Abstract
We assessed whether comparative efficacy and safety of biphasic insulin aspart 30 (BIAsp 30) plus metformin versus BIAsp 30 monotherapy differed for patients with type 2 diabetes mellitus (T2DM) inadequately controlled with oral antidiabetic drugs with different cardiovascular risk scores and different body mass indexes (BMI) by performing a post hoc analysis of the randomized controlled MERIT study. In the MERIT study, eligible patients were randomized 1:1 to receive BIAsp 30 plus metformin or BIAsp 30 for 16 weeks. Patients in the 2 treatment groups were classified into “low” and “high” risk subgroups based on their GloboRisk scores and into “BMI ≤ 26 kg/m2”and “BMI > 26 kg/m2” subgroups. Primary efficacy endpoint was between-treatments comparison of HbA1c changes from baseline for these 2 sets of subgroups. Between-treatments comparisons of secondary efficacy and safety endpoints were also performed. We found that BIAsp 30 plus metformin led to significantly higher percentage of high-risk patients achieving HbA1c target < 7% than BIAsp 30 monotherapy, with an overall comparable safety profile for high-risk patients. Meanwhile, for patients with BMI ≤ 26 kg/m2, compared with BIAsp 30 monotherapy, BIAsp 30 plus metformin led to significantly higher percentages of patients achieving HbA1c target (47.83% vs 28.17%, P = 0.0165) and composite target of HbA1c < 7% without hypoglycemia or weight gain (20.29% vs 6.85%, P = 0.0187) and have a slightly better safety profile. In conclusion, for T2DM patients at high CV risk or with BMI ≤ 26 kg/m2, BIAsp 30 plus metformin was preferable to BIAsp 30 monotherapy.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease characterized by continuing decline of pancreatic islet β-cell function and subsequent decrease in endogenous insulin secretion1,2,3. In China, T2DM had an estimated prevalence of 10.9% among adult in 20134,5,6. Strict glycemic control by lowering glycated hemoglobin (HbA1c) is at the center of T2DM management7,8. The current American Diabetes Association (ADA) consensus statement recommends metformin as the preferred initial pharmacological treatment for T2DM9. It also recommends that metformin should be continued so long as it can be tolerated and not contraindicated and that other pharmacological agents such as insulin should be added to metformin9. In China, oral antidiabetic (OAD) monotherapy was the most common therapy (51.2%), followed by insulin in combination with OAD (27.0%) and insulin monotherapy (21.8%)5,10. In addition, most patients on insulin therapy in China received premixed insulin such as biphasic insulin aspart 30 (BIAsp 30)10. The 16-week randomized, parallel-controlled MERIT study was designed to mimic the real-life practice in China and to assess efficacy and safety of BIAsp 30 with and without metformin for patients with T2DM inadequately controlled with OADs5. It found that BIAsp30 plus metformin was not inferior to BIAsp 30 monotherapy in safely reducing HbA1c level, that BIAsp 30 plus metformin was associated with a significantly higher proportion of patients achieving HbA1c < 7% and achieving HbA1c < 7% without hypoglycemia or weight gain5.
ADA guidelines recommend a patient-centered approach in choosing proper T2DM treatment for individual patient, and one important consideration is cardiovascular (CV) comorbidities9,11. Cardiovascular disease (CVD) is the leading cause of death in T2DM patients, and the constantly evolving T2DM treatment algorithm includes better control of CV risk in T2DM patients at high CV risks12. It has been reported that metformin may reduce the risk of CV events and death9, as well as improve CV risk profile7,13,14. Meanwhile, numerous studies have assessed the effects of various CV risk factors such as total cholesterol (TC), body mass index (BMI) and blood pressure on efficacy of OADs such as metformin15,16,17,18,19,20. However, the effect of a patient’s composite CV risk score on comparative efficacy and safety of insulin with and without metformin has not been studied. GloboRisk is a tool for predicting a person’s10-year risk of CVD that can be recalibrated and updated for application in 182 countries with readily available information21,22. Unlike other risk scores such as the Framingham equation, GloboRisk allows the age patterns of CVD risk to vary by gender and across different populations and also allows for age-related attenuation of the effect of risk factors on CVD outcomes21,22. Laboratory-based GloboRisk includes smoking, systolic blood pressure (SBP), diabetes, TC, gender and age as variables21,22. A subject in a low and middle-income country such as China with ≥ 20% 10-year CVD risk is considered to have high CV risk22. One aim of the current study was to perform a post hoc analysis of the MERIT study to assess whether and how comparative efficacy and safety of BIAsp 30 with and without metformin differed for patients with different 10-year CVD risk score calculated based on laboratory-based GloboRisk.
BMI was not one of the variables included in the laboratory-based GloboRisk21,22. However, BMI is commonly considered an important CV risk factor23. Whether a patient’s baseline BMI affected efficacy of various OADs including metformin remains controversial17,18,19,20. One post hoc analysis of the MERIT study revealed similar HbA1c changes from baseline associated with BIAsp30 plus metformin treatment among the 3 BMI subgroups: normal weight (BMI, 18.5–23.9 kg/m2), overweight (BMI, 24.0–27.9 kg/m2) and obese (BMI, ≥ 28 kg/m2)5. In the current study, we did a preliminary analysis regarding whether comparative efficacy and safety of BIAsp 30 with and without metformin differed for patients with BMI ≤ 26 kg/m2 versus BMI > 26 kg/m2.
Methods
Design, participants and interventions
This was a post hoc analysis of the MERIT study, a 16-week, randomized, open-label, multicenter, parallel-controlled study conducted at 6 medical centers in China5. Details of the design and method of the MERIT study were described previously5. The MERIT study (registered at the Chinese Clinical Trial Registry (http://www.who.int/ictrp/network/chictr2/en/; CTR-IPR-15006834) assessed efficacy and safety of BIAsp 30 with and without metformin for patients with T2DM inadequately controlled with OADs. Briefly, patients 18–79 years old diagnosed with T2DM with BMI ≥ 18.5 kg/m2 and HbA1c ≥ 7% despite treatment with two or more OADs for more than 3 months from 6 medical centers in China were recruited for this study5. Details of the exclusion criteria were described previously5. The study was approved by the institutional review board of each participating center (Beijing Hospital, Tianjin Medical University Metabolic Diseases Hospital, Qilu Hospital of Shandong University, The First Affiliated Hospital of Fujian Medical University, Sir Run Run Shaw Hospital of Nanjing Medical University, and Shanghai East Hospital affiliated to Tongji University), and was conducted in accordance with the Declaration of Helsinki24 and Good Clinical Practice of China25. All patients provided written informed consent before any trial-related activities. The study was registered at the Chinese Clinical Trial Registry (http://www.who.int/ictrp/network/chictr2/en/): CTR-IPR-15006834.
The MERIT study consisted of a 2-week screening period and a 16-week treatment period, wherein the treatment period was divided into a 4-week titration period and a 12-week maintenance period. After screening, eligible patients were randomized 1:1 to receive BIAsp 30 (NovoMix30) subcutaneous injection plus oral metformin hydrochloride (Glucophage) or only BIAsp 30 subcutaneous injection. Both drugs were manufactured by Merck Serono Pharmaceutical R&D Co., Ltd (Beijing, China). Details of the BIAsp 30 and metformin adjustment and titration schedules and final doses were described previously5. Briefly, during the titration period, doses of metformin and insulin for each patient were adjusted to achieve fasting plasma glucose (FPG) target of 4.4–7.0 mmol/L and 2-h postprandial glucose (PPG) target of < 10 mmol/L (180 mg/dL). The initial dose of metformin during the titration phase was 500 or 850 mg once daily and the dose was titrated to a maximum tolerated dose of ≤ 2500 mg/day (850 mg administered 3 times daily). The initial doses of BIAsp 30 were 0.2 U/kg/day and 0.3 U/kg/day for the BIAsp 30 plus metformin group and the BIAsp 30 monotherapy group, respectively. The BIAsp 30 dose for the BIAsp 30 plus metformin group was titrated until optimal metformin dose was reached. For both treatment groups, the daily BIAsp 30 dose was split into 2 portions that were administered 5 min before breakfast and dinner. The dose of the pre-breakfast BIAsp 30 were adjusted according to FPG and post-breakfast PPG, and the dose of pre-dinner BIAsp 30 were adjusted according to and FPG and PPG before meals5.
Patient populations, efficacy and safety endpoints of the present post hoc analysis
Laboratory-based GloboRisk score was used to calculate the 10-year CVD risk of each patient. China equation (http://www.globorisk.org/calc/labform) was used for GloboRisk score calculation. Variables included age, gender, TC, SBP, current smoking and diabetes21,22. The calculation could be performed at http://www.globorisk.org/calc/labform. As we did not record whether each patient in our study was a smoker or non-smoker, we ran the calculation twice, assuming all patients were smokers and then assuming all were non-smokers, and came up with 2 GloboRisk scores for each patient. Patients with ≥ 20% CVD risk were considered high-risk patients, while patients with < 20% CVD risk were considered low-risk patients21,22. Patients in the 2 treatment groups were classified into the low-risk and high-risk subgroups based on their GloboRisk scores. Additionally, patients in the 2 treatment groups were classified into the “BMI ≤ 26 kg/m2” and “BMI > 26 kg/m2” subgroups.
Primary efficacy endpoint was HbA1c changes from baseline compared between the 2 treatment groups (between-groups comparison) for the low-risk and high-risk subgroups as well as the “BMI ≤ 26 kg/m2” and “BMI > 26 kg/m2” subgroups.
Secondary efficacy endpoints included between-groups comparisons of percentages of patients who achieved HbA1c target (< 7%) and percentages of patients who achieved a pre-specified composite target (HbA1c < 7% without hypoglycaemia or weight gain) for the 2 pairs of subgroups.
Safety outcomes included between-groups comparisons of percentages of patients who had adverse events (AEs) and treatment-related adverse reactions, as well as between-groups comparisons of weight gains from baseline and percentages of patients who experienced at least 1 hypoglycemic episode and mean number of hypoglycemic episode(s) per patient for the 2 pairs of subgroups.
Statistical analysis
Efficacy analyses were conducted on the intent-to-treatment (ITT) population (all randomized patients receiving ≥ 1 dose of study drug) with last observation carried forward (LOCF), while safety analysis were conducted on the safety set (SS) (all randomized patients who received ≥ 1 dose of study drug and had baseline safety data and safety data for at least 1 follow-up visit).
Data were presented as mean ± standard deviation (SD) or N (percentage). To evaluate between-groups differences, t test or Wilcoxon rank sum test was used for continuous variables while Chi-square test was used for categorical variables. Statistical significance was accepted with a P value < 0.05. SAS version 9.1.3 for Windows (SAS Institute Inc., Cary, North Carolina, USA) was used for all statistical analyses.
Results
For patients at high 10-year CV risk, compared with BIAsp 30 monotherapy, BIAsp 30 plus metformin led to significantly higher percentage of patients achieving HbA1c target of < 7%
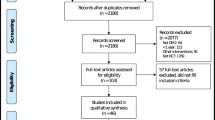
Flow diagram of the MERIT study was depicted in Fig. 1. The ITT populations included 130 and 127 patients in the BIAsp 30 plus metformin group and the BIAsp 30 monotherapy group, respectively. Among them, records of 103 patients in the BIAsp plus metformin group and 107 patients in the BIAsp 30 monotherapy group were complete for the purpose of GloboRisk calculation. Assuming all patients were non-smokers, the BIAsp 30 plus metformin group included 39 patients at low 10-year CV risk and 64 patients at high 10-year CV risk, while the BIAsp 30 monotherapy group included 56 low-risk patients and 51 high-risk patients. Assuming all patients were smokers, the BIAsp 30 plus metformin group included 14 low-risk patients and 89 high-risk patients, while the BIAsp monotherapy group included 17 low-risk patients and 90 high-risk patients (Table 1).
Flow diagram of the MERIT study. ITT, intent-to treat (created using Microsoft Word 2010, https://www.microsoft.com/en-us/microsoft-365/previous-versions/microsoft-word-2010).
Regardless of whether we assumed all patients were non-smokers or smokers, the 2 treatments led to comparable HbA1c changes from baseline and comparable percentages of patients achieving the composite endpoint of HbA1c < 7% without hypoglycemia or weight gain in both low-risk patients and high-risk patients (All P > 0.05) (Table 1).
Meanwhile, regardless of whether we assumed all patients were non-smokers or smokers, BIAsp 30 plus metformin led to significantly higher percentage of patients achieving HbA1c target of < 7% than BIAsp 30 monotherapy in high-risk patients (54.55% vs 34.78%, P = 0.0470; and 53.33% vs 36.71%, P = 0.0381 assuming all were non-smokers and smokers, respectively), while comparable percentages of low-risk patients in the 2 treatment groups achieved HbA1c target of < 7% (P > 0.05) (Table 1).
BIAsp 30 plus metformin and BIAsp 30 monotherapy had overall comparable safety profiles for both low-risk and high-risk patients
Safety analyses were performed on the SS which included 128 and 125 patients in the BIAsp 30 plus metformin and the BIAsp monotherapy groups, respectively. Among them, records of 101 and 106 patients in the BIAsp 30 plus metformin group and the BIAsp 30 monotherapy group were complete for the purpose of GloboRisk calculation, respectively.
Assuming all patients were non-smokers, the BIAsp 30 plus metformin group included 38 low-risk patients and 63 high-risk patients, while the BIAsp 30 monotherapy group included 55 low-risk patients and 51 high-risk patients. Assuming all patients were smokers, the BIAsp 30 plus metformin group included 14 low-risk patients and 87 high-risk patients, while the BIAsp monotherapy group included 16 low-risk patients and 90 high-risk patients (Table 2).
Regardless of whether all patients were assumed non-smokers or smokers, the 2 treatment groups had comparable percentages of patients who had AE(s) and patients experiencing at least 1 hypoglycemic episode as well as comparable numbers of hypoglycemic episode per patients in both low-risk and high-risk patients (P all > 0.05) (Table 2).
Meanwhile, regardless of whether all patients were assumed non-smokers or smokers, low-risk patients in the 2 treatment groups had comparable percentages of patients experiencing treatment-related adverse reaction(s) as well as comparable weight gains from baseline (All P > 0.05) (Table 2).
High-risk patients in the BIAsp 30 plus metformin group had significantly less weight gain than patients in the BIAsp 30 monotherapy group assuming all patients were non-smokers (0.25 ± 1.96 kg vs 1.37 ± 1.95, P = 0.0117), whereas when we assumed all patients were smokers, high-risk patients in the 2 treatment groups had comparable weight gains (P > 0.05) (Table 2).
Finally, when we assumed all patients were smokers, significantly higher percentage of high-risk patients in the BIAsp plus metformin group experienced treatment-related adverse reactions than patients in the BIAsp monotherapy group (21.84% vs 8.89%, P = 0.0166), while when all patients were assumed non-smokers, the 2 treatment groups had comparable percentages of high-risk patients with treatment-related adverse reactions (P > 005) (Table 2).
Compared with BIAsp 30 monotherapy, BIAsp 30 plus metformin led to significantly higher percentages of patients with BMI ≤ 26 kg/m2 achieving HbA1c target of < 7% and achieving the composite target of HbA1c < 7% without hypoglycemia or weight gain
The BIAsp 30 plus metformin group included 84 patients with BMI ≤ 26 kg/m2 and 46 patients with BMI > 26 kg/m2 in the ITT population, while the BIAsp 30 monotherapy group included 85 patients with BMI ≤ 26 kg/m2 and 42 patients with BMI > 26 kg/m2 in the ITT population (Table 3).
The 2 treatment groups had comparable HbA1c changes from baseline in both patients with BMI ≤ 26 kg/m2 and patients with BMI > 26 kg/m2 (both P > 0.05) (Table 3). On the other hand, for patients with BMI ≤ 26 kg/m2, compared with BIAsp 30 monotherapy, BIAsp 30 plus metformin led to significantly higher percentages of patients achieving HbA1c target of < 7% (47.83% vs 28.17%, P = 0.0165) and achieving the composite target of HbA1c < 7% without hypoglycemia or weight gain (20.29% vs 6.85%, P = 0.0187), though not for patients with BMI > 26 kg/m2 (both P > 0.05) (Table 3).
A slightly better safety profile was associated with BIAsp 30 plus metformin than BIAsp 30 monotherapy for patients with BMI ≤ 26 kg/m2 and a slightly worse safety profile associated with BIAsp 30 plus metformin than BIAsp 30 monotherapy for patients with BMI > 26 kg/m2
The BIAsp 30 plus metformin group included 82 patients with BMI ≤ 26 kg/m2 and 46 patients with BMI > 26 kg/m2 in the SS, while the BIAsp 30 monotherapy group included 83 patients with BMI ≤ 26 kg/m2 and 42 patients with BMI > 26 kg/m2 in the SS (Table 4).
For patients with BMI ≤ 26 kg/m2, compared with BIAsp 30 monotherapy, the BIAsp 30 plus metformin treatment led to significantly smaller weight gain (0.75 ± 2.46 kg vs 1.51 ± 2.40, P = 0.0449), comparable percentages of patients experiencing AEs and treatment-related adverse reactions, comparable percentage of patients having hypoglycemic episode(s) and comparable mean number of hypoglycemic episode per patient (All P > 0.05) (Table 4).
Meanwhile, for patients with BMI > 26 kg/m2, compared with BIAsp 30 monotherapy, BIAsp 30 plus metformin led to significantly greater percentage of patients having hypoglycemic episode(s) (26.05% vs 9.52%, P = 0.0442) and significantly more hypoglycemic episode per person (0.46 ± 0.91 vs 0.10 ± 0.30, P = 0.0329), as well as comparable percentages of patients experiencing AEs and treatment-related adverse reactions and comparable weight gain from baseline (All P > 0.05) (Table 4).
Discussion
In this post hoc analysis of the MERIT study, we found that for patients at high 10-year CV risk (GloboRisk score ≥ 20%), compared with BIAsp 30 monotherapy, BIAsp 30 with metformin had a somewhat better efficacy profile (significantly higher percentage of patients achieving HbA1c target of < 7%, comparable HbA1C change from baseline and comparable percentage of patients achieving the composite target of HbA1c < 7% without hypoglycemia or weight gain) and an overall comparable safety profile, while BIAsp 30 with and without metformin had comparable efficacy and safety profiles for patients at low 10-year CV risk. Meanwhile, compared with BIAsp 30 monotherapy, for patients with BMI ≤ 26 kg/m2, BIAsp 30 plus metformin led to significantly higher percentages of patients achieving HbA1c target of < 7% and achieving the composite target of HbA1c < 7% without hypoglycemia or weight gain, and comparable HbA1c change from baseline. It also had a better safety profile (significantly less weight gain) than BIAsp 30 monotherapy in patients with BMI ≤ 26 kg/m2. As for patients with BMI > 26 kg/m2, the two treatments had comparable efficacy profiles and BIAsp 30 monotherapy had a somewhat better safety profile than BIAsp with metformin (lower percentage of patients having hypoglycemic episode[s] and less hypoglycemic episode per patient associated with BIAsp 30 monotherapy).
Metformin is the preferred initial pharmacological treatment for T2DM5,9. It has been recognized that adding metformin to insulin allows for insulin dose reduction and could reduce insulin-associated side effects such as weight gain9,26, a point also made evident by the MERIT study5. In the MERIT study, during the treatment period, patients in the BIAsp 30 plus metformin group received significantly lower daily insulin dose than patients in the BIAsp 30 monotherapy group (0.38 ± 0.14 U/kg/day vs 0.47 ± 0.15 U/kg/day) as well as experienced significantly less weight gain (0.33 ± 2.68 kg vs 1.16 ± 2.45 kg), although the 2 group of patients experienced comparable number of hypoglycemic events5. The MERIT study and others have shown that insulin plus metformin was both effective and safe5,26,27,28,29. Removing metformin from insulin—metformin combination therapy led to significant deterioration of glycemic control26,30.
An individualized, patient-centered and multi-factorial approach in managing T2DM is recommended, and better control of CV risk is one important feature of this approach12,14. Besides glycemic control, it was also important to modify other CV risk factors such as obesity, high blood pressure and hyperlipidemia in order to reduce risk of macrovascular and microvascular complications commonly associate with T2DM14. Therefore, the effects of various antidiabetic agents on CV risk factors should be considered when choosing a treatment for T2DM patients9,14. Studies have shown that metformin could improve CV risk profile in patients with T2DM as reflected by its ability to reduce TC, triglyceride, BMI, waist circumference and blood pressure7,13,14,26. Knowing that metformin could confer some cardiovascular benefits, there is still the question of exactly what kind of patients could benefit most from metformin. It has been reported that high blood pressure and hypertension phenotype suppressed the efficacy of metformin in lowering HbA1c15. A lower baseline TC level was reported to be a predictor for higher HbA1c level 6 months after metformin initiation, while at 18 months, baseline cholesterol level ceased to be a predictor16. At the same time, studies assessing whether a patient’s baseline BMI affected efficacy of metformin generated inconsistent results17,18,19,20. However, whether a patient’s composite CV risk affected efficacy and safety of metformin or comparative efficacy and safety of insulin with and without metformin has not been assessed, such a study could provide a comprehensive picture as to what kind of patients would benefit most from a particular treatment and could potentially help clinicians choose a treatment tailored to individual patient’s CV risk.
Our post hoc analysis of the MERIT study was the first to use GloboRisk, a tool for predicting a person’s 10-year risk of CVD21,22, to assess whether a patient’s composite CV risk affected comparative efficacy and safety of 2 treatments. Our results indicated that for patients with high CV risk, BIAsp 30 plus metformin combination therapy was preferable to BIAsp 30 monotherapy. For patients with low CV risk, the two treatments were comparable. Prescribing BIAsp 30 plus metformin combination therapy instead of BIAsp 30 monotherapy to patients at high CV risk could also take advantage of metformin’s known ability to improve a patient’s CV risk profile7,13,14,26. Our analysis demonstrated that GloboRisk could be a useful tool to help clinicians choose a proper treatment for patients according to their composite CV risk.
Laboratory-based GloboRisk does not include a patient’s baseline BMI as a variable21,22, although BMI is a commonly known CV risk factor23. Therefore we also assessed whether comparative efficacy and safety of BIAsp 30 with and without metformin differed for patients with BMI ≤ 26 kg/m2 and patients with BMI > 26 kg/m2 and found that BIAsp 30 plus metformin had better efficacy as well as led to significantly less weight gain than BIAsp 30 monotherapy for patients with BMI ≤ 26 kg/m2, while the 2 treatment had comparable efficacy profiles for patients with BMI > 26 kg/m2. Although this result seemed to somewhat differ from our findings that BIAsp 30 plus metformin had better efficacy than BIAsp 30 monotherapy for patients at high CV risk, it must be noted that although high BMI is a CV risk factor, high BMI alone does not represent high composite CV risk. Of course, more studies are needed.
What are the clinical implications of our findings? Our results suggested when choosing between BIAsp 30 plus metformin and BIAsp 30 monotherapy, a clinician could take into consideration a patient’s CV risk such as laboratory-based Globorisk score and BMI. For patients at high CV risk and/or with BMI ≤ 26 kg/m2, BIAsp 30 plus metformin combination is preferred, while for patients with low CV risk and/or BMI > 26 kg/m2, either treatment could be prescribed. Our analysis and finding could be especially helpful for optimizing treatment protocols for Chinese patients. Due to social, economic and psychological factors, delay in insulin initiation and insulin overuse are both common in China31,32,33,34,35. The Observational Registry for BI Treatment (ORBIT) on 19,894 patients with T2DM in China found that prior to insulin initiation, mean duration of diabetes was 6.4 ± 5.3 years and mean HbA1c level was 9.6 ± 2.0%32. In addition, patients with shorter T2DM duration at insulin initiation might also have better glycemic control than patients with longer T2DM duration10. The MERIT study underlying our analysis included patients with a mean T2DM duration of approximately 9 years and HbA1c level of around 9% prior to insulin initiation, and as such it reflected the real-world practice in China5. As it was known that better glycemic control could reduce a patient’s CV risk13,14 and long T2DM duration itself was an additional CV risk factor for patients with diabetes36, patients deemed to be at high CV risk according to their GloboRisk scores (≥ 20%) would have even higher CV risk if they have poor glycemic control and/or long T2DM duration, for these patients, BIAsp 30 and metformin combination therapy could potentially be especially advantageous. Of course, our study is a preliminary analysis, more researches are needed to further elucidate the role of T2DM duration in comparative profiles of BIAsp 30 and metformin combination therapy and BIAsp 30 monotherapy.
Our study was limited by the fact that it was a post hoc analysis of a randomized controlled study not originally designed to test whether a patient’ composite CV risk score and BMI affected comparative efficacy and safety of BIAsp 30 with and without metformin, therefore, our results might not carry the same weight as results obtained from a perspective, pre-specified analysis, and should be viewed as being hypothesis-generating and needing further confirmation by perspective pre-specified studies. Secondly, since we did not record whether our patients were smokers or non-smokers, we assumed all patients were smokers and then all were non-smokers and calculated their GloboRisk scores twice, therefore the accuracy of our results regarding the effect of patient’s CV risk on comparative efficacy and safety of BIAsp with and without metformin could be reduced. However, since regardless of whether all patients were assumed smokers or non-smokers, we got consistent results regarding comparative efficacy of the 2 treatments, we think our findings were solid. Thirdly, as a post hoc analysis, the sample size of our study was not pre-calculated to make sure it was adequately powered. In addition, the MERIT study underlying our analysis was a medium-term 16-week study rather than a long-term study5, therefore our post-hoc analysis only address the impact of CV risk and BMI on comparative medium-term efficacy and safety profiles of the 2 treatments. 3 months are generally enough to properly assess the efficacy and safety of an antidiabetic treatment, and antidiabetic studies lasting 3–4 months were common26,28,29,37. Of course, a long-term study could better address the long-term efficacy and safety of an antidiabetic treatment, however, due to limited funding, we decided that a 16-week study was proper for our purpose of comparing the 2 treatment regimens. On the other hand, our study was the first to use GloboRisk to analyze whether and how patient’s composite CV risk affected comparative efficacy and safety of BIAsp 30 with and without metformin, such a study could help a clinician choose a tailored treatment for a patients from several available treatment regimens according to the patient’s CV risk and/or BMI.
In conclusion, for Chinese patients at high CV risk (≥ 20% GloboRisk score) and/or with BMI ≤ 26 kg/m2, BIAsp 30 and metformin combination therapy could be preferable to BIAsp 30 monotherapy as BIAsp 30 and metformin combination therapy had a better efficacy profile as well as a comparable or even better safety profile than BIAsp 30 monotherapy. Meanwhile, for Chinese patients at low CV risk and/or with BMI > 26 kg/m2, either treatment could be prescribed, as the 2 treatment regimens had comparable efficacy profiles, although BIAsp 30 monotherapy had a slightly better safety profile than the combination therapy for patients with BMI > 26 kg/m2.
Data availability
The data used for this manuscript are available on reasonable request from the corresponding author.
References
Zinman, B. Initial combination therapy for type 2 diabetes mellitus: Is it ready for prime time?. Am. J. Med. 125, S19–S34 (2011).
Alavudeen, S. S. et al. Comparative evaluation of biphasic insulin with metformin and triple oral hypoglycemic agents (OHA) in type 2 diabetes patients. Saudi Pharm. J. 28, 210–214 (2020).
Kann, P. H. et al. Starting insulin therapy in type 2 diabetes: Twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp. Clin. Endocrinol. Diabetes 114, 527–532 (2006).
World Health Organization. Global status report on noncommunicable diseases 2014. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf. (2014).
Guo, L. et al. A randomized, open-label, multicentre, parallel-controlled study comparing the efficacy and safety of biphasic insulin aspart 30 plus metformin with biphasic insulin aspart 30 monotherapy for type 2 diabetes patients inadequately controlled with oral antidiabetic drugs: The merit study. Diabetes Obes. Metab. 20, 2740–2747 (2018).
Wang, L. et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 317, 2515–2523 (2017).
Mourão-Júnior, C. A., Sá, J. R., Guedes, O. M. & Dib, S. A. Effects of metformin on the glycemic control, lipid profile, and arterial blood pressure of type 2 diabetic patients with metabolic syndrome already on insulin. Braz. J. Med. Biol. Res. 39, 489–494 (2006).
American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin. Diabetes 33, 97–111 (2015).
American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2019. Diabetes Care 42, S90–S102 (2019).
Pan, C., Yang, W., Jia, W., Weng, J. & Tian, H. Management of Chinese patients with type 2 diabetes, 1998–2006: The diabcare-China surveys. Curr. Med. Res. Opin. 25, 39–45 (2009).
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41, 2669–2701 (2018).
Cahn, A., Cernea, S. & Raz, I. Outcome studies and safety as guide for decision making in treating patients with type 2 diabetes. Rev. Endocr. Metab. Disord. 17, 117–127 (2016).
Giugliano, D. et al. Metformin for obese, insulin-treated diabetic patients: Improvement in glycaemic control and reduction of metabolic risk factors. Eur. J. Clin. Pharmacol. 44, 107–112 (1993).
Ovalle, F. Cardiovascular implications of antihyperglycemic therapies for type 2 diabetes. Clin. Ther. 33, 393–407 (2011).
Tomioka, S. et al. Clinical characteristics influencing the effectiveness of metformin on Japanese type 2 diabetes receiving sulfonylureas. Endocr. J. 54, 247–253 (2007).
Martono, D. P. et al. Predictors of HbA1c levels in patients initiating metformin. Curr. Med. Res. Opin. 32, 2021–2028 (2016).
Han, J. et al. Different clinical prognostic factors are associated with improved glycaemic control: Findings from MARCH randomized trial. Diabet. Med. 34, 490–499 (2017).
Cai, X. et al. Baseline body mass index and the efficacy of hypoglycemic treatment in type 2 diabetes: A meta-analysis. PLoS ONE 11, e0166625 (2016).
Ji, L. et al. Impact of baseline BMI on glycemic control and weight change with metformin monotherapy in Chinese type 2 diabetes patients: Phase IV open-label trial. PLoS ONE 8, e57222 (2013).
Sumitani, S. et al. Effectiveness of metformin and lifestyle interventions as an initial treatment in Japanese patients with newly diagnosed type 2 diabetes: A prospective observational study. J. Med. Investig. 59, 166–173 (2012).
Hajifathalian, K. et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): A pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 3, 339–355 (2015).
Ueda, P. et al. Laboratory-based and office-based risk scores and charts to predict 10-year risk of cardiovascular disease in 182 countries: A pooled analysis of prospective cohorts and health surveys. Lancet Diabetes Endocrinol. 5, 196–213 (2017).
McGurnaghan, S. et al. Cardiovascular disease prevalence and risk factor prevalence in Type 2 diabetes: A contemporary analysis. Diabet. Med. 36, 718–725 (2019).
World Medical Association. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. http://www.wma.net/ (2008).
China Food and Drug Administration. Good clinical practice of China. http://www.sda.gov.cn/WS01/CL0053/24473.html (2003).
Kvapil, M., Swatko, A., Hilberg, C. & Shestakova, M. Biphasic insulin aspart 30 plus metformin: An effective combination in type 2 diabetes. Diabetes Obes. Metab. 8, 39–48 (2006).
Douek, I. F. et al. Continuing metformin when starting insulin in patients with Type 2 diabetes: A double-blind randomized placebo-controlled trial. Diabet. Med. 22, 634–640 (2005).
Ushakova, O. et al. Comparison of biphasic insulin aspart 30 given three times daily or twice daily in combination with metformin versus oral antidiabetic drugs alone in patients with poorly controlled type 2 diabetes: A 16-week, randomized, open-label, parallel-group trial conducted in Russia. Clin. Ther. 29, 2374–2384 (2007).
Velojic-Golubovic, M. et al. Biphasic insulin aspart 30: Better glycemic control than with premixed human insulin 30 in obese patients with Type 2 diabetes. J. Endocrinol. Investig. 32, 23–27 (2009).
Tong, P. C., Chow, C. C., Jorgensen, L. N. & Cockram, C. S. The contribution of metformin to glycaemic control in patients with type 2 diabetes mellitus receiving combination therapy with insulin. Diabetes Res. Clin. Pract. 57, 93–98 (2002).
Chinese Society of Endocrinology. Chinese expert consensus on the clinical application of insulin for adult type 2 diabetes. Chin. J. Endocrinol. Metab. 29, 1–6 (2013).
Ji, L. et al. Observational registry of basal insulin treatment (ORBIT) in patients with type 2 diabetes uncontrolled by oral hypoglycemic agents in China-study design and baseline characteristics. Diabetes Technol. Ther. 17, 735–744 (2015).
Ji, L., Tsai, S. T., Lin, J. & Bhambani, S. National variations in comorbidities, glycosylated hemoglobin reduction, and insulin dosage in Asian patients with type 2 diabetes: The FINE-Asia registry. Diabetes Ther. 6, 519–530 (2015).
Lee, K. P. Psycholosocial factors associated with psychological insulin resistance in primary care patients in Hong Kong. J. Clin. Transl. Endocrinol. 2, 157–162 (2015).
Yiu, M. P., Cheung, K. L. & Chan, K.W. A questionnaire study to analyze the reasons of insulin refusal of DM patients on maximum dose of oral hypoglycemic agents (OHA) among 3 GOPC in Kowloon West Cluster. http://www.ha.org.hk/haconvention/hac2010/proceedings/pdf/Poster/spp-p5-38.pdf (2010).
Bragg, F. et al. Risks and population burden of cardiovascular diseases associated with diabetes in China: A prospective study of 05 million adults. PLoS Med. 13, e1002026 (2016).
Gu, Y. et al. The impact of initiating biphasic human insulin 30 therapy in type 2 diabetes patients after failure of oral antidiabetes drugs. Diabetes Technol. Ther. 14, 244–250 (2009).
Acknowledgements
The current post hoc analysis of the MERIT trial did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors, although the MERIT trial was sponsored by Merck Serono Co. Ltd. China, an affiliate of Merck KGaA, Darmstadt, Germany. Finally, the authors thank the patients who participated in this trial.
Author information
Authors and Affiliations
Contributions
L.G. was involved in the study design. All authors conducted the MERIT study and collected data need for the current post hoc analysis, contributed to the analysis and interpretation of the data, were involved in drafting the manuscript or revising it critically for important intellectual content and have given final approval of the version to be published. Finally, all authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
L. G. received research funding from Merck Serono Co. Ltd. China. All other authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, L., Chang, B., Chen, L. et al. Composite cardiovascular risk and BMI affected comparative profiles of BIAsp 30 + metformin vs BIAsp 30 monotherapy: a MERIT post-hoc analysis. Sci Rep 11, 4131 (2021). https://doi.org/10.1038/s41598-021-83410-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83410-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.