on stained thick and thin blood films in research settings (version 1.0): procedure: methods manual. World Health Organization. https://apps.who.int/iris/handle/10665/163782 (2015).
Organización Mundial de la Salud. Diagnóstico microscópico del paludismo Bases del Parte I. Guía del alumno Segunda edición. https://apps.who.int/iris/bitstream/handle/10665/164468/9789243547824_spa.pdf;jsessionid=BA33B7B105A67E54725B12D6B3395147?sequence=1 (2014).
Rougemont, M. et al. Detection of four plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J. Clin. Microbiol. 42, 5636–5643 (2004).
Singh, B. et al. A genus-and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am. J. Trop. Med. Hyg. 60, 687–692 (1999).
Arévalo-Herrera, M. et al. Complicated malaria in children and adults from three settings of the Colombian Pacific Coast: a prospective study. PLoS ONE 12, e0185435. https://doi.org/10.1371/journal.pone.0185435 (2017).
Lopez-Perez, M. et al. Malaria-related anemia in patients from unstable transmission areas in Colombia. Am. J. Trop. Med. Hyg. 92, 294–301 (2015).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163 (2016).
Giavarina, D. Understanding bland altman analysis. Biochem. Medica 25, 141–151 (2015).
Stevenson, M. et al. epiR: Tools for the Analysis of Epidemiological Data. R package version 1.0–14. https://cran.r-project.org/web/packages/epiR/epiR.pdf (2020).
Stock, C. & Hielscher, T. DTComPair: comparison of binary diagnostic tests in a paired study design. R package version 1.0.3. http://CRAN.R-project.org/package=DTComPair (2014).
Datta, D. blandr: a Bland-Altman Method Comparison package for R. Zenodo. https://cran.r-project.org/web/packages/blandr/blandr.pdf (2017).
Gamer, M., Lemon, J., Fellows, I. & Singh, P. irr: Various Coefficients of Interrater Reliability and Agreement. R package version 0.84.1. https://cran.r-project.org/web/packages/irr/irr.pdf (2019).
Revelle, W. psych: Procedures for Personality and Psychological Research, Northwestern University, Evanston, Illinois, USA. https://cran.r-project.org/web/packages/psych/psych.pdf (2019).
Guerra, C. A. et al. The limits and intensity of plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 5, e38. https://doi.org/10.1371/journal.pmed.0050038 (2008).
Guerra, C. A. et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl. Trop. Dis. 4, e774. https://doi.org/10.1371/journal.pntd.0000774 (2010).
D’Acremont, V. et al. Beyond malaria-causes of fever in outpatient Tanzanian children. N. Engl. J. Med. 370, 809–817 (2014).
malERA Refresh Consultative Panel on Tools for Malaria Elimination T. malERA: an updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication. PLoS Med. 14, e1002455. https://doi.org/10.1371/journal.pmed.1002455 (2017).
Malaria Policy Advisory Committee Meeting. Meeting report of the WHO Evidence Review Group on Low-Density Malaria Infections. https://www.who.int/malaria/mpac/mpac-oct2017-erg-malaria-low-density-infections-session2.pdf?ua=1 (2017).
Berzosa, P. et al. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malar. J. 17, 333. https://doi.org/10.1186/s12936-018-2481-4 (2018).
Acknowledgments
This study would not have been possible without the willingness of the community of Quibdó (Chocó, Colombia) and the medical and administrative staff of the Local Hospital Ismael Roldán Valencia. We appreciate the hard work of Tomasa Santos, who participated in the enrollment of participants, and of Luisa Carbal, Andrés Holguin and Juan David Puerta, who carried out the DNA extraction and molecular diagnostic tests. We thank PhD. Associate Professor Daniel Camilo Aguirre-Acevedo at the University of Antioquia for advisory on the statistical analysis of the data.
Funding
Sysmex Corporation and University of Antioquia (Vice-Rectory of Research) funded the design of the study, field work, laboratory tests, data analysis and writing of the manuscript. LZI received support from the postdoctoral fellowship program Colciencias (Nro 811/2018).
Author information
Authors and Affiliations
Contributions
L.Z.I., A.T.C., T.L.M., and K.U. designed the study. L.Z.I., T.L.M., Y.T. and M.I. directed its implementation. L.Z.I. and T.L.M. led the field data collection activities, as well as laboratory test. V.S.C. and E.G. participated in field work activities. AR supported the study logistics for field work, participated in microscopy read and molecular diagnostic tests. L.Z.I. analyzed the data and prepared draft the manuscript. I.T. and Y.T. reviewed the data analysis. All authors contributed to the manuscript edit and revising. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors LZI, ATC, VS, EG, AR and TLM from University of Antioquia declare that they have no competing interest. KU, YT, MI and IT are employees of Sysmex Corporation. KU holds a patent for Lysercell M (Patent No. US2006-0223137 A1).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zuluaga-Idárraga, L., Rios, A., Sierra-Cifuentes, V. et al. Performance of the hematology analyzer XN-31 prototype in the detection of Plasmodium infections in an endemic region of Colombia. Sci Rep 11, 5268 (2021). https://doi.org/10.1038/s41598-021-84594-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84594-y
This article is cited by
-
Potential application of the haematology analyser XN-31 prototype for field malaria surveillance in Kenya
Malaria Journal (2022)
-
Clinical performance testing of the automated haematology analyzer XN-31 prototype using whole blood samples from patients with imported malaria in Japan
Malaria Journal (2022)
-
Fast detection and quantification of Plasmodium species infected erythrocytes in a non-endemic region by using the Sysmex XN-31 analyzer
Malaria Journal (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.