Abstract
Plasmodium knowlesi is the major cause of zoonotic malaria in Southeast Asia. Rapid and accurate diagnosis enables effective clinical management. A novel malaria diagnostic tool, Gazelle (Hemex Health, USA) detects haemozoin, a by-product of haem metabolism found in all Plasmodium infections. A pilot phase refined the Gazelle haemozoin identification algorithm, with the algorithm then tested against reference PCR in a larger cohort of patients with P. knowlesi mono-infections and febrile malaria-negative controls. Limit-of-detection analysis was conducted on a subset of P. knowlesi samples serially diluted with non-infected whole blood. The pilot phase of 40 P. knowlesi samples demonstrated 92.5% test sensitivity. P. knowlesi-infected patients (n = 203) and febrile controls (n = 44) were subsequently enrolled. Sensitivity and specificity of the Gazelle against reference PCR were 94.6% (95% CI 90.5–97.3%) and 100% (95% CI 92.0–100%) respectively. Positive and negative predictive values were 100% and 98.8%, respectively. In those tested before antimalarial treatment (n = 143), test sensitivity was 96.5% (95% CI 92.0–98.9%). Sensitivity for samples with ≤ 200 parasites/µL (n = 26) was 84.6% (95% CI 65.1–95.6%), with the lowest parasitaemia detected at 18/µL. Limit-of-detection (n = 20) was 33 parasites/µL (95% CI 16–65%). The Gazelle device has the potential for rapid, sensitive detection of P. knowlesi infections in endemic areas.
Similar content being viewed by others
Introduction
The public health implications of emerging Plasmodium knowlesi infections across most Southeast Asia countries1,2,3 is exemplified by the significant ongoing burden of zoonotic malaria in Malaysia, with 17,125 cases and 48 deaths since 20174 despite near-elimination of other human Plasmodium species5,6. Rapid and accurate detection remains a crucial pillar of effective clinical management for zoonotic and human-only malaria as recommended by the WHO in all endemic settings7. Delays in treatment for P. knowlesi infections can lead to the development of severe disease and increased risk of fatal complications8.
Microscopy is the current standard for malaria point-of-care (POC) diagnosis in most endemic countries in Southeast Asia but is unreliable for P. knowlesi due to misidentification with other Plasmodium species9,10. Microscopy also requires trained laboratory technicians and equipment, necessitating ongoing quality assurance11. In Malaysia, the implementation of routine polymerase chain reaction (PCR) in Plasmodium species confirmation for all malaria cases since 201212 has enabled accurate public health reporting for P. knowlesi13. Although demonstrating an increasing P. knowlesi case incidence, particularly in East Malaysia14, routine PCR is not feasible for immediate clinical management and requires expensive laboratory infrastructure and trained personnel.
The World Health Organization (WHO) Evidence Review group first recommended developing and improving POC P. knowlesi detection methods in 2017 in response to the increasing public health threat posed by zoonotic malaria transmission with an intractable monkey parasite reservoir15. One novel diagnostic approach utilizes rotating magneto-optical technology for the detection of haemozoin crystals, a paramagnetic by-product found in all malaria species16. The Gazelle™ (Hemex Health, USA) is a battery-operated device. It functions by detecting changes in light signal intensity as it passes through magnetically-induced reorientated haemozoin crystals found in Plasmodium-infected blood samples collected from a finger prick or venous sampling. Previous studies have demonstrated the efficacy of the Gazelle™ in detecting P. falciparum17 and P. vivax18 when compared to nested PCR as the reference standard19. In addition to reporting detection limits for Plasmodium species infections as low as 40 parasites per microliter of blood20, the Gazelle™ was able to detect haemozoin in malaria-infected whole blood across early and late asexual stages of the parasite life-cycle21. Existing haemozoin-based detection assays have not been designed or evaluated for P. knowlesi human infections.
In this study, the performance of the Gazelle™ in detecting clinical P. knowlesi infections compared to reference PCR was assessed in a setting endemic for P. knowlesi.
Methods
Study site and subjects
Malaria patients were enrolled between July 2020 and November 2021 at Ranau District Hospital in Sabah, Malaysia (Fig. 1). Ranau District Hospital is a secondary referral center servicing the administrative district (area of 3609 km2), including 16 primary health clinics, with all cases of malaria admitted for in-patient management according to national guidelines12. Patients presenting at the hospital study site were included if they were positive for P. knowlesi by microscopy, were aged > 1 year, and they or their guardian provided appropriate written informed consent. Appropriate written informed consent was obtained from febrile individuals aged more than 12 years with negative malaria microscopy at the same healthcare facility, prospectively enrolled as controls. Patient blood samples were collected, and their demographic and clinical information were recorded on standardized case record forms as part of an ongoing prospective observational malaria study. Severe knowlesi was defined using WHO 2014 research criteria22 for P. knowlesi, including hyperparasitaemia threshold of 100,000/μL, and jaundice defined as bilirubin > 50 μmol/L with parasite count > 20,000/μL and/or creatinine > 132 μmol/L23,24.
Ethical approval
The study was approved by the Medical Research and Ethics Committee of the Ministry of Health, Malaysia (NMRR-10-584-6684) and Menzies School of Health Research, Northern Territory, Australia (HREC 10-1431). Written informed consent was obtained from all participants and/or their legal guardians. All methods were performed in accordance with relevant guidelines and regulations.
Blood sample procedures
Venous whole blood samples were collected in ethylenediaminetetraacetic acid (EDTA) vacutainers prior to antimalarial treatment where possible. Thick and thin blood films were generated to verify parasite count by microscopy. Microscopic quantification of P. knowlesi parasitaemia was conducted by an experienced research microscopist equivalent to WHO Level 1 competency. The final parasite count per microlitre was calculated from the number of parasites counted to 200 white blood cells on a thick blood film, multiplied by the individual patient’s total white cell count25 obtained from routine hospital laboratory automated counts. The percentage of each parasite developmental stage26 was determined based on the number of early rings, late rings, mature trophozoites, schizonts, and gametocytes in 40 high-powered fields (HPF, × 1000 magnification) on thin blood films.
A 30 µL blood sample (as specified in the manufacturers’ instructions) was used to evaluate the Gazelle™17,27. The remaining volume of EDTA whole blood samples were stored at − 80 °C for subsequent malaria PCR species confirmation.
Plasmodium species confirmation by real-time PCR
Malaria PCR was conducted on all microscopically diagnosed malaria cases and microscopy-negative controls for the following Plasmodium species: P. knowlesi, P. falciparum, P. vivax, P. malariae and P. ovale spp. Genomic DNA was extracted from 200 μL of whole blood using QIAamp DNA Blood Mini Kits (Cat. No.: 51106; QIAGEN) according to the manufacturer’s manual, with a final elution volume of 200 μL. PCR was performed by laboratory research members blinded to the microscopy results. A real-time PCR assay, QuantiFast™ targeting the 18S SSU rRNA gene was conducted once for each sample as per the manufacturer’s and Sabah Public Health Laboratory protocols, using the Bio-Rad CFX96 Touch™ PCR machine (Bio-Rad, USA). QuantiFast™ real-time PCR was carried out over two separate reactions. The first was for Plasmodium genus screening, and if positive, was followed by subsequent P. knowlesi and other human Plasmodium species-specific detection28.
Study procedures
An initial pilot phase was conducted to refine the Gazelle™ detection algorithm for P. knowlesi. Whole blood samples of microscopy-identified positive P. knowlesi, which were later confirmed via PCR, were placed into separate test cartridges, consisting of 30 µL of whole blood sample and 65 µL of assay buffer, then loaded into the Gazelle™ device17,27. Test results were displayed on the device screen within a minute and systematically documented by a trained research staff member. Each sample was tested only once. In the event of an invalid test result, a new cartridge was prepared to repeat the test. Daily calibration of the Gazelle™ device was conducted prior to testing of clinical samples and comprised running a haemozoin positive control (active ingredients: Triton X-100 [assay buffer], propylene glycol, FD&C Red 40, citric acid, sodium benzoate [food dye] and concentrated artificial haemozoin solution), negative control (ingredients: assay buffer and dye), and an empty cartridge.
Pilot data from the Gazelle™ were uploaded to Hemex servers in real-time. Algorithm refinement was conducted at the completion of this initial study phase by reducing background noise, updating algorithm threshold values used for positive or negative detection, and optimizing the speciation algorithm for P. knowlesi against P. falciparum.
The refined detection algorithm was then tested on a subsequent larger prospective cohort of patients diagnosed with P. knowlesi malaria as well as malaria-negative febrile controls. A single empty cartridge was run daily as a blank sample negative control prior to testing clinical samples. Similar to the pilot phase, all patient samples were tested only once on the device.
The secondary study analysis evaluated the P. knowlesi parasite density limit of detection for the Gazelle™ device. Whole blood samples in EDTA from P. knowlesi infected patients with research microscopy parasite quantification were diluted using ABO blood-group matched (Lorne Laboratories Ltd., UK) whole blood from healthy donors at twofold serial dilutions. Sample testing was performed up to the dilution whereby two consecutive negative readings were obtained. The limit of detection was defined as parasite concentration of the dilution tested prior to consecutive negative results. To increase the number of samples in this analysis based on the availability of matched blood group donors, the limit of detection was also tested on a subset of P. knowlesi clinical samples taken from patients who had already received anti-malarial treatment. To confirm and improve the quality and reliability of the limit of detection findings, research microscopy readings were also carried out on individual samples at each of the LOD dilutions.
Statistical analysis
The diagnostic accuracy of the Gazelle™ to detect P. knowlesi clinical infections for both the pilot phase and primary analysis were compared against reference PCR results using STATA v16 (TX, USA). Wilcoxon rank-sum test compared differences between the sex of patients (% of males) with age (median) as a dependent variable. The distribution of microscopy parasite counts for infections within defined groups were summarised and compared using the geometric mean. As defined below using the number of true positive (TP), false negative (FN), false positive (FP) and true negative (TN) results, diagnostic tests for sensitivity (TP/TP + FN), specificity (TN/TN + FP), and positive and negative predictive values were calculated29. Exact binomial confidence intervals of 95% for each of the above diagnostic metrics were calculated and reported. Overall device performance was compared by testing equality of the receiver operating characteristic (ROC) areas.
Independent comparisons between the sensitivity of the Gazelle™ device versus reference PCR were conducted using Fisher’s exact test for: (a) parasitaemia of above or below 200 parasites/µL, and (b) samples collected before versus after administration of antimalarial treatment. For the latter, logistic regression was used to compare the device sensitivity further when controlling for median time in hours post-treatment. Logistic regression was used to confirm log-transformed parasite counts influenced Gazelle™ test positivity. For the limit of detection analysis, geometric mean LOD with 95% confidence intervals were estimated and reported using GraphPad Prism v9.4.1 (San Diego, CA). A p-value of less than 0.05 for 2-sided tests was considered significant.
Results
Study participant screening and enrolment
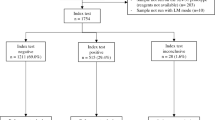
From July 2020 to November 2021, 1757 febrile patients presented at the hospital study site, including 540 individuals diagnosed with P. knowlesi malaria using screening by microscopy. Of this number, 513 (95%) received species confirmation via real-time PCR, as required by the Malaysian Ministry of Health guidelines for malaria.
A total of 287 febrile patients were enrolled into this study (Table 1), with a pilot cohort comprising of 40 P. knowlesi cases, and a validation cohort with 203 P. knowlesi cases and 44 malaria-negative controls. Overall, 45% (110/243) of P. knowlesi cases and 80% (32/44) negative controls presented directly at the hospital and were not referred from catchment area clinics as mandated for all cases of microscopically confirmed malaria. Extrapolating the proportion of PCR-confirmed P. knowlesi cases and malaria-negative controls directly presenting at Ranau hospital yielded a crude estimate of background malaria prevalence of 18.8% (95% CI 16.7–21.1%) for this febrile cohort.
Pilot phase cohort demographics and Gazelle™ performance
The 40 P. knowlesi-infected patients in the pilot phase had a geometric mean parasite count of 577/µL (95% CI 331–1006/µL), ranging from 34 to 14,888/µL (Table 1). Patients had a median age of 28 years (range 9–69 years), with males being the majority (75%). There were 27 samples (68%) which were collected after the patient had been administered anti-malarial treatment.
For patients with no prior treatment, the Gazelle™ achieved a sensitivity of 100% (13/13, 95% CI 75.3–100%) for P. knowlesi detection when compared to reference PCR, including a sample with low parasite count of 52 parasites/µL. When samples collected from patients who had already received anti-malarial treatment were included, sensitivity decreased to 92.5% (37/40, 95% CI 78.6–97.6%) (Table 2). The median time post-treatment for the pilot phase enrolments was 3.6 h (IQR 2.8–5.3). There were three false negative results, from samples collected at 13.7, 13.3 and 3.2 h after first anti-malarial treatment dose, and with parasite counts of 34/µL, 38/µL and 105/µL, respectively.
Validation cohort demographics and clinical status
The validation phase prospectively recruited a further 247 febrile patients; 203 (82%) were confirmed P. knowlesi malaria cases and 44 were malaria-negative controls. All patients had their malaria status confirmed via reference PCR (Table 1). The median age of patients with P. knowlesi malaria was 36 (range 4–87) years, similar to the febrile controls who had a median age of 40 (range 12–80) years. The majority of malaria patients and controls were male, 80% and 70%, respectively. A statistically significant age difference was observed between the sex of the malaria patients, with a median age of 33 (IQR 22–48) years for males and 44 (IQR 29–56) years for females (p = 0.018).
The median duration of self-reported fever prior to presentation was 4 days (range 1–14 days and 2–21 days) for both malaria cases and negative controls respectively. Eighty-three malaria patients (41%) and 5 febrile controls (11%) had a self-reported history of malaria but none within the 2 months prior to study enrolment. The geometric mean parasitaemia of the 203 P. knowlesi-infected patients was 837/µL (95% CI 638–1099/µL), ranging between 18 and 331,727/µL. There were 2 adults enrolled with severe malaria according to WHO criteria22: one patient had jaundice with a parasite count of 30,906/μL, the other had both jaundice and hyperparasitaemia with a parasite count of 331,727/μL. All other patients were admitted with uncomplicated malaria.
Of the P. knowlesi-positive samples tested on the Gazelle™, 143 were collected from patients prior to anti-malarial treatment. There were 60 patients with blood samples collected after administration of anti-malarial treatment, from which 95% (57/60) received at least one dose of oral artemether-lumefantrine and another 5% (3/60) received intravenous artesunate.
Primary analysis of Gazelle™ diagnostic performance for P. knowlesi
From the 143 P. knowlesi samples collected and tested before any anti-malarial treatment, the reported test sensitivity was 96.5% (138/143, 95% CI 92.0–98.9%) (Table 2). The geometric mean parasitaemia of these samples was 986/µL (95% CI 719–1352/µL), with the lowest detected parasite count of 18/µL. There were five P. knowlesi isolates with false-negative results on Gazelle™ testing, with a median parasitaemia of 42/µL (IQR 41–59, range 23–251/µL). The Gazelle™ recorded a sensitivity of 92.0% (95% CI 84.3–96.7%) for the 81 P. knowlesi patients with no previous self-reported malaria history.
There were 60 additional P. knowlesi-infected patient samples evaluated after the initial administration of weight-based antimalarial treatment. The median time between treatment and blood sampling for Gazelle™ testing was 2.6 h (IQR 1.5–4.2 h). The geometric mean parasitaemia of samples tested in this group was slightly lower than those tested prior to treatment at 567/µL (95% CI 334–963/µL). When comparing test performance in this group to pre-treatment samples, a trend towards lower test sensitivity of 90.0% (54/60, 95% CI 79.5–96.2%) was observed (p = 0.062). The median parasitaemia of six post-treatment P. knowlesi clinical samples with a false-negative result was 40 parasites/µL (IQR 24–52, range 20–244 parasites/µL), with no statistically significant difference in the distribution of parasite counts between pre-and post-treatment groups.
The combined sensitivity for P. knowlesi detection including both pre-treatment and post-treatment samples was 94.6% (192/203, 95% CI 90.5–97.3%). The improvement in sensitivity for the validation cohort after refinement of the detection algorithm was not statistically significant compared to the overall pilot phase sensitivity of 92.5%.
The specificity of the Gazelle™ was 100% (95% CI 92.0–100%) for malaria diagnosis, as demonstrated by negative test results for all malaria-negative febrile controls. A high diagnostic accuracy was observed with a ROC of 0.97 (95% CI 0.96–0.99), a positive predictive value (PPV) of 100% (95% CI 55.0–100%) and a negative predictive value (NPV) of 98.8% (95% CI 97.7–99.3%).
Sensitivity at low parasite counts
Combining both pilot and validation phase cohorts, 47 P. knowlesi-infected patients (23%) had parasite counts less than 200/µL, with a median parasitaemia of 60 (IQR 40–120) parasites/µL. The Gazelle™ achieved a diagnostic sensitivity of 80.9% (95% CI 66.7–90.9%) in this group (Fig. 2). Sensitivity improved to 87.1% (95% CI 78.0–93.3%) for the 85 (42%) P. knowlesi malaria cases with a parasitaemia below 500 parasites/µL.
(a) Parasite count distribution of P. knowlesi clinical samples and corresponding test outcomes, grouped according to antimalarial treatment status at sampling point, further sub-grouped by pilot and validation testing phases; horizontal dotted line denotes 200 parasites/µL cutoff. (b) Sensitivity of the Gazelle device accounting for all clinical samples and subset of samples with parasite counts less than 200 parasites/µL, grouped according to antimalarial treatment status at sampling point, further sub-grouped by pilot and validation testing phases; vertical bars represent 95% confidence intervals.
Sub-microscopic P. knowlesi samples
Four patients were censored from the primary analysis for not meeting study inclusion criteria despite initially enrolled as microscopy-negative febrile controls, as subsequent reference PCR confirmed submicroscopic P. knowlesi infections. The Gazelle™ did not successfully detect any of these 4 infections, which were below the research microscopy limit of detection of approximately 30 parasites/µL. The inclusion of these submicroscopic P. knowlesi infections in the primary analysis would not have resulted in a statistically significant difference in the reported overall Gazelle™ sensitivity.
Sensitivity of Gazelle™ compared to microscopy
Against reference PCR results, sensitivity of the Gazelle™ (96.5%) was comparable to that of microscopy (97.2%) for P. knowlesi detection on samples collected prior to treatment, including the 4 febrile patients with submicroscopic parasitaemia. When compared against all 207 PCR-positive P. knowlesi infections before and after treatment, sensitivity of microscopy was higher than the Gazelle™ (95.7% vs 92.8% respectively, p = 0.014).
Parasite life-stages and Gazelle detection
Microscopic evaluation of parasite life-stages was carried out on the 147 pre-treatment P. knowlesi samples. The mean proportions of early trophozoite (rings), late trophozoite and schizont asexual life-stages in a single infection were 2.2% (95% CI 1.4–3.4%), 92.9% (95% CI 90.5–95.2%) and 5.1% (95% CI 3.3–7.8%), respectively. The mean proportion of rings was 1.5% in those positive by the Gazelle™ compared to 6.6% for those that were negative, with no statistically significant difference between groups. Gazelle™ test positivity correlated with parasitaemia (Spearman’s rho = 0.272, p = 0.001), however it did not correlate with the proportion of ring, trophozoite or schizont life-stages within individual P. knowlesi infections.
Detection limits of the Gazelle™ for clinical P. knowlesi infections
A subset of 20 P. knowlesi-positive patient samples was included in the LOD analysis with serial parasitaemia dilutions. Of the 20 clinical samples, 8 (40%) were obtained from patients who had anti-malarial treatment prior to blood sampling (median time post-treatment 2.7 h, range 0.3–3.7 h). The LOD geometric mean was calculated to be 33 parasites/µL (95% CI 16–65 parasites/µL) (Fig. 3). The individual LOD for the 20 P. knowlesi samples evaluated ranged from 4 to 558 parasites/µL. The LOD geometric mean was comparable for pre- versus post-treatment samples: 34 vs 32 parasites/µL, respectively.
Discussion
Highly sensitive, rapid, and affordable diagnostic tools are urgently needed to detect and improve the clinical management of P. knowlesi infections in endemic regions30. Sensitive PCR-based assays for detection of P. knowlesi have enabled a greater understanding of the heterogenous distribution and increasing incidence of P. knowlesi in Malaysia6. However, in other endemic areas of Southeast Asia, accurate PCR detection has not been utilised outside targeted malaria research surveys or in returned travelers31. The widespread deployment and clinical utility of PCR remain constrained due to availability, cost, and delay in obtaining results. Low density infections for both zoonotic and human-only Plasmodium species continue to challenge diagnostic accuracy and hamper national malaria case reporting and elimination efforts32. In the current study, the haemozoin-based malaria diagnostic Gazelle™ device demonstrated excellent performance in POC detection of P. knowlesi, with a sensitivity of 96.5% for samples tested prior to antimalarial treatment. The high reported sensitivity of the Gazelle™ was above the 95% threshold deemed sufficient by the WHO as an alternative to microscopy for first-line diagnostic use33, confirming potential utility of this device in endemic regions where first-line microscopy may not be available. Additionally, a sensitivity of 96% was observed for detection of P. knowlesi infections with parasite counts of more than 200/µL, which was previously reported in over 88% of patients passively presenting to health facilities with symptomatic P. knowlesi infections24.
Previous studies evaluating the clinical performance of the Gazelle™ for malaria diagnosis (P. falciparum, P. vivax, P. malariae and P. ovale spp) reported sensitivities ranging from 72 to 82% compared to PCR, out-performing current lateral flow malaria RDTs17,27,34. The utility of the Gazelle™ as a diagnostic screening tool previously demonstrated 100% specificity for malaria from a survey of 440 febrile individuals in Sri Lanka, although in comparison to light microscopy was unable to differentiate between the small number of P. falciparum, P. ovale, P. vivax and P. malariae cases included in the study34. Improved sensitivity of the Gazelle™ for P. vivax and P. falciparum detection has also been reported in patients with no prior history of malaria or treatment17, potentially indicating a greater or more rapid degree of haemozoin phagocytosis in individuals with previous malaria exposure. However, this phenomenon was not observed in the current evaluation for P. knowlesi, although the small numbers of those with previous P. knowlesi infections limited this analysis.
The sensitivity of the Gazelle™ of 81% for P. knowlesi infections with parasite counts of less than 200/µL is insufficient for reliable diagnosis in this group, limiting use of the Gazelle™ as an alternative first-line diagnostic tool in areas where microscopy is currently deployed. The performance of the Gazelle™ at low parasite counts was variable overall, highlighted by the lowest recorded parasite count detected at 18/µL, and a mean limit of detection of 33 parasites/µL, similar to microscopy thresholds despite poorer comparative sensitivity against PCR overall. The inconsistent Gazelle™ performance at very low parasite densities likely relates to both inherent variability in the reference standard of microscopic quantification, and also the relative composition of parasite life-stages present in individual infections. A higher proportion of mature trophozoite and schizont life-stages should correspond to a comparatively larger amount of detectable haemozoin in peripheral blood at similar parasite densities. Dark field microscopy image processing algorithms have demonstrated that ring stages older than 6 h begin to show detectable haemozoin, and rings between 10 and 16 h reliably contain detectable haemozoin16, implying hemozoin-based detection tools may have limited utility for detection of early infections. Previous evaluations of two other haemozoin-based tools have claimed detection of ring-stage infections of P. falciparum is possible at below 40 parasites/µL20,35. Despite P. falciparum infections intrinsically consisting of only ring-stage parasites in peripheral blood due to cytoadherence of later life-stages in the microvasculature, a separate study assessing the Gazelle™ for clinical P. falciparum infections reported false negative at parasite counts up to 1188/µL34, with follow-up investigations to rectify this still ongoing.
Recent improvements in lateral flow point-of-care diagnostics for P. knowlesi have been based on either non-specific Plasmodium species parasite lactate dehydrogenase (pLDH) targets, or cross-reactivity with epitopes for P. vivax-specific pLDH36,37. However, adapting technology for P. knowlesi detection designed for more common human malaria species requires trade-offs between test accuracy, complexity, and cost. The Gazelle™ was tested on a similar parasite density distribution sample set of clinical P. knowlesi infections as a previously published large evaluation of 10 pLDH-based malaria rapid diagnostic tests37. The best performing Plasmodium species pan-pLDH and P. vivax-pLDH tests for P. knowlesi detection reported sensitivities of 87.0% and 92.0%, respectively37, comparable to the overall Gazelle™ performance in the current study. Other antigen- and antibody-based novel diagnostic assays are being designed to detect multiple infectious causes of acute febrile illness, including the DPP® Fever Panel II Asia for diagnosis of falciparum and non-falciparum malaria, dengue and melioidosis38. Automated microscopy technology utilising machine learning software to visualize malaria blood films is being developed to detect Plasmodium species39. Although these devices demonstrate promising alternatives to conventional first-line malaria diagnostics, they have not been evaluated for clinical P. knowlesi infections to date. They would ideally require validation to either encompass P. knowlesi detection or confirm initial reported specificity for other Plasmodium species before field deployment31.
A limitation of this study was that the background prevalence of malaria could only be a crude estimation based on patients who presented directly at the referral hospital site. Despite this, a prevalence estimate that is lower by 5% would not significantly affect the NPV (99.1% [95% CI 98.5–99.6%]).
The current Gazelle™ version evaluated in this study provides qualitative malaria diagnosis without the current ability for Plasmodium species differentiation and quantification. This study provides important additional data on the first systematic evaluation of a haemozoin-based detection tool for detection of one zoonotic malaria species in an endemic clinical setting. The Gazelle™ device is not able to differentiate between Plasmodium species, which is crucial for targeted management in areas where multiple endemic Plasmodium species are present. In its current form, the Gazelle™ only allows for rapid screening to differentiate malaria due to any Plasmodium species infection from other causes of acute febrile illness for those presenting to health facilities with laboratory capacity, as demonstrated by the consistent 100% specificity from febrile malaria-negative controls observed in this study and other previous evaluations18,34,40. Improvements to the Gazelle™ are ongoing, with subsequent versions expected to distinguish between clinically important Plasmodium spp. via algorithms developed based on species-specific differences in haemozoin morphology41.
In addition to detection of malaria based on paramagnetic properties of hemozoin crystals, the current iteration of the Gazelle™ has a separate slot for identifying common haemoglobin variants42 using cellulose acetate-based cartridges on a microchip electrophoresis platform. This device has been evaluated for point-of-care screening for other blood-related diseases and hemoglobinopathies such as sickle cell43,44 and thalassemia44 traits. In malaria endemic areas with a high prevalence of thalassaemia, a single strategically placed device may be able to perform routine screening of thalassemia in addition to rapid detection of clinical malaria infections, thus maximizing its cost-effectiveness in the field.
In terms of diagnostic limitations, there remains the possibility that haemozoin detection methods may give false positive results in cases where circulating haemozoin persist despite the complete clearance of parasites from a previously resolved infection. Murine models have demonstrated the persistent presence of haemozoin for an extended period of 196–270 days post-Plasmodium species infection in many organs, including the liver, spleen and bone marrow45,46. Haemozoin isolated from cultured P. falciparum isolates showed evidence of extreme resistance to phagocytic degradation and was not easily digested by a single process. This suggests haemozoin may be actively redistributed in the body for long periods of time through repeated phagocytosis, not only at the time of liberation from erythrocytes47. To our knowledge, the duration by which haemozoin crystals persist in successfully treated P. knowlesi infected patients has not been reported. The potential persistence of haemozoin in P. knowlesi-infected humans may include retention within the circulatory system or intravascularly in extracellular fluids. It remains unclear what possible immune-modulatory effects they illicit over multiple rounds of phagocytosis, or if the forms in which they persist may be detectable by currently available tools such as the Gazelle™. Despite this theoretical possibility, the current study did show that the Gazelle™ gave 100% specificity for P. knowlesi mono-infections; this high specificity may not be achievable in settings of high malaria transmission.
The Gazelle™ offers several major advantages in point-of-care functional utility for malaria diagnosis. This includes minimal time-to-detection of about 1–2 min for the actual blood analysis test component on all study participants. Peripheral blood samples are easily obtainable, particularly given the low blood volume required for testing. This is possible through more acceptable and better tolerated finger-prick sampling of patients compared to more invasive venepuncture. Improving end-user utility would be aided by future designs allowing, i.e., self-loading of 30 µL whole blood directly from finger pricks into cartridge containing the buffer solution, as opposed to requiring pipettes in a laboratory setting. The device is potentially suitable for remote primary health care settings where power supply and laboratory consumable cold chain processes may not be available. In addition, the Gazelle™ requires no pre-requisite manual sample preparation such as Giemsa-staining which may lower overall sensitivity and specificity of test48. The Gazelle™ device also provides a comparably quick result compared to that of a typical RDT, which takes about 15 min after loading the blood sample. An economic limitation would be the cost involved in supplying devices to many small clinics. The optimal utility of the Gazelle™ for most low-income countries would be limited to district referral hospitals frequented by many febrile patients daily.
Conclusion
The Gazelle™ shows potential for use as a non-species-specific tool for malaria diagnosis encompassing P. knowlesi detection. The most relevant context would be in countries where other human Plasmodium species are approaching elimination and high-quality microscopy is not used as the first-line diagnostic tool. P. knowlesi parasitaemia limit of detection of the Gazelle™ was comparable to expert microscopy. Further development of the Gazelle™ is required to accurately differentiate between Plasmodium species, including validation for other zoonotic malaria, and to guide appropriate treatment.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information file.
References
Setiadi, W. et al. A zoonotic human infection with simian malaria, Plasmodium knowlesi, in Central Kalimantan, Indonesia. Malar. J. 15, 218. https://doi.org/10.1186/s12936-016-1272-z (2016).
Coutrier, F. N. et al. Laboratory challenges of Plasmodium species identification in Aceh Province, Indonesia, a malaria elimination setting with newly discovered P. knowlesi. PLoS Negl. Trop. Dis. 12, e0006924. https://doi.org/10.1371/journal.pntd.0006924 (2018).
Zaw, M. T. & Lin, Z. Human Plasmodium knowlesi infections in South-East Asian countries. J. Microbiol. Immunol. Infect. 52, 679–684. https://doi.org/10.1016/j.jmii.2019.05.012 (2019).
World Health Organization. World Malaria Report 2022 (World Health Organization, 2022).
World Health Organization. World Malaria Report 2021 (World Health Organization, 2021).
Cooper, D. J. et al. Plasmodium knowlesi Malaria in Sabah, Malaysia, 2015–2017: Ongoing increase in incidence despite near-elimination of the human-only Plasmodium species. Clin. Infect. Dis. 70, 361–367. https://doi.org/10.1093/cid/ciz237 (2020).
World Health Organization. Global Technical Strategy for Malaria 2016–2030, 2021 Update 40 (2021).
Rajahram, G. S. et al. Deaths from Plasmodium knowlesi malaria: Case series and systematic review. Clin. Infect. Dis. 69, 1703–1711. https://doi.org/10.1093/cid/ciz011 (2019).
Barber, B. E., William, T., Grigg, M. J., Yeo, T. W. & Anstey, N. M. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar. J. 12, 8. https://doi.org/10.1186/1475-2875-12-8 (2013).
Herdiana, H. et al. Two clusters of Plasmodium knowlesi cases in a malaria elimination area, Sabang Municipality, Aceh, Indonesia. Malar. J. 17, 1. https://doi.org/10.1186/s12936-018-2334-1 (2018).
World Health Organization. Microscopy for the Detection, Identification and Quantification of Malaria Parasites on Stained Thick and Thin Blood Films in Research Settings (Version 1.0): Procedure: Methods Manual 33 (World Health Organization, 2015).
Ministry of Health, M. (Vector Borne Disease Sector, Disease Control Division, Ministry of Health, Malaysia, 2014).
Hussin, N. et al. Updates on malaria incidence and profile in Malaysia from 2013 to 2017. Malar. J. 19, 55. https://doi.org/10.1186/s12936-020-3135-x (2020).
Chin, A. Z. et al. Malaria elimination in Malaysia and the rising threat of Plasmodium knowlesi. J. Physiol. Anthropol. 39, 36. https://doi.org/10.1186/s40101-020-00247-5 (2020).
Abeysinghe, R. Outcomes from the evidence review group on Plasmodium knowlesi. Present. Malar. Policy Advis. Comm. Meet 22–24 (2017).
Delahunt, C., Horning, M. P., Wilson, B. K., Proctor, J. L. & Hegg, M. C. Limitations of haemozoin-based diagnosis of Plasmodium falciparum using dark-field microscopy. Malar. J. 13, 147. https://doi.org/10.1186/1475-2875-13-147 (2014).
Kumar, R. et al. First successful field evaluation of new, one-minute haemozoin-based malaria diagnostic device. EClinicalMedicine 22, 100347. https://doi.org/10.1016/j.eclinm.2020.100347 (2020).
Valdivia, H. O. et al. Field validation of a magneto-optical detection device (Gazelle) for portable point-of-care Plasmodium vivax diagnosis. PLoS ONE 16, e0253232. https://doi.org/10.1371/journal.pone.0253232 (2021).
Snounou, G. & Singh, B. Nested PCR analysis of Plasmodium parasites. Methods Mol. Med. 72, 189–203. https://doi.org/10.1385/1-59259-271-6:189 (2002).
Orbán, Á. et al. Evaluation of a novel magneto-optical method for the detection of malaria parasites. PLoS ONE 9, e96981. https://doi.org/10.1371/journal.pone.0096981 (2014).
Pukáncsik, M. et al. Highly sensitive and rapid characterization of the development of synchronized blood stage malaria parasites via magneto-optical hemozoin quantification. Biomolecules 9, 579. https://doi.org/10.3390/biom9100579 (2019).
World Health Organization. Severe malaria. Trop. Med. Int. Health 19, 7–131. https://doi.org/10.1111/tmi.12313_2 (2014).
Barber, B. E. et al. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: High proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin. Infect. Dis. 56, 383–397. https://doi.org/10.1093/cid/cis902 (2013).
Grigg, M. J. et al. Age-related clinical spectrum of Plasmodium knowlesi malaria and predictors of severity. Clin. Infect. Dis. 67, 350–359. https://doi.org/10.1093/cid/ciy065 (2018).
Ba, E. H. et al. Microscopy for the Detection, Identification and Quantification of Malaria Parasites on Stained Thick and Thin Blood Films in Research Settings: Procedure: Methods Manual (2015).
Lee, K. S., Cox-Singh, J. & Singh, B. Morphological features and differential counts of Plasmodium knowlesi parasites in naturally acquired human infections. Malar. J. 8, 73. https://doi.org/10.1186/1475-2875-8-73 (2009).
De Melo, G. C. et al. Performance of a sensitive haemozoin-based malaria diagnostic test validated for vivax malaria diagnosis in Brazilian Amazon. Malar. J. 20, 1. https://doi.org/10.1186/s12936-021-03688-0 (2021).
Nuin, N. A. et al. Comparative evaluation of two commercial real-time PCR kits (QuantiFast and abTES) for the detection of Plasmodium knowlesi and other Plasmodium species in Sabah, Malaysia. Malar. J. 19, 306. https://doi.org/10.1186/s12936-020-03379-2 (2020).
McNeil, B. J., Keeler, E. & Adelstein, S. J. Primer on certain elements of medical decision making. N. Engl. J. Med. 293, 211–215 (1975).
Abeysinghe, R. Malaria Policy Advisory Committee Meeting 22–24.
Drakeley, C. et al. Advances in Parasitology Vol. 113, 77–130 (Academic Press, 2021).
World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges (World Health Organization, 2020).
World Health Organization. New Perspectives: Malaria Diagnosis, Report of a Joint WHO/USAID: Informal Consultation Held on 25–27 October 1999 Vol. 1999, 4–48 (World Health Organization, 2000).
Fernando, D. et al. Evaluation of a haemozoin-based rapid diagnostic test for diagnosis of imported malaria during the phase of prevention of reestablishment in Sri Lanka. Malar. J. 21, 7. https://doi.org/10.1186/s12936-022-04283-7 (2022).
Baptista, V. et al. The future in sensing technologies for malaria surveillance: A review of hemozoin-based diagnosis. ACS Sensors 6, 3898–3911. https://doi.org/10.1021/acssensors.1c01750 (2021).
McCutchan, T. F., Piper, R. C. & Makler, M. T. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg. Infect. Dis. 14, 1750–1752. https://doi.org/10.3201/eid1411.080840 (2008).
Tan, A. F. et al. Diagnostic accuracy and limit of detection of ten malaria parasite lactate dehydrogenase-based rapid tests for Plasmodium knowlesi and P. falciparum. Front. Cell. Infect. Microbiol. 12, 1023219 (2022).
Amornchai, P. et al. Sensitivity and specificity of DPP® Fever Panel II Asia in the diagnosis of malaria, dengue and melioidosis. J. Med. Microbiol. 71, 001584 (2022).
Rees-Channer, R. R. et al. Evaluation of an Automated Microscope Using Machine Learning for the Detection of Malaria in Travelers Returned to the UK (2022).
Fontecha, G. et al. Field evaluation of a hemozoin-based malaria diagnostic device in Puerto Lempira, Honduras. Diagnostics 12, 1206. https://doi.org/10.3390/diagnostics12051206 (2022).
Noland, G. S., Briones, N. & Sullivan, D. J. The shape and size of hemozoin crystals distinguishes diverse Plasmodium species. Mol. Biochem. Parasitol. 130, 91–99. https://doi.org/10.1016/S0166-6851(03)00163-4 (2003).
Hasan, M. N. et al. Paper-based microchip electrophoresis for point-of-care hemoglobin testing. Analyst 145, 2525–2542. https://doi.org/10.1039/c9an02250c (2020).
Segbefia, C. et al. American Journal of Tropical Medicine and Hygiene 21.
Shrivas, S. et al. Evaluation of microchip-based point-of-care device “Gazelle” for diagnosis of sickle cell disease in India. Front. Med. 8, 639208. https://doi.org/10.3389/fmed.2021.639208 (2021).
Frita, R., Carapau, D., Mota, M. M. & Hänscheid, T. In vivo hemozoin kinetics after clearance of Plasmodium berghei infection in mice. Malaria Res. Treat. 2012, 1–9 (2012).
Levesque, M. A., Sullivan, A. D. & Meshnick, S. R. Splenic and hepatic hemozoin in mice after malaria parasite clearance. J. Parasitol. 85, 570. https://doi.org/10.2307/3285800 (1999).
Boura, M., Frita, R., Góis, A., Carvalho, T. & Hänscheid, T. The hemozoin conundrum: Is malaria pigment immune-activating, inhibiting, or simply a bystander? Trends Parasitol. 29, 469–476. https://doi.org/10.1016/j.pt.2013.07.005 (2013).
Wongsrichanalai, C., Barcus, M. J., Muth, S., Sutamihardja, A. & Wernsdorfer, W. H. A review of malaria diagnostic tools: Microscopy and rapid diagnostic test (RDT). In Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77 (6) of American Journal of Tropical Medicine and Hygiene (2007).
Acknowledgements
The authors thank the study participants, the IDSKKS malaria research team (Maizatul Farina binti Abd Mutalib, Noorazela binti Mohamed Yassin, Mohd Rizan Osman, Danshy Alaza, and Azielia Elastiqah), Ranau District Hospital director and clinical/laboratory staff, Dr. Fiona Bizini of Ranau Public Health District (PKD Ranau), Dr. Dyang Ruksuna Md Ruhul, Dr. Wan Mohamad Haziman Bin Wan Taib and Dr. Mohd Fakhrurizal Bin Matdiris as well as the Sabah State Public Health Laboratory. They would like to thank the Director General of Health Malaysia for the permission to publish this article. This work was supported by the Australian Centre of Research Excellence in Malaria Elimination, the National Health and Medical Research Council, Australia (Grant Numbers 1037304 and 1045156, fellowships to NMA [1042072], MJG [1138860]), the National Institutes of Health, USA (R01AI160457-01) and Malaysian Ministry of Health (Grant Number BP00500/117/1002) awarded to GSR. SK is supported by a Menzies Future Leaders Fellowship. AFT is supported via a Malaysia Australia Colombo Plan Commemoration (MACC) and Australian Government Research Training Program (RTP) Scholarship at Charles Darwin University. MJG is supported by the Australian Centre for International Agricultural Research, and Indo-Pacific Centre for Health Security, DFAT, Australian Government (LS-2019-116).
Author information
Authors and Affiliations
Contributions
M.J.G., P.T., D.B. and N.M.A. conceived the study design; A.F.T., S.S.S. and Y.L.L. performed data collection and conducted the experiments; A.F.T. performed data analysis and wrote the original draft; P.T., G.S.R., T.W., B.E.B., S.K., N.M.A., D.B. and M.J.G. provided supervision, funding acquisition and resources; All authors read, reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
P.T. and D.B. are employees of Hemex Health. The other authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, A.F., Thota, P., Sakam, S.S.B. et al. Evaluation of a point-of-care haemozoin assay (Gazelle device) for rapid detection of Plasmodium knowlesi malaria. Sci Rep 13, 4760 (2023). https://doi.org/10.1038/s41598-023-31839-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31839-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.