Abstract
Regarded as doped binary hydrides, ternary hydrides have recently become the subject of investigation since they are deemed to be metallic under pressure and possibly potentially high-temperature superconductors. Herein, the candidate structure of Li5MoH11 is predicted by exploiting the evolutionary searching. Its high-pressure phase adopts a hexagonal structure with P63/mcm space group. We used first-principles calculations including the zero-point energy to investigate the structures up to 200 GPa and found that the P63cm structure transforms into the P63/mcm structure at 48 GPa. Phonon calculations confirm that the P63/mcm structure is dynamically stable. Its stability is mainly attributed to the isostructural second-order phase transition. Our calculations reveal the electronic topological transition displaying an isostructural second-order phase transition at 160 GPa as well as the topology of its Fermi surfaces. We used the projected crystal orbital Hamilton population (pCOHP) to examine the nature of the chemical bonding and demonstrated that the results obtained from the pCOHP calculation are associated with the electronic band structure and electronic localized function.
Similar content being viewed by others
Introduction
The success of experimental syntheses of metal hydrides has attracted considerable attention especially amongst the high-pressure research community, a corollary to discoveries—both experimental and theoretical—of a variety of interesting physical properties of these materials1,2,3,4,5,6,7,8. Also, metal hydrides have long been considered as one of the best candidates for high-temperature superconductors. The superhydride Ce-H system, for instance, was successfully synthesized using the laser-heated diamond anvil cell (DAC) accompanied with synchrotron X-ray diffraction, which was theoretically confirmed by the evolutionary variable-composition simulation indicating that CeH9 adopts a hexagonal clathrate structure with the P63/mmc symmetry and potentially produces high-temperature superconductivity with estimated \(T_{c}\) of 105–117 K at 200 GPa9. Another metal-hydride superconductor of LaH10 with \(T_{c}\) = 260 K, approaching room temperature, was synthesized and experimentally observed under pressure between 180–200 GPa10, while it was suggested by a subsequent theoretical study to adopt a sodalite-like clathrate structure with \(Fm\overline{3}m\) symmetry and exhibit a decreasing trend in \(T_{c}\) under an increase of pressure11. Additionally, LaH11 is expected to produce high \(T_{c}\) as theoretically predicted12 as well as several other materials13,14,15,16,17,18,19.
More recently, ternary superhydrides (compounds each consisting of two substituted metal elements and hydrogen atoms) have been proposed to achieve metallicity and that one can expect a near room-temperature superconductivity out of them10,11,17,18. However, the ternary hydride Li5MoH11 does not at all exhibit high-temperature superconductivity under high pressure, only with its maximum \(T_{c}\) of 6.5 K at 160 GPa belonging to the high-pressure phase20. Li5MoH11 being one of the family of the hydrogen-rich metals Li5MH11, where M = Mo, W, Nb, and Ta21, adopts a hexagonal structure with the P63cm space group at ambient pressure, which is constructed via the ionic bonds of i.e. Li+, H−, and the ninefold-hydrogen-coordinated [MoH9]\(^{3-}\). The structure was also predicted in theory to exhibit an insulator–metal transition under compression21. As for Li5MoH11, it was revealed that the P63cm structure transforms into the Cc and Pc structures at 5 GPa and 94 GPa, respectively21. Not long ago an experimental study utilizing high-pressure synchrotron x-ray diffraction (XRD) cast doubt on the ambiguity in structural determination of this ternary compound. According to the observation, the Cc and Pc structures do not exist at the predicted pressures (5 GPa and 94 GPa, respectively) as well as the fact that at higher pressure the crystal structure of Li5MoH11 cannot also be determined experimentally20.
In this work, we aimed to identify the unknown structure of Li5MoH11 above 160 GPa, taking the crystal structure obtained from experiment as the starting structure throughout the entire calculation, by using the Universal Structure Predictor: Evolutionary Xtallography (USPEX). It is well-known that the standard spin-polarized DFT calculation, specifically within the generalized gradient approximation (GGA functional), cannot successfully describe the d-orbital of a transition metal22, e.g. Mo atom23, due highly to the strongly correlated orbitals. In order to fully take into account the valence states of Mo atom, we performed the GGA+U calculations with the objective of correctly determining the reliable values of \(U_{\text {eff}}\) for Li5MoH11. Regarding its potential for superconductivity, the electronic structure and the nature of the chemical bonding observed by the experimental study20 were shown to propound a possibility of \(T_{c}\). Again, the aforementioned experimental observation found that Li5MoH11 has two different superconducting phases, indicating that a phase transition at 160 GPa might be expected to be an isostructural second-order phase transition, while the possibility of which is supported by means of an electronic topological transition (ETT). The concept of ETT has successfully been used to describe the nature of the electronic structures in several materials24,25,26,27,28, particularly the metal hydride class24. It was also shown that ETT plays a crucial role in electronic band structure via the association with the topology of the Fermi surface (FST)29,30. In effect, the relationship between ETT and FST provides new insight into the nature of the electronic structure of Li5MoH11, as will be discussed later. We also support results of electronic band structure and electron localized function by demonstrating the projected crystal orbital Hamilton population (pCOHP) method.
Results and discussion
We used USPEX to predict the crystal structure of the hydrogen-rich metal Li5MoH11 under high pressure and found that there exist two low-enthalpy structures adopting structurally identical hexagonal structures with R3c and P63/mcm space groups. The predicted structures are shown in Fig. 1, while the optimized structural parameters for the R3c and the P63/mcm structures are presented in Table 1. Subsequently the calculated structural parameters have been fitted to the Birch-Murnaghan equation of state (EOS), as summarized in Table 2. We initially conjectured that Mo− might accept electrons from the surrounding Li+ cations under pressure23. Also, Mo atom is reported to have an unusual spin-coupling that eventually modifies the oxidation state31. We thus applied the GGA and GGA+\(U_{\text {eff}}\) methods to investigate the possibility of this effect. In both cases, Mo− was found to not accept any electron from the surrounding Li+ cations due to the fact that the predicted structure of Li5MoH11 structure is verified as being nonmagnetic (NM), since its magnetization is equal to zero. The selected value of \(U_{\text {eff}}\) plays a decisive role in structural stability as well32,33,34,35. In the case of Li5MoH11, we thus evaluated the corresponding structural phase transition and electronic properties without incorporating \(U_{\text {eff}}\).
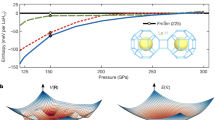
The structural phase transitions of Li5MoH11 are presented by the relative enthalpy, which is obtained from the difference between the enthalpy calculated and that of the P63cm structure, as a function of pressure. According to our calculations, the P63cm structure transforms into the R3c structure at 48 GPa, followed by the existence of the P63/mcm structure at 64 GPa, as shown in Fig. 2a. Addition to this, the corresponding structural stability is further confirmed by the incorporation of the zero-point energy (ZPE) of the nuclei estimation, indicating that the R3c structure is not energetically favored at all throughout the whole pressure range, as shown in Fig. 2b. Rather, the P63cm crystal only transitions into the P63/mcm structure at 48 GPa, which was previously pointed out that the R3c structure becomes unstable when taking into account the effect of ZPE36,37,38. The P63/mcm structure, therefore, is thermodynamically stable over a wide range of pressures. When it comes to dynamical stability, the harmonic approximation incorporating ZPE of nuclei scheme is used to investigate the nature of the lattice dynamics of Li5MoH11. Reported in Fig. 3a, the phonon dispersions accounting for P63cm structure at 40 GPa was computed and the structure is demonstrated to be stable, whereas upon a compression up to 50 GPa this symmetry is dynamically unstable due to the presence of negative frequency branches, as evidenced in Fig. 3b. In effect, the P63cm structure has a tendency to transform into the P63/mcm structure at this very pressure. The inclusion of ZPE calculation apparently plays a crucial role in determining the stable structure of Li5MoH11 under pressure, as previously demonstrated in other metal hydride systems39,40. Regarding the R3c phase, the responsible pressure-dependent phonon dispersion displays a tiny soft-mode at the \(\Gamma\)-point which is shown in Fig. 3c, accompanied by a zoomed-in \(K-\Gamma -M\) path in Fig.3d. This suggests a possibility of becoming a meta-stable structure for the R3c phase, while the really stable phase belongs to the P63/mcm structure.
The crystal structures of Li5MoH11. The green, purple, and pink spheres represent, respectively, Li, Mo, and H atoms. (drawn by VESTA (ver. 3.4.7)65 (URL https://jp-minerals.org/vesta/en/download.html)).
Upon a series of compression, the P63/mcm structure is clearly confirmed to be dynamically stable by the absence of negative frequency in the phonon spectra, as can be seen in Fig. 4, which implies that the compression promoted the global minimum enthalpy of the structure. At this point, we found that the P63/mcm structure is dynamically favored over the P63cm and R3c structures under a wide range of pressure, i.e., beginning at 80 GPa. According to the phonon density of states responsible for both Li and H atoms at around 25 THz (Fig. 4b–d), it is obvious that there exists coupling phonon branches, while they each tend to separate and eventually result in the intermediate optical phonon modes. It is worth noting that there remains the plausibility for Li5MoH11 to have a phase transition due to the fact that it was previously observed to superconduct with two different superconducting phases at 160 GPa20. At this point, however, it is difficult to verify whether there exists another phase of Li5MoH11 aside from the P63/mcm structure, which, as a consequence, is expected to undergo the isostructural second-order phase transition above 160 GPa, as previously pointed out by means of the appearance of the intermediate optical phonon modes41 .
The electronic band structures of the P63/mcm Li5MoH11 under varying pressures are plotted in Fig. 5a–d. The Mo atom’s 4d orbitals split into two energy levels, i.e, E\(_{2g}\) (d\(_{xy}\), d\(_{x^{2}-y^{2}}\)) and E\(_{1g}\) (d\(_{yz}\), d\(_{xz}\)), whereas the Li atom’s 2p orbitals yield the E\(_{1u}\) (p\(_{x}\), p\(_{y}\)) and the A\(_{2u}\) (p\({_z}\)) orbitals as well as into the A\(_{1g}\) orbital arising from the H atom’s 1s orbital. The band structure evidences a coupling between the E\(_{2g}\) of Mo atom and the E\(_{1u}\) orbitals of Li atom and reveals a Dirac-like cone, as also found recently42,43, at the \(\Gamma\)-point in Fig. 5a, which is originated from the coupling between the \(\delta\)- and \(\pi\)-bonds. Upon an increase of pressure, particularly at 100 GPa (see Fig. 5b), the separation of the E\(_{2g}\) orbitals of Mo and the E\(_{1u}\) orbitals of Li become apparent. This implies the electronic topological transition, which is referred to as the Lifshitz transition44. The following dispersion (see Fig. 5c) displays a downward shift of the A\(_{2u}\) orbitals of Li, which in turn leads to the coupling between the E\(_{1u}\) orbital of Li and the E\(_{1g}\) orbital of Mo that exhibit the \(\sigma\)–\(\pi\) bonding, at 160 GPa, when eventually the latter is roughly level with the E\(_{2g}\) orbitals of Mo atom at 200 GPa also exhibiting \(\sigma\)–\(\delta\) bonding. Likewise, the A\(_{2u}\) orbitals of Li continue to couple with the E\(_{1u}\) orbitals of Li despite the increase of pressure, which results in \(\sigma\)–\(\pi\) bonding. As mentioned earlier, isostructural second-order phase transition induced by the P63/mcm structure can be made possible by the Lifshitz transition. Moreover, the electronic band structures show no sign of flat bands near the Fermi level. Even though a set of flat bands can be spotted near the Fermi level as well as the presence of van Hove singularities (vHs) within H3S13, YH6 and YH1014 systems, leading to the possibility of achieving high values of \(T_{c}\), this can be alternatively explained by the shape of the electronic band structure or density of states of our system. Our system’s electronic structures are similar to those found in YSH615, LaSH615, CeH916, and CeH1019, in terms of steep branches near the Fermi level. However, we found that Li5MoH11 does not exhibit high-temperature superconductivity. As an explanation, the energy level of the H atom (the A\(_{1g}\) orbitals) is lower than the Fermi level by -2.605 to -2.538 eV at 80 to 200 GPa, respectively, which further supports the implication that the high-pressure phase observed by experiment does not give high-temperature superconductivity20.
Another key finding that needs to be mentioned is the Fermi surface topology (FST). It is well-known that the FST is associated with an electronic topological transition (ETT), which originates from a given electronic band structure24,25,26,27,28, that plays a key role in promoting the second-order phase transition45. This was previously emphasized in previous studies29,30 the change of material’s topology is promoted by a compression. As depicted in Fig. 6, the characteristics of the FST clearly increase the likelihood of isostructural second-order phase transition: there are individual variations in surface#2 and surface#3 at 160 GPa , compared to those at 80 GPa. The topological changes in FST corresponds to the electronic band structures calculated. Thus the isostructural second-order phase transition of Li5MoH11 can be described by the presence of ETT. More interestingly, surface#2 and surface#3 both form the Fermi surface nesting at the pockets surrounding the \(\Gamma\)-point at 160 GPa, holding a vital clue to superconductivity29.
Sketch of FST of P63/mcm at 80 and160 GPa, respectively (drawn by XCrySDen program (ver. 1.5.60)66 (URL http://www.xcrysden.org/Download.html#_toc_1)).
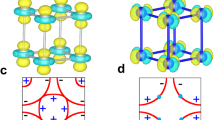
(a) The 3D-electron localization function (ELF) in Li5MoH11 structure at 0 and 160 GPa, respectively. (drawn by VESTA (ver. 3.4.7)65 (URL https://jp-minerals.org/vesta/en/download.html)) (b) Projected crystal orbital Hamilton populations (pCOHPs) in Li5MoH11 structure at 160 GPa.
The likelihood of finding an electron in the neighborhood space of Li5MoH11 can be measured by the electron localization function (ELF)46, as reported in Fig. 7a. The tendency of electron localization in the P63/mcm structure is described by uniform electron gas of the same density19,47,48,49,50. The calculated ELF reveals a set of chemical bonding at 0 GPa. The distances between the first (Li–Mo), second (Li–H), third (Mo–H), and fourth (H–H) nearest neighbors (NN) read 2.94826 Å, 1.83412 Å, 1.90874 Å, and 2.24193 Å, respectively. There exists electronic probability weakly accumulating between the first NN Li–Mo, the second NN Li–H, and the third NN Mo–H, particularly the bonding between the fourth NN H–H which indicates that it is likely to be a strong bonding at 0 GPa. Upon further compression up to 160 GPa, the ELF displays that the distance between the first NN Li–Mo, second NN Li–H, third NN Mo–H, and fourth NN H–H are 2.38216 Å, 1.55862 Å, 1.70037 Å, and 1.27095 Å, respectively. It is worth noting that there is also a strong bonding between H and H, and the increase of electronic distribution between Mo and H at 0 GPa, implying that H–H bonding is likely to be a strong covalent bonding. We further investigated the nature of the chemical bonding near the Fermi level by with the aid of the projected crystal orbital Hamilton populations (pCOHP) calculation, which enables the determination of anti-bonding and bonding characteristics, e.g., covalent bonds, along energy range51,52,53. Illustrated in Fig. 7b, it is obvious that the projected wave function of the Li–Mo bonding displays anti-bonding, in a good agreement with ELF and corresponds to anti-bonding in the electronic band structure54. Our calculations also reveal that the E\(_{1u}\) orbitals and the E\(_{1g}\) orbitals exhibit the \(\sigma\)–\(\pi\) bonding, by a determination of the electronic band structure. The projected wave function of the Li–H bonding is proved to be an ionic bond, which is confirmed by the ELF calculation, while those of the Mo–H and H–H bondings are indicated to be covalent bonds. In particular, the ELF calculation remarkably suggests a a strong covalent bond as a result of the H–H bonding. So, the characteristic of the Li–Mo anti-bonding implies that Li5MoH11 cannot achieve a high value of \(T_{c}\).
Conclusion
In this work, we identify the high-pressure phases of Li5MoH11 by performing an evolutionary searching. Our calculations show that by incorporating the zero-point energy evaluation the P63/mcm structure is thermodynamically and dynamically favored over the P63cm and R3c structures above 50 GPa The perspective of theoretical inspection points out that the P63mcm structure exists under high pressure adopting the hexagonal basis. Phonon dispersion calculations and the electronic topological transition reveal that the P63/mcm structure exhibits an isostructural second-order phase transition. Also, we have shown that the topology of the Fermi surface is associated with the electronic band structure, which nonetheless does not exhibit any flat bands near the Fermi level. This reflects that fact that Li5MoH11 does not have a high value of \(T_{c}\). Here, our calculations support the experimental observations of \(T_{c}\) in the previous study20. The nature of the chemical bonding is associated with the electronic band structure, implying that the characteristics of the chemical bonding entail the value of \(T_{c}\).
Methods
The searching for the structures of the hydrogen-rich metal Li5MoH11 was performed by USPEX55. In all subsequent generations, the random symmetric algorithm employed 40% heredity, 20% random symmetric, 20% soft mutation, and 20% transmutation operators in the pressure range from 160 to 200 GPa with structures containing up to four formula units. All structures were fully relaxed using the generalized gradient approximation of the Perdew–Burke–Ernzerhof (GGA-PBE) functional56 as the exchange-correlation functional. We selected the projector augmented wave (PAW) method57 to describe the core and valence electrons as well as the conjugate gradient scheme, as implemented in the Vienna ab initio simulation package (VASP)58. A plane-wave basis set up to a cutoff energy of 700 eV and a 10 × 10 × 4, 12 × 12 × 4, and 12 × 12 × 10 k-point meshes generated by the Monkhorst–Pack (MP) method59 were used for the P63cm structure, the R3c structure, and the P63/mcm structure, respectively. The pseudocore radii of Li, Mo, and H account for 1.7, 2.50, and 0.80 Bohrs, respectively, which are small enough to ensure that no overlap of spheres will occur under applied pressure. The zero-point energy of the nuclei (ZPE) is estimated within the harmonic approximation, which was included in the enthalpies as a function of the pressure. All values of enthalpy of all structures obtained were fitted by a Birch-Murnaghan equation of state (EOS). The dynamic stable structures were calculated by using the ab initio lattice dynamics with the supercell approach, as implemented in the VASP code together with the PHONOPY package60.The plane-wave energy cutoff of 60 Ry was selected as well as a 12 × 12 × 10 k-point mesh was used for the P63/mcm structure. Based on the linear response theory61, the GGA+U was selected as a means to perform the Hubbard U calculation which is implemented in Quantum Espresso (QE) package62. The effective interaction parameters of which were tested to be \(U_{\text {eff}}\) = \(U-J\) = 1.2 eV. The projected crystal orbital Hamilton population63 (pCOHP) was used to describe the chemical bonding of the P63/mcm structure, as implemented in LOBSTER code64.
References
Lebègue, S., Alouani, M., Arnaud, B. & Pickett, W. E. Pressure-induced simultaneous metal-insulator and structural-phase transitions in LiH: a quasiparticle study. Europhys. Lett. 63, 562–568 (2003).
Zhang, S. et al. Nonmetallic FeH6 under high pressure. J. Phys. Chem. C 122, 12022–12028 (2018).
Batalović, K., Radaković, J., Belošević-Čavor, J. & Koteski, V. Transition metal doping of Mg2FeH6 - a DFT insight into synthesis and electronic structure. Phys. Chem. Chem. Phys. 16, 12356–12361 (2014).
Liu, Y. et al. Stability and properties of the Ru-H system at high pressure. Phys. Chem. Chem. Phys. 18, 1516–1520 (2016).
Muramatsu, T. et al. Metallization and superconductivity in the hydrogen-rich ionic salt BaReH9. J. Phys. Chem. C 119, 18007–18013 (2015).
Shao, Z. et al. Unique phase diagram and superconductivity of calcium hydrides at high pressures. Inorg. Chem. 58, 2558–2564 (2019).
Pluengphon, P., Bovornratanaraks, T., Tsuppayakorn-aek, P., Pinsook, U. & Inceesungvorn, B. High-pressure phases induce H-vacancy diffusion kinetics in tm-doped MgH2: Ab initio study for hydrogen storage improvement. Int. J. Hydrogen Energy 44, 21948–21954 (2019).
Pluengphon, P., Tsuppayakorn-aek, P., Inceesungvorn, B. & Bovornratanaraks, T. Pressure-induced structural stability of alkali trihydrides and H2-desorption occurrence: Ab initio study for hydrogen storage improvement. International Journal of Hydrogen Energy (2020).
Salke, N. P. et al. Synthesis of clathrate cerium superhydride CeH9 at 80–100 GPa with atomic hydrogen sublattice. Nat. Commun. 10, 4453 (2019).
Somayazulu, M. et al. Evidence for superconductivity above 260 K in lanthanum superhydride at megabar pressures. Phys. Rev. Lett. 122, 027001 (2019).
Wang, C., Yi, S. & Cho, J.-H. Pressure dependence of the superconducting transition temperature of compressed \({\rm LaH}_{10}\). Phys. Rev. B 100, 060502 (2019).
Otto, H. H. Super-hydrides of lanthanum and yttrium: On optimal conditions for achieving near room temperature superconductivity. World J. Condens. Matter Phys. 9, 22–36 (2019).
Quan, Y. & Van Pickett, W. E. hove singularities and spectral smearing in high-temperature superconducting \({\text{H}_{3}\text{S}}\). Phys. Rev. B 93, 104526 (2016).
Heil, C., di Cataldo, S., Bachelet, G. B. & Boeri, L. Superconductivity in sodalite-like yttrium hydride clathrates. Phys. Rev. B 99, 220502 (2019).
Liang, X. et al. First-principles study of crystal structures and superconductivity of ternary YSH6 and LASH6 at high pressures. Phys. Rev. B 100, 184502 (2019).
Li, X. et al. Polyhydride CeH9 with an atomic-like hydrogen clathrate structure. Nat. Commun. 10, 3461 (2019).
Drozdov, A. P. et al. Superconductivity at 250 K in lanthanum hydride under high pressures. Nature 569, 528–531 (2019).
Sukmas, W., Tsuppayakorn-aek, P., Pinsook, U. & Bovornratanaraks, T. Near-room-temperature superconductivity of Mg/Ca substituted metal hexahydride under pressure. J. Alloys Compd. 849, 156434 (2020).
Tsuppayakorn-aek, P., Pinsook, U., Luo, W., Ahuja, R. & Bovornratanaraks, T. Superconductivity of superhydride CeH10 under high pressure. Mater. Res. Express 7, 086001 (2020).
Meng, D. et al. Superconductivity of the hydrogen-rich metal hydride Li5MoH11 under high pressure. Phys. Rev. B 99, 024508 (2019).
Takagi, S. et al. Formation of novel transition metal hydride complexes with ninefold hydrogen coordination. Sci. Rep. 7, 44253 (2017).
Sit, P. H. L., Car, R., Cohen, M. H. & Selloni, A. Simple, unambiguous theoretical approach to oxidation state determination via first-principles calculations. Inorg. Chem. 50, 10259–10267 (2011).
Lutfalla, S., Shapovalov, V. & Bell, A. T. Calibration of the DFT/GGA+U method for determination of reduction energies for transition and rare earth metal oxides of Ti, V, Mo, and Ce. J. Chem. Theory Comput. 7, 2218–2223 (2011).
Zhou, F. et al. Pnma metal hydride system LiBH: a superior topological semimetal with the coexistence of twofold and quadruple degenerate topological nodal lines. J. Phys.: Condens. Matter 32, 365502 (2020).
Yang, T., Cheng, Z., Surucu, G. & Wang, X. Coexistence of parabolic and linear band crossings and electron-doped spin-gapless properties in rhombohedral type YbBO3. J. Alloys Compd. 823, 153835 (2020).
Wang, X. et al. Rich topological nodal line bulk states together with drum-head-like surface states in NaAlGe with anti-PbFCl type structure. J. Adv. Res. 23, 95–100 (2020).
Wang, X. et al. Novel topological nodal lines and exotic drum-head-like surface states in synthesized CsCl-type binary alloy tios. J. Adv. Res. 22, 137–144 (2020).
Tsuppayakorn-aek, P. et al. Bain deformation mechanism and Lifshitz transition in magnesium under high pressure. Phys. Status Solidi B 2000279 (2020).
Xu, C. Q. et al. Topological phase transition under pressure in the topological nodal-line superconductor \({\rm PbTaSe}_{2}\). Phys. Rev. B 96, 064528 (2017).
Zhang, Y. et al. Pressure effect on kohn anomaly and electronic topological transition in single-crystal tantalum. Phys. Rev. B 100, 075145 (2019).
Bjornsson, R. et al. Identification of a spin-coupled Mo(iii) in the nitrogenase iron-molybdenum cofactor. Chem. Sci. 5, 3096–3103 (2014).
Tian, M.-F., Deng, X., Fang, Z. & Dai, X. Intermediate-pressure phases of cerium studied by an LDA + Gutzwiller method. Phys. Rev. B 84, 205124 (2011).
Zhou, L. et al. Structural stability and thermodynamics of CrN magnetic phases from ab initio calculations and experiment. Phys. Rev. B 90, 184102 (2014).
Hou, L., Li, W.-D., Wang, F., Eriksson, O. & Wang, B.-T. Structural, electronic, and thermodynamic properties of curium dioxide: Density functional theory calculations. Phys. Rev. B 96, 235137 (2017).
Isaacs, E. B. & Marianetti, C. A. Compositional phase stability of strongly correlated electron materials within DFT+\(U\). Phys. Rev. B 95, 045141 (2017).
Abe, K. Ab initio study of metallic aluminum hydrides at high pressures. Phys. Rev. B 100, 174105 (2019).
Ye, X., Zarifi, N., Zurek, E., Hoffmann, R. & Ashcroft, N. W. High hydrides of scandium under pressure: Potential superconductors. J. Phys. Chem. C 122, 6298–6309 (2018).
Zhang, J. et al. High-temperature superconductivity in the Ti-H system at high pressures. Phys. Rev. B 101, 134108 (2020).
Bianco, R., Errea, I., Calandra, M. & Mauri, F. High-pressure phase diagram of hydrogen and deuterium sulfides from first principles: Structural and vibrational properties including quantum and anharmonic effects. Phys. Rev. B 97, 214101 (2018).
Olea-Amezcua, M. A., De la Peña Seaman, O. & Heid, R. Superconductivity by doping in alkali-metal hydrides without applied pressure: An ab initio study. Phys. Rev. B 99, 214504 (2019).
Pereira, A. L. J. et al. Isostructural second-order phase transition of \(\beta\)-Bi2O3 at high pressures: An experimental and theoretical study. J. Phys. Chem. C 118, 23189–23201 (2014).
Mack, S. A., Griffin, S. M. & Neaton, J. B. Emergence of topological electronic phases in elemental lithium under pressure. Proc. Natl. Acad. Sci. 116, 9197 (2019).
Elatresh, S. F. et al. High-pressure lithium as an elemental topological semimetal. Phys. Rev. Mater. 3, 044203 (2019).
Lifshitz, I. et al. Anomalies of electron characteristics of a metal in the high pressure region. Sov. Phys. JETP 11, 1130–1135 (1960).
Kubo, K. Ferromagnetism and Fermi surface transition in the periodic Anderson model: Second-order phase transition without symmetry breaking. Phys. Rev. B 87, 195127 (2013).
Becke, A. D. & Edgecombe, K. E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 92, 5397–5403 (1990).
Tsuppayakorn-aek, P., Luo, W., Watcharatharapong, T., Ahuja, R. & Bovornratanaraks, T. Structural prediction of host-guest structure in lithium at high pressure. Sci. Rep. 8, 5278 (2018).
Tsuppayakorn-aek, P. et al. The ideal commensurate value of Sc and the superconducting phase under high pressure. J. Appl. Phys. 124, 225901 (2018).
Bovornratanaraks, T., Tsuppayakorn-aek, P., Luo, W. & Ahuja, R. Ground-state structure of semiconducting and superconducting phases in xenon carbides at high pressure. Sci. Rep. 9, 2459 (2019).
Tsuppayakorn-Aek, P. et al. Route to high-Tc superconductivity of BC7 via strong bonding of boron-carbon compound at high pressure. Sci. Rep. 10, 1–8 (2020).
Zhao, Z. et al. Potentially superhard hcp \(\text{Cr}{{N}}_{2}\) compound studied at high pressure. Phys. Rev. B 93, 214104 (2016).
Peng, F. et al. Hydrogen clathrate structures in rare earth hydrides at high pressures: Possible route to room-temperature superconductivity. Phys. Rev. Lett. 119, 107001 (2017).
Kotmool, K. et al. Structural phase transitions, electronic properties, and hardness of RuB4 under high pressure in comparison with FeB4 and OsB4. J. Phy. Chem. C (2020).
Dubois, S. M. M., Zanolli, Z., Declerck, X. & Charlier, J. C. Electronic properties and quantum transport in graphene-based nanostructures. Eur. Phys. J. B 72, 1–24 (2009).
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 124, 244704 (2006).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Togo, A. & Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 108, 1–5 (2015).
Cococcioni, M. & de Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in the \(\text{ LDA }+\text{ U }\) method. Phys. Rev. B 71, 035105 (2005).
Giannozzi, P. et al. Quantum espresso: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter 21, 395502 (2009).
Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. Crystal orbital hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 115, 5461–5466 (2011).
Maintz, S., Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. Lobster: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 37, 1030–1035 (2016).
Momma, K. & Izumi, F. Vesta: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41, 653–658 (2008).
Kokalj, A. Xcrysden—a new program for displaying crystalline structures and electron densities. J. Mol. Graph. Model. 17, 176–179 (1999).
Acknowledgements
We are deeply grateful for the kindness of Dezhong Meng, Shigeyuki Takagi, and Shin-ichi Orimo for suggestions. We gratefully acknowledge the NSC (National Supercomputer Centre, Linköping, Sweden) in Sweden for providing computing time. This research project is supported by Second Century Fund (C2F), Chulalongkorn University. This project is funded by National Research Council of Thailand (NRCT): (NRCT5-RSA63001-04) and Chulalongkorn University, Grant for Research. R.A. and W.L. thank the Swedish Research Council and Swedish Research Links for financial support.
Author information
Authors and Affiliations
Contributions
Author contributions: P.T. and T.B. designed the research; P.T. and T.B performed the research; P.T. and T.B. analysed the data; and P.T., R.A., W.L, and T.B. wrote the paper; W.S. and T.B corrected the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsuppayakorn-aek, P., Sukmas, W., Ahuja, R. et al. Stabilization and electronic topological transition of hydrogen-rich metal Li5MoH11 under high pressures from first-principles predictions. Sci Rep 11, 4079 (2021). https://doi.org/10.1038/s41598-021-83468-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83468-7
This article is cited by
-
Stabilizing superconductivity of ternary metal pentahydride \(\hbox {CaCH}_{{5}}\) via electronic topological transitions under high pressure from first principles evolutionary algorithm
Scientific Reports (2022)
-
Effect of substitution on the superconducting phase of transition metal dichalcogenide Nb(Se\(_{x}\)S\(_{1-x}\))\(_{2}\) van der Waals layered structure
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.