Abstract
Blood pressure variability (BPV) is associated with higher cardiovascular morbidity risks; however, its association with cognitive decline remains unclear. We investigated whether higher BPV is associated with faster declines in cognitive function in ischemic stroke (IS) patients. Cognitive function was evaluated between April 2010 and August 2015 using the Mini-mental State Examination (MMSE) and Montreal Cognitive Assessment in 1,240 Korean PICASSO participants. Patients for whom baseline and follow-up cognitive test results and at least five valid BP readings were available were included. A restricted maximum likelihood–based Mixed Model for Repeated Measures was used to compare changes in cognitive function over time. Among a total of 746 participants (64.6 ± 10.8 years; 35.9% female). Baseline mean-MMSE score was 24.9 ± 4.7. The median number of BP readings was 11. During a mean follow-up of 2.6 years, mean baseline and last follow-up MMSE scores were 25.4 ± 4.8 vs. 27.8 ± 4.4 (the lowest BPV group) and 23.9 ± 5.2 vs. 23.2 ± 5.9 (the highest BPV group). After adjusting for multiple variables, higher BPV was independently associated with faster cognitive decline over time. However, no significant intergroup difference in cognitive changes associated with mean systolic BP was observed. Further research is needed to elucidate how BPV might affect cognitive function.
Similar content being viewed by others
Introduction
Stroke survivors often experience cognitive impairment1, and post-stroke dementia is associated with poor outcomes2. Approximately 65% of stroke survivors experience cognitive decline, with 30% developing dementia1. Some demographic factors, including increased age, lower education level3, and stroke-related factors including hemiplegia, pre-stroke antiplatelet use4, and imaging factors including white matter hyperintensities (WMHs) and medial temporal lobe atrophy5, are associated with cognitive decline after stroke. Baseline cognitive impairment before stroke2 is an important predictor for post-stroke dementia6,7.
Blood pressure variability (BPV), the normal oscillation of blood pressure (BP), is associated with higher risks of cardiovascular events8 and morbidity9. Additionally, previous studies10,11,12,13,14,15,16,17 demonstrated an association between high BPV and low cognition in various populations, although a causal relationship has not been established. A possible hypothesis is that BP fluctuations provoke ischemic damage to the white matter through cerebral hypoperfusion18. Carotid astherosclerosis19 and increased large arterial stiffness might reduce baroreceptor sensitivity and contribute to cerebral damage20. Considering that stroke patients’ auto-regulatory capacities are already impaired, making them more vulnerable to BP fluctuations, a high BPV might be a predictive factor for post-stroke cognitive decline.
Since cognitive function varies with time after the index stroke, physicians should evaluate cognitive outcomes serially rather than at a single point. Regarding the methodological aspect of cognitive evaluation, prior studies were limited in that they did not reflect cognitive changes over multiple visits in a longitudinal follow-up study10,12,14.
Therefore, in this study, we sought to determine whether BPV affects cognitive decline in patients with acute ischemic stroke (IS) by assessing BPV during each visit interaction. We hypothesized that a higher visit-to-visit BPV is associated with a faster decline in cognitive function in IS patients.
Results
Baseline characteristics
A total of 746 participants were included in the MMSE analysis (mean age, 64.6 ± 10.8 years; 35.9% female). Included patients were younger (64.6 ± 10.8 versus 67.8 ± 10.4 years). The mean education length was 8.6 ± 4.8 years. Baseline stroke severity was milder (NIHSS 1.8 ± 2.0 versus 2.6 ± 3.0). The baseline characteristics for participants included in and excluded from the analysis are shown in Table S1.
Among the included participants, initial median NIHSS score was 1 (interquartile range [IQR], 0–3). The mean MMSE score was 24.9 ± 4.7. The mean follow-up period was 2.6 years (IQR, 2.0–3.1), while the mean number of BP readings was 11 (IQR, 8–14).
Detailed baseline characteristics were stratified according to BPV-SD (Table 1). Compared with patients in the T1 group, patients in higher tertile groups were older and had fewer education years, lower cognitive function, and higher mean baseline BP.
Trajectories of cognition test scores over follow-up period
Analysis I: BPV and cognition test scores
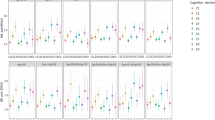
We adjusted for age, sex, educational years, probucol treatment, baseline NIHSS score, baseline cognition test scores, diabetes and index of high risk of ICH, and mean SBP. Variables with p < 0.1 in univariate analysis or biological relevance were included in the multivariable model. During a mean follow-up of 2.6 years, MMSE scores from baseline to last follow up were 25.4 ± 4.8 vs. 27.8 ± 4.4 for the lowest BPV group, and 23.9 ± 5.2 vs. 23.2 ± 5.9 for the highest BPV group. The MMRM analysis showed that the cognition test scores over the follow-up period according to the BPV-SD group decreased rapidly. The trend using MMRM including BPV by visit interaction was statistically significant (p < 0.05) (Table 2). After adjusting for baseline cognitive status, linear trends of cognition test scores over the follow-up period were also significant across the groups (p for trend < 0.001) (Fig. 1A–D). The results of analysis using secondary BPV parameters (SDreg and VIM) were similar to those using BPV-SD, at least in the analysis using MMSE (Tables S2 and S3).
The cognition test scores from the Mixed-Model Repeated Measures analysis over the follow-up period according to the BPV-SD group. (A) MMSE, Crude (B) MMSE, Adjusted (C) MoCA, Crude (D) MoCA, Adjusted. BPV-SD intra-individual standard deviation of systolic blood pressure, MMSE Mini-mental State Examination, MoCA Montreal Cognitive Assessment.
Analysis II: Mean SBP and cognition test scores
The detailed baseline characteristics between the tertile groups stratified according to mean SBP are presented in Table S4. After adjusting for multiple co-variables, MMRM analysis showed that the temporal cognitive changes over the follow-up period according to mean SBP were not statistically significant for the MMSE or MoCA test (Fig. 2A,B and Table S5).
Analysis III: Subgroup analysis stratified by baseline cognitive status
The relationship between high BPV and diagonal cognitive decline was also evident in the analysis of the subgroups stratified according to baseline cognitive status (MMSE score ≤ 24 vs. MMSE score > 24). Unlike patients with MMSE scores ≤ 24, there was a significant cognitive decline according to the BPV groups among patients with MMSE scores > 24 (p < 0.0001) (Fig. 3A–D and Table S6). After adjusting for initial cognitive differences between the two groups, cognitive function showed a tendency to deteriorate over time, especially in the higher BPV groups.
The cognition test scores from the Mixed-Model Repeated Measure analysis over the follow-up period according to initial cognitive function (MMSE ≤ 24 vs. MMSE > 24). (A) MMSE ≤ 24, Crude (B) MMSE ≤ 24, Adjusted (C) MMSE > 24, Crude (D) MMSE > 24, Adjusted. MMSE mini-mental state examination, MoCA Montreal Cognitive Assessment.
Discussion
Our study showed that high BPV may be related to cognitive decline in a cohort of IS patients. This trend was also evident in the subgroups stratified according to baseline cognitive function, especially in patients with normal baseline cognitive function (MMSE score > 24). However, mean SBP was not significantly associated with cognitive decline.
Previous reports demonstrated a positive relationship between cognitive decline and high BPV11,12,13,14. Although the exact patho-mechanism remains unknown, there is speculation about why BPV is an independent predictor for cognitive decline. First, recurrent cerebral hypoperfusion may play a role. Cerebral blood flow remains stable when systemic BP changes within the lower and upper pressure limits of autoregulation. However, high BPV may accompany a BP below the lower limit, which results in recurrent cerebral hypoperfusion. Patients with IS have more diminished autoregulatory capacities21,22. Hypoperfusion may lead to selective collapse of key proteins within the paranodal axon–glial junctions that are critical in white matter function23. Second, BP fluctuation may contribute to amyloid-beta (Aβ) deposits and brain atrophy23. In healthy elderly individuals, greater sleep-systolic BPV24,25 and chronic hypoperfusion26 is associated with aggravated brain atrophy. Furthermore, transgenic mice with amyloid precursor protein mutations (TgAPP) model demonstrated that blood flow reductions promote amyloid deposition compared with wild-type mice27. A comorbid amyloid pathology could mediate cognitive impairment after stroke28. Besides cerebral hypoperfusion, endothelial dysfunction, inflammation, neurohumoral activation, and oxidative stress have been suggested as underlying mechanisms29,30,31.
It remains to be determined why mean BP was not associated with cognitive decline. Consistent with our results, although researchers observed no correlation between mean BP and dementia, an increase of 1-SD in CV was associated with a 10% increased risk of dementia16. Among 24,593 patients without preexisting cognitive dysfunction, the CVSBP was independent predictors of cognitive decline, whereas mean SBP was not10. In 240 patients with Alzheimer disease, only systolic BPV showed a decrease in MMSE score15. Because the brain has autoregulation, a constant pressure is maintained even in patients with some degree of hypertension. Therefore, a mildly to moderately elevated BP might not affect cognitive decline. Our findings may suggest that mean BP has less impact on cognitive function than BPV.
One notable point is that BPV would have impacted cognitive decline before the index stroke. Although the study population from PICASSO was randomized, the differences in baseline cognitive function among the tertile groups might be meaningful. Since BPV does not occur at the time of the index IS, we proposed that the diminished initial cognitive status was also caused by the influence of BPV over a long period of time.
Higher BPV is an independent predictor of cognitive decline in patients without preexisting cognitive dysfunction10,13. To clarify it, we classified patients into two groups (MMSE score ≤ 24 vs. MMSE score > 24). It is noteworthy that high BPV was associated with significant cognitive decline in those with MMSE scores > 24 (Fig. 4). We suspect that those patients with a relatively preserved baseline cognitive function are more likely to have been influenced by BPV since they were not yet influenced by other amyloid or vascular pathologies. These findings suggest that BPV has relatively low impact on cognitive decline in patients with pathological conditions.
Our study has some specific strengths and limitations. The main study (PICASSO)32 had a double-blind randomized design with a large population. Second, our study utilized repeated cognition tests, whereas previous studies conducted one or two evaluations during follow-up10,11,13. Third, regarding methodology, compared to previous studies using linear-to-linear regression or cross-sectional methods, MMRM has been beneficially used in the analysis of longitudinal data, especially when randomly missed values are assumed33. Finally, we utilized both MMSE and MoCA to detect cognitive decline after stroke. In a longitudinal study, preliminary exploration using MMSE as a screening test is reasonable15. However, MoCA is considered a well-validated tool for screening, particularly in terms of executive function34. Since MoCA involves more demanding visual construction tasks, it had a lesser ceiling effect34.
An important limitation is that this population with multiple microbleeds and prior ICH is not necessarily representative of all IS patients. Additionally, the patients included in this study had very mild stroke severity (median NIHSS 1). Therefore, the interpretation of the results may not be generalized. Second, BP was not assessed at the same time of day for all subjects during each follow-up appointment. This limitation might contribute to the random error of BPV measurements that may attenuate the associations. Third, attrition bias may affect our results, as it was present in previous longitudinal cognitive studies35. Fourth, because we did not find definite evidence of a causative relationship, high BPV may be a marker of disease rather than a contributing factor. Fifth, probucol, a non-statin cholesterol-lowering agent, prevented hippocampal synaptic impairment36 as well as blood–brain barrier dysfunction37. However, to reduce the influences of probucol, we adjusted for probucol treatment in the MMRM analysis. Sixth, we did not investigate anti-hypertensive or anti-diabetic medication type, which may affect BPV. Although we have all the data on anti-hypertensive drugs, anti-diabetic drugs, and HbA1C levels, drug-types and doses are very arbitrary. Furthermore, we believe that BPV is the result of reflecting all of these factors and our aim is to evaluate the effect of BPV on cognitive decline in patients with IS. Seventh, several insights were suggested that the relationships among BPV, stroke location and cognitive impairment. However, we did not analyze those interactions in this study38,39,40. Finally, it is generally true that an increased BPV is associated with vascular events41. Although we evaluated the associations between BPV and stroke or composite outcomes of vascular events (Table S7), the BPV parameters showed inconsistent results. Various indices of BPV have different prognostic relevance for vascular outcomes42. Accordingly, a recent review suggested that BPV parameters remain a research tool rather than an index for risk stratification43.
Our results suggest that high BPV is closely linked to faster decline of cognitive function in a cohort of IS patients. Current treatment guidelines for high BP are based on mean values44. However, our findings suggest that physicians should more pay attention to BPV to prevent cognitive decline. Further investigations are required to elucidate the correlation between BPV and cognitive function.
Methods
This study is a sub-analysis of the PICASSO trial32, a randomized controlled trial conducted in 67 centers in three countries (South Korea, the Philippines, and Hong Kong, China). PICASSO was a two-by-two factorial study designed to determine the efficacy and safety of cilosatzol and probucol. Between April 2010 and August 2015, this study included 1,534 patients with non-cardioembolic IS or transient ischemic attack (TIA) within 180 days, who had prior intracerebral hemorrhage (ICH) or multiple cerebral microbleeds on gradient echo imaging. Almost all patients were having significant burden of small vessel disease; approximate 70% had moderate-to-severe WMH (Fazekas scale 2–3), and the median Fazekas scale was 2. The study rationale, design, and relevant information were described previously45. All methods were carried out in accordance with relevant guidelines (STROBE guidelines).
The institutional review board (IRB) of each participating center approved this study, and all participants gave written informed consent (Asan medical center IRB No. 2009-0189).
Subjects
A total of 1534 patients participated in the PICASSO trial. Among the 1382 Korean PICASSO participants, 1240 participated in the PICASSO-COG sub-study. Because neither the Mini-mental State Examination (MMSE) nor the Montreal Cognitive Assessment (MoCA) have been validated by cross-cultural studies in each language, we excluded non-Korean PICASSO participants. Detailed information about the design of the PICASSO-COG sub-study has been published previously46. The definitions of the baseline cognition tests and valid BP evaluations are described below. Of the PICASSO-COG participants, those for whom baseline and follow-up cognition test results were unavailable were excluded. MMSE and MoCA data for baseline and one or more follow-up tests were available for 892 and 877 patients. Among these patients, those for whom at least five valid BP readings were unavailable were excluded. Thus, the final subjects included 746 patients evaluated with MMSE and 733 analyzed using MoCA (Fig. 4A,B).
Measurement of cognitive function
We assessed cognition at the 1-month, 4-month, annual (13, 25, 37 and 49 months after enrollment), and final visits. In patients with vascular events, we used results of the cognitive tests at the time of the event as the final cognitive outcome. To minimize the influence of acute cognitive decline by the index stroke47, we defined baseline cognitive tests as those conducted at 4–7 months after stroke. Baseline cognitive function was assessed at the 4-month visit after enrollment in subjects randomized within 3 months after stroke onset or at the 1-month visit after enrollment for those randomized at 4–6 months after stroke onset46. Cognitive tests were performed by the local investigator or the study coordinators, who were pretrained at the time of study initiation and received continued training at regular investigator meetings46.
BP measurements and definition of valid blood pressure value
After randomization, participants were scheduled to visit outpatient clinics 1-month later and every 3-months thereafter that until study completion. At every scheduled and unscheduled visit, researchers at each hospital who were trained and experienced in BP measurement measured patients’ BP using an automatic sphygmomanometer with patients in a sitting position after sufficient rest. The automatic sphygmomanometer which had been calibrated in each institution was used. However, we could not validate the automatic devices for BP measurements between research centers.
We defined the first “valid” BP measurement as the BP value measured at the randomization visit when the index event was a TIA or when the time interval from the onset of the index IS to randomization was more than 30 days. When the time interval from stroke onset to randomization was 30 days or less, the first valid BP measurement was the BP level measured at the 1-month visit after randomization. By defining the valid BP this way, we minimized the influence of acute stroke on the BP value. We defined the final valid BP as the BP level measured at the closing visit when cardiovascular endpoints had not occurred. To reduce the influence of acute cardiovascular outcomes, if cardiovascular endpoints had occurred, the final valid BP was the BP value measured at the last visit before development of the endpoints. We included all BP levels measured at unscheduled and scheduled visits between the first and final valid BP measurements.
The predictive power of visit-to-visit BPV increased with more BP readings, i.e. the BPV values were not predictive if there were 4 or fewer BP readings8. Thus, we excluded participants for whom fewer than five valid BP measurements were available.
Calculation of visit-to-visit BPV
We used the intra-individual standard deviation (SD) of systolic BP (SBP) as the primary parameter of BPV (BPV-SD). Although traditional measures of BPV, including the SD and coefficient of variation (CV), are often used, absolute levels of BPV are often positively correlated with mean BP levels48. Therefore, we calculated a transformed parameter of SD that is defined to be uncorrelated with mean BP levels: variation independent of the mean (VIM). VIM is proportional to SD/meanx, with x derived from curve fitting in this cohort. As a secondary parameter of BPV, we also calculated SDreg, the SD of the participant’s regression line with SBP regressed across visits41. Conceptually, SD is the “average” of the deviations of the mean (which is assumed to be static over time), while SDreg is the “average” of the deviations of the regression line (which assumes a linear change over time).
Confounding variables
For each participant, we collected data on demographics, years of education, vascular risk factors, initial National Institutes of Health Stroke Scale (NIHSS) score, mean SBP, and severity of WMH on magnetic resonance imaging (MRI) using the Fazekas scale. The composite outcomes of vascular events included stroke, myocardial infarction, and vascular death.
Statistical analysis
Baseline characteristics are expressed as number of participants (%), mean ± SD, or median (interquartile range). Participants were classified into three groups according to BPV tertiles (T1, lowest; T2, middle; and T3, highest). Differences in the distribution of baseline characteristics between the BPV tertiles were identified using the chi-square test, analysis of variance (ANOVA), or Kruskal–Wallis test as appropriate.
To compare changes in cognitive function over time between the BPV groups, a Mixed-Model Repeated Measures (MMRM) approach was used. MMRM has been extensively used in the analysis of longitudinal data, especially when missing data are a concern and some randomly missed values are assumed33. In this study, MMRM allowed within-subject correlation because of the repeated evaluations of cognitive test scores and different numbers of measurements among patients at the follow-up visits. The effect of different study sites was adjusted to be random in the model using an unstructured variance–covariance matrix. Subgroup analyses were then performed according to baseline cognitive function (MMSE score of > 24 vs. MMSE score of ≤ 24). Stroke or composite outcomes of vascular events were assessed using Poisson regression or the Cox proportional hazards model.
A two-sided 5% level was used to indicate statistical significance, and all statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Data availability
The datasets analysed during the present study are available from the corresponding author on reasonable request.
References
Hachinski, V. et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37, 2220–2241. https://doi.org/10.1161/01.Str.0000237236.88823.47 (2006).
Sibolt, G. et al. Poststroke dementia is associated with recurrent ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 84, 722–726. https://doi.org/10.1136/jnnp-2012-304084 (2013).
Leys, D., Henon, H., Mackowiak-Cordoliani, M. A. & Pasquier, F. Poststroke dementia. Lamcet Neurol. 4, 752–759. https://doi.org/10.1016/s1474-4422(05)70221-0 (2005).
Bejot, Y. et al. Prevalence of early dementia after first-ever stroke: a 24-year population-based study. Stroke 42, 607–612. https://doi.org/10.1161/strokeaha.110.595553 (2011).
Yang, J. et al. Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimer’s Dementia 11, 16–23. https://doi.org/10.1016/j.jalz.2014.01.003 (2015).
Pendlebury, S. T. & Rothwell, P. M. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: Analysis of the population-based Oxford Vascular Study. Lancet Neurol. 18, 248–258. https://doi.org/10.1016/s1474-4422(18)30442-3 (2019).
Allan, L. M. et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain 134, 3716–3727. https://doi.org/10.1093/brain/awr273 (2011).
Rothwell, P. M. et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 375, 895–905. https://doi.org/10.1016/S0140-6736(10)60308-X (2010).
Whittle, J. et al. Visit-to-visit variability of BP and CKD outcomes: Results from the ALLHAT. Clin. J. Am. Soc. Nephrol. 11, 471–480. https://doi.org/10.2215/cjn.04660415 (2016).
Bohm, M. et al. Systolic blood pressure variation and mean heart rate is associated with cognitive dysfunction in patients with high cardiovascular risk. Hypertension 65, 651–661. https://doi.org/10.1161/HYPERTENSIONAHA.114.04568 (2015).
Nagai, M., Hoshide, S., Ishikawa, J., Shimada, K. & Kario, K. Visit-to-visit blood pressure variations: New independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J. Hypertens. 30, 1556–1563. https://doi.org/10.1097/HJH.0b013e3283552735 (2012).
Qin, B. et al. Visit-to-visit variability in blood pressure is related to late-life cognitive decline. Hypertension 68, 106–113. https://doi.org/10.1161/HYPERTENSIONAHA.116.07494 (2016).
Sabayan, B. et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: Prospective cohort study. BMJ 347, f4600. https://doi.org/10.1136/bmj.f4600 (2013).
Yano, Y. et al. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension 64, 983–988. https://doi.org/10.1161/HYPERTENSIONAHA.114.03978 (2014).
Lattanzi, S., Luzzi, S., Provinciali, L. & Silvestrini, M. Blood pressure variability predicts cognitive decline in Alzheimer’s disease patients. Neurobiol. Aging 35, 2282–2287. https://doi.org/10.1016/j.neurobiolaging.2014.04.023 (2014).
Alperovitch, A. et al. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimer’s Dementia 10, S330-337. https://doi.org/10.1016/j.jalz.2013.05.1777 (2014).
Kwon, K. Y., Pyo, S. J., Lee, H. M., Seo, W. K. & Koh, S. B. Cognition and visit-to-visit variability of blood pressure and heart rate in de novo patients with Parkinson’s disease. J. Mov. Disord. 9, 144–151. https://doi.org/10.14802/jmd.16012 (2016).
Brickman, A. M. et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch. Neurol. 67, 564–569. https://doi.org/10.1001/archneurol.2010.70 (2010).
Nagai, M., Hoshide, S., Ishikawa, J., Shimada, K. & Kario, K. Visit-to-visit blood pressure variations: New independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J. Am. Soc. Hypertens. 5, 184–192. https://doi.org/10.1016/j.jash.2011.03.001 (2011).
Kingwell, B. A., Cameron, J. D., Gillies, K. J., Jennings, G. L. & Dart, A. M. Arterial compliance may influence baroreflex function in athletes and hypertensives. Am. J. Physiol. 268, H411-418. https://doi.org/10.1152/ajpheart.1995.268.1.H411 (1995).
Aoi, M. C. et al. Impaired cerebral autoregulation is associated with brain atrophy and worse functional status in chronic ischemic stroke. PLoS ONE 7, e46794. https://doi.org/10.1371/journal.pone.0046794 (2012).
Immink, R. V. et al. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke 36, 2595–2600. https://doi.org/10.1161/01.STR.0000189624.06836.03 (2005).
Duncombe, J. et al. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 131, 2451–2468. https://doi.org/10.1042/CS20160727 (2017).
Goldstein, I. B., Bartzokis, G., Guthrie, D. & Shapiro, D. Ambulatory blood pressure and brain atrophy in the healthy elderly. Neurology 59, 713–719 (2002).
Goldstein, I. B., Bartzokis, G., Guthrie, D. & Shapiro, D. Ambulatory blood pressure and the brain: A 5-year follow-up. Neurology 64, 1846–1852. https://doi.org/10.1212/01.Wnl.0000164712.24389.Bb (2005).
Lambert, C. et al. Longitudinal patterns of leukoaraiosis and brain atrophy in symptomatic small vessel disease. Brain 139, 1136–1151. https://doi.org/10.1093/brain/aww009 (2016).
Okamoto, Y. et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 123, 381–394. https://doi.org/10.1007/s00401-011-0925-9 (2012).
Liu, W. et al. Influence of amyloid-beta on cognitive decline after stroke/transient ischemic attack: Three-year longitudinal study. Stroke 46, 3074–3080. https://doi.org/10.1161/strokeaha.115.010449 (2015).
Di Flaviani, A. et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care 34, 1605–1609. https://doi.org/10.2337/dc11-0034 (2011).
Diaz, K. M. et al. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens. Res. 35, 55–61. https://doi.org/10.1038/hr.2011.135 (2012).
Kim, K. I. et al. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ. J. 72, 293–298 (2008).
Kim, B. J. et al. Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): A multicentre, randomised controlled trial. Lancet Neurol. 17, 509–518. https://doi.org/10.1016/s1474-4422(18)30128-5 (2018).
Mallinckrodt, C. et al. Recent developments in the prevention and treatment of missing data. Ther. Innov. Regul. Sci. 48, 68–80. https://doi.org/10.1177/2168479013501310 (2014).
Nakaoku, Y. et al. Montreal Cognitive Assessment score correlates with regional cerebral blood flow in post-stroke patients. Clin. Neurol. Neurosurg. 174, 68–74. https://doi.org/10.1016/j.clineuro.2018.09.004 (2018).
Pendlebury, S. T. et al. Methodological factors in determining rates of dementia in transient ischemic attack and stroke: (I) impact of baseline selection bias. Stroke 46, 641–646. https://doi.org/10.1161/strokeaha.114.008043 (2015).
Santos, D. B. et al. Probucol, a lipid-lowering drug, prevents cognitive and hippocampal synaptic impairments induced by amyloid beta peptide in mice. Exp. Neurol. 233, 767–775. https://doi.org/10.1016/j.expneurol.2011.11.036 (2012).
Mamo, J. C. et al. Probucol prevents blood-brain barrier dysfunction and cognitive decline in mice maintained on pro-diabetic diet. Diabetes Vasc. Dis. Res. 16, 87–97. https://doi.org/10.1177/1479164118795274 (2019).
Nagai, M. et al. Visit-to-visit blood pressure variability and Alzheimer’s disease: Links and risks. J. Alzheimer’s Dis. 59, 515–526. https://doi.org/10.3233/jad-161172 (2017).
Kitamura, J. et al. The insular cortex, Alzheimer disease pathology, and their effects on blood pressure variability. Alzheimer Dis. Assoc. Disord. 34, 282–291. https://doi.org/10.1097/wad.0000000000000340 (2020).
Kitamura, J. et al. Blood pressure variability in acute ischemic stroke: Influence of Infarct location in the insular cortex. Eur. Neurol. 79, 90–99. https://doi.org/10.1159/000486306 (2018).
Shimbo, D. et al. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: Data from the Women’s Health Initiative. Hypertension 60, 625–630. https://doi.org/10.1161/hypertensionaha.112.193094 (2012).
Parati, G., Stergiou, G. S., Dolan, E. & Bilo, G. Blood pressure variability: clinical relevance and application. J. Clin. Hypertens. 20, 1133–1137. https://doi.org/10.1111/jch.13304 (2018).
Asayama, K. et al. Does blood pressure variability contribute to risk stratification? Methodological issues and a review of outcome studies based on home blood pressure. Hypertens. Res. 38, 97–101. https://doi.org/10.1038/hr.2014.153 (2015).
Chobanian, A. V. et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42, 1206–1252. https://doi.org/10.1161/01.HYP.0000107251.49515.c2 (2003).
Hong, K. S., Kim, B. J., Lee, J. Y., Kwon, S. U. & Investigators, P. Rationale and design of the PreventIon of CArdiovascular events in iSchemic Stroke patients with high risk of cerebral hemOrrhage (PICASSO) study: A randomized controlled trial. Int. J. Stroke 10, 1153–1158. https://doi.org/10.1111/ijs.12519 (2015).
Yu, K. H. et al. Design and rationale for a cognitive outcome substudy in ischemic stroke patients with high risk of cerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 25, 2061–2066. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.04.028 (2016).
Levine, D. A. et al. Trajectory of cognitive decline after incident stroke. JAMA 314, 41–51. https://doi.org/10.1001/jama.2015.6968 (2015).
Dolan, E. & O’Brien, E. Blood pressure variability: Clarity for clinical practice. Hypertension 56, 179–181. https://doi.org/10.1161/hypertensionaha.110.154708 (2010).
Acknowledgements
We wish to thank the PICASSO investigators.
Funding
While the original study was supported by Korea Otsuka Pharmaceutical Company (S.U. Kwon), there are no conflicts of interests for this study.
Author information
Authors and Affiliations
Contributions
S.U.K. and J.-H.L. devised the original study concept and design. J.-S.L., M.S.O., K.-H.Y., J.-H.P., Y.-J.K., J.-H.R., Y.-H.H., S.H.H., and S.H.A. participated the acquisition of data. J.S.L. performed statistical analyses. Y.K. wrote the manuscript. Y.K., S.U.K. and J.-H.L. interpreted the results and made an intellectual contribution.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y., Lim, JS., Oh, M.S. et al. Blood pressure variability is related to faster cognitive decline in ischemic stroke patients: PICASSO subanalysis. Sci Rep 11, 5049 (2021). https://doi.org/10.1038/s41598-021-83945-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83945-z
This article is cited by
-
Blood pressure variability supersedes heart rate variability as a real-world measure of dementia risk
Scientific Reports (2024)
-
Allopurinol and blood pressure variability following ischemic stroke and transient ischemic attack: a secondary analysis of XILO-FIST
Journal of Human Hypertension (2024)
-
Role of ambulatory blood pressure monitoring in elderly hypertensive patients
Clinical Hypertension (2022)
-
Burden and correlates of cognitive impairment among hypertensive patients in Tanzania: a cross-sectional study
BMC Neurology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.