Abstract
The effects of antihyperglycemic medications on cardiovascular events and mortality are heterogeneous and their effects on intermediate factors might explain these differences. This systematic review explores the relationship between metabolic factors, mechanism of action, and mortality effects of antihyperglycemic medications in type 2 diabetes. Randomized trials assessing the effects of antihyperglycemic medications on all-cause or cardiovascular mortality in type 2 diabetes were included. Myocardial infarction, stroke, and heart failure were secondary outcomes. The effects of medications on HbA1c, severe hypoglycemia (SH), body weight, systolic blood pressure (SBP), and mechanism of action were evaluated. Meta-analyses and meta-regressions were performed grouping studies according to the above-cited factors. All-cause mortality was lower for medications that reduced HbA1c, SH, body weight, and SBP. Decreased cardiovascular mortality was associated with lower HbA1c, SH, SBP. Myocardial infarction and stroke were also associated with favorable metabolic profile. These findings were not confirmed in meta-regression models. Medications associated with lower SH, body weight and SBP had a lower risk of heart failure. In conclusion, medications with better metabolic profile were associated with reduced all-cause and cardiovascular mortality. These findings are based on indirect comparisons and must be applied cautiously.
Similar content being viewed by others
Introduction
Subjects with type 2 diabetes have a high risk of cardiovascular disease, which is the leading cause of death and disability. Many factors influence this risk, such as glucose control, hypoglycemia frequency, body weight, and blood pressure1,2,3.
Unexpectedly, studies analyzing the role of intensified glucose control on cardiovascular mortality were able to achieve significant differences in glycemic control but did not find a reduction in events4,5,6. This lack of benefit may be attributable to the increased number of hypoglycemic events and weight gain associated with strict glycemic control4,5,6.
Several classes of antihyperglycemic medications have been approved for the treatment of type 2 diabetes, but, until recently, metformin was the only medication proven to reduce cardiovascular events and mortality in patients with this condition7. In the last years, cardiovascular safety trials showed that some sodium-glucose-linked cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) are also capable of reducing cardiovascular events and all-cause mortality in high-risk populations8,9,10,11. Although these medications have different mechanisms of action and effects on glucose values, they share similar favorable metabolic effects, such as weight loss, and reduced risk of hypoglycemia12. Furthermore, the mechanism of action may not be the only determinant of the effects of a medication on cardiac events, as different representatives of the same class seem to have diverse effects on cardiovascular events8,11,13,14.
Joint guideline from the European Association for the Study of Diabetes and American Diabetes Association recommend a patient-centered approach15. This is based on the selection of medications in type 2 diabetes based on patient factors and considering the heterogeneity of treatment options, such as weight effects, hypoglycemia risk, and previous cardiovascular events15. Also, medications are classified as pharmacological classes, and not according to individual representatives or their effects on the above factors15. Whether this approach leads to better outcomes to patients is unknown and is mainly based on expert’s opinion.
Considering the heterogeneity between antihyperglycemic agents, we hypothesized that antihyperglycemic medications that lead to better glycemic control, lower severe hypoglycemia risk, lower body weight, and lower blood pressure might reduce the risk of death (all-cause or cardiovascular). We also explored if these outcomes are influenced by the mechanism of action of the medications. The objective of this systematic review with meta-analysis is to evaluate the relationship between the above factors, the treatment with antihyperglycemic medications in type 2 diabetic subjects, and mortality and cardiovascular events.
Methods
We registered this review and meta-analysis in the International Prospective Register of Systematic Reviews (PROSPERO) under number CRD42016043895. This report follows the PRISMA statement for systematic reviews16. Ethical approval was exempted.
Eligibility, data sources, and searches
Studies were eligible if were performed in subjects with type 2 diabetes (patients), evaluated any antihyperglycemic medication (intervention), reported all-cause or cardiovascular mortality (outcome) and were randomized controlled parallel trials (study); no specific comparator was defined. We searched PubMed, EMBASE, the Cochrane Library, and clinicaltrials.org from inception up to May 2020 for randomized controlled trials, performed in patients with type 2 diabetes, that reported any of the main outcomes (all-cause or cardiovascular mortality). There was no additional restriction on the searches. The search terms were type 2 diabetes AND mortality OR cardiovascular mortality AND randomized controlled trial. A hand search of reference lists of previous systematic reviews and key articles was also performed, and all potentially eligible studies were considered for review.
Study selection
Two investigators (DVR and CV) independently screened potentially relevant studies based on titles and abstracts. Studies that met inclusion criteria were thoroughly reviewed. Consensus resolved disagreements. Articles were included if they were randomized controlled trials, included antihyperglycemic medications approved for the treatment of type 2 diabetes, had lasted more than one year and reported at least one of the outcomes of interest (all-cause or cardiovascular mortality).
Data extraction
Two investigators (DVR and CV) independently extracted relevant data from studies. Change in glycated hemoglobin (HbA1c), body weight, systolic blood pressure, and severe hypoglycemia events, were also extracted. We evaluated all-cause mortality and cardiovascular mortality as primary outcomes and the incidence of cardiovascular events (acute myocardial infarction, stroke, and heart failure) as secondary outcomes. As recommended, when the study comprised more than two groups, we either (a) spliced the intervention group to compare with each of the control groups; or (b) combined the control groups to compare with the intervention group, to perform pair-wise comparisons17. Discrepancies in extracted data were resolved by consensus.
Quality assessment
The Cochrane Collaboration tool was used to assess the bias risk of individual studies18. Using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) method, we ranked the quality of the evidence of each outcome as high, moderate, low, or very low17. Summary of findings table was constructed using GRADEPRO software19.
Data synthesis and analysis
To analyze the effects of the factors (glycemic control, severe hypoglycemia risk, body weight, and blood pressure changes) on the outcomes of interest, we adopted the following approach. First, we extracted each variable numerical change from baseline to end of follow-up in the experimental arm. Second, we also extracted the differences between study arms (variable delta of the intervention group minus variable delta of the control group). At last, the effects of an experimental intervention in the factor was classified as “reduction” of the factor (HbA1c, body weight or blood pressure) or “no reduction”. The variable was classified as “reduction” if both changes (variation during follow-up in the experimental arm and between experimental and control arms) were equal or greater than: (a) 0.3% for HbA1c; (b) 1 kg for body weight or; (c) 1 mmHg for systolic blood pressure. We opted this approach as it allows to select interventions that showed real improvements in glycemic control, body weight, or systolic blood pressure, and not a deterioration in the experimental arm that was only smaller than the observed in control group. For example, in UKPDS33 patients in the sulfonylurea group had a mean body weight gain of 2.15 kg, and in the insulin group patients gained 4 kg20. With this approach, we avoided classifying this difference as “weigh reduction”. For severe hypoglycemia, the absolute risk difference between intervention and control arms was used. These categories (“reduction” and “no reduction”) were used to stratify the meta-analyses. As an additional and exploratory analysis, we evaluated if the mechanism of action would influence the results and medications were classified as: (a) insulin / secretagogues; (b) insulin-sensitizing; (c) incretins and; (d) SGLT2i.
The outcomes were combined with Mantel–Haenszel relative risks (RR) with random-effects model17. We evaluated statistical heterogeneity with Cochran’s Q and the I2 test (P < 0.1 and I2 > 50% indicated elevated heterogeneity, respectively). Small study bias was assessed with the funnel plot asymmetry and with the Begg and Egger tests; if bias was identified, we performed a trim-and-fill computation to evaluate the potential effect of unpublished studies on the results21,22. Our objective involved the evaluation and comparison of subgroup analyses in meta-analysis. We followed Cochrane Handbook recommendations for comparing subgroups in random effects model, besides performing stratified analyses, we also compared the subgroups (factors and mechanisms of action) with metaregressions using dichotomous variables17,23. Analyses were performed using Stata 13.0 software (StataCorp, College Station, USA). Graphics were constructed with forestplot package using Rstudio program.
Results
Database review and characteristics of selected studies
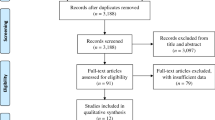
Electronic and manual searches retrieved 2,180 potential studies, of which 2077 were excluded based on titles and abstracts and 103 were selected for full-text review. In total, 46 studies met the inclusion criteria. A complete flow diagram of study inclusion is shown in Fig. 1.
The included studies represent a total of 216,575 patients and a total of 15,304 all-cause deaths and 8,994 cardiovascular deaths. Summarized study characteristics are presented in Table 1; detailed characteristics of the included studies are presented in Table S1 and S2 (Supplementary material). Studies evaluated the effects of almost every class of antihyperglycemic agents; incretin-based therapies were the most common medications assessed (thirteen studies of dipeptidyl peptidase-4 inhibitors and eight studies of GLP-1RA). About half of the studies (24 from 46) had active comparators, of which sulfonylurea was the predominant class.
Three studies had more than two arms and needed group splicing or combining to be included in this meta-analysis. In the ADOPT and PIONEER 4 studies24,25, we spliced one study arm (ADOPT: metformin; PIONEER 4: placebo) and compared it to remainder arms. In the 4-T study26, we combined the groups receiving bolus insulin (prandial insulin and biphasic insulin).
Overall, the risk of bias was low, especially for randomization and blinding. For incomplete outcome data, six studies had high risk of bias (mostly due to an unbalanced number of patients without follow-up information). Detailed assessment information on the risk of bias of individual studies is presented in the supplementary appendix (Table S3, Supplementary material).
Main results
As shown in Fig. 2 and Table S4 (Supplementary material), antihyperglycemic medications are associated with reduced risk of all-cause and cardiovascular mortality overall. Stratifying the analysis according to glycemic control, severe hypoglycemia risk, body weight, and systolic blood pressure showed that treatments that lead to reduction of these factors (except weight change for cardiovascular mortality) were associated with lower all-cause mortality. Glycemic control was the factor with the greatest numeric reduction of all-cause mortality (RR 0.90 [95% CI 0.84–0.96]) and cardiovascular mortality (RR 0.88 [95% CI 0.80–0.96]); Table S3, Supplementary material. In the meta-regression models, none of these comparisons were statistically significant.
Regarding analyses evaluating mechanisms of action of medications (Table S4 and Figure S1A and S1B, Supplementary material), only incretins were associated with reduced all-cause or cardiovascular mortality. Meta-regression models found that this difference was not statistically significant.
Statistical heterogeneity was low (Table S4, Supplementary material). Small study bias was not identified with Egger and Begg tests and funnel plot inspection (Supplementary material, Figure S2 A and B).
Secondary outcomes
Treatments that lead to lower HbA1c, severe hypoglycemia risk, body weight, and systolic blood pressure were associated with a lower risk for myocardial infarction (Table S4 and Figures S1C and S1D, Supplementary material). Meta-regressions did not confirm these findings. Statistical heterogeneity was low. Small study bias risk was detected for myocardial infarction (Figure S2C, Supplementary material), but trim and fill computation did not change the results.
Reduction of HbA1c, body weight, and systolic blood pressure was associated with lower stroke risk (Table S4 and Figures S1E and S1F, Supplementary material). Meta-regression analyses also did not confirm these findings. Statistical heterogeneity was low, and small study bias was not identified (Figure S2D, Supplementary material). Insulin sensitizers and incretins were associated with lower stroke risk. Although meta-regression did not confirm these associations, for insulin sensitizers the results were borderline (P = 0.055).
As shown in Table S4 and Figures S1G and S1H (Supplementary material), reduction of severe hypoglycemia risk, body weight, and systolic blood pressure factors were associated with a lower risk of heart failure. None of this factors were confirmed in meta-regression. Of note, statistical heterogeneity was elevated in heart failure analyses. Small study bias was not identified (Figure S2E, Supplementary material). Regarding mechanism of action, insulin sensitizers were associated with a higher risk of heart failure and SGLT2i were associated with lower risk, both in stratified and meta-regression analyses.
Grading quality of evidence
The GRADE quality of evidence for all outcomes was moderate and the summary of findings are presented in supplementary material (Table S5, Supplementary material). Despite being based on high-quality studies with low risk of bias, our observations on the effects of clinical factors on the outcomes of interest (all-cause and cardiovascular mortality, myocardial infarction, stroke, and heart failure) are indirect due to the nature of the analysis17.
Discussion
Based on data from high-quality RCTs, we found that antihyperglycemic agents that lead to lower HbA1c, severe hypoglycemia risk, body weight, and systolic blood pressure were associated with reduced risk of all-cause and myocardial infarction in patients with type 2 diabetes. Cardiovascular mortality was associated with the same factors except body weight. As the analyses are indirect, based on aggregated patient data and meta-regression models did not confirm the findings, this information must be applied and generalized cautiously.
The results for secondary outcomes are also interesting. The association of SGLT2i with reduced risk for heart failure and insulin sensitizers with a higher risk for heart failure has been already shown27. However, the relation between severe hypoglycemia and heart failure is novel. The mechanism may be a true effect of hypoglycemia on myocardial cells28, however it is more likely to represent medications with safer cardiovascular profile. The association between insulin sensitizers and stroke has been observed previously7,29. Lower insulin resistance may be the promoter of this benefit, and numerically has a greater effect on stroke than myocardial infarction risk30.
These findings are based on an extensive and systematic literature review. The studies identified had mostly low risk of bias. This allowed us to explore our objective with high quality data and with a large number of patients. The definition of “reduction” of a metabolic factor combined the differences within and between arms to improve confidence in the classification. We also followed the recommended statistical approach to compare the subgroups of medications. These complementary analyses allowed the identification of the limitations of our data.
Previous systematic reviews with meta-analysis explored the effects of different antihyperglycemic medications on mortality or cardiovascular events. Two recent network meta-analysis showed contradictory results: one failed to identify evident superiority of any drug class, and the other found that SGLT2i and GLP-1RAs were associated with reduced risk of cardiovascular events and mortality31,32. We believe these results reinforce the importance of the hypothesis explored in our systematic review. Individually, several medication classes were assessed for benefit or harm in systematic reviews of RCTs and also failed to show a consistent effect on mortality and cardiovascular events33,34,35,36. Our review broadens these findings and explored the relationship between metabolic profile and mechanism of action of these medications and mortality. In the stratified meta-analyses, our findings suggest that the metabolic effects of medications must be considered in treatment selection of patients with type 2 diabetes. Also, these results agree with current guidelines, that recommend considering other factors besides the simple glycemic effects of antihyperglycemic agents15.
This is the first systematic review exploring this topic, but some primary studies evaluated this research question recently. An observational study from a Swedish database showed that glycated hemoglobin, systolic blood pressure, and body mass index are all important risk factors for all-cause mortality in patients with type 2 diabetes37. Individual data from large RCTs also indicate the same pattern. An additional analysis of Look AHEAD indicates that patients in the category of greatest weight loss in the first year had decreased risk of cardiovascular death and non-fatal cardiovascular events independent from the arm they were originally randomized38. This Look AHEAD analysis, which corroborates our findings, reinforces that diabetes treatments must focus on other targets (such as avoidance of hypoglycemia, lower weight gain, and lower blood pressure) in addition to glycemic control. Data from EMPA-REG OUTCOME trial suggest that plasma volume contraction may be the main determinant of benefits from empagliflozin treatment39. However, glycemic control, systolic blood pressure, and body weight were also associated with a lower risk of cardiovascular death39. These studies reinforce the reliability of our findings. Further analyses of primary data from RCTs (individually or through individual patient data meta-analysis) could also provide valuable information17,40.
From a mechanistic point of view, our results are biologically plausible, as better glycemic control, less severe hypoglycemic events, lower weight and blood pressure were all associated with better outcomes in subjects with type 2 diabetes in observational or interventional studies1,2,3. However, this systematic review does not determine if: (a) the factors are predictors of a beneficial effect of these medications or; (b) there is a mechanistic relationship between favorable overall medication profile and mortality and cardiovascular events. As we observed small changes in the metabolic factors, it seems more likely that there is association rather than causation. That is, small changes on intermediate outcomes (glycemic control, severe hypoglycemia risk, body weight, and blood pressure) are not expected to be the determinants of reduced mortality risk. We also could not explore the effects of medications on cholesterol, as reporting was available only in a few studies.
This study has some limitations inherent to the design used. We aimed to explore the new hypothesis that other factors along with mechanism of action of each antihyperglycemic agent might explain the variability on its clinical effects. Cochrane Handbook states that subgroup analyses are observational17, so our findings must be considered indirect. They are susceptible to bias, such as confounding. Our results represent an association between the study variables and outcomes; so they may not represent causation. Associations between metabolic factors, mechanism of action, and outcomes must be confirmed, as most of the meta-regressions did not explain the heterogeneity within study results. However, we cannot assume the lack of association between studied factors and outcomes only based on meta-regression results as “one should never use a nonsignificant finding to conclude that the true means in subgroups are the same, or that a covariate is not related to effect size”23. Also, as this meta-analysis deals with study-level characteristics, only summarized effects of each study are evaluated and relevant association between factors and outcomes may be missed17. In other words, the link between “better metabolic profile” of antihyperglycemic medications and reduced mortality must be further explored and studied, ideally with meta-analysis from individual patient data17,40.
Another issue of this review is the combination of different classes of medications. This problem may be considered the main limitation as there is unquestionable clinical heterogeneity in this approach. However, we explored this limitation by considering the mechanisms of action of medication in our analyses. Also, this limitation is inherent to addressing the hypothesis that determinants other than the mechanism of action of antihyperglycemic agents may mediate the clinical effects of antihyperglycemic agents. The use of different comparators is a similar problem. We tried to partially control this limitation by using the differences between study arms rather than using the absolute values. Network meta-analysis might deal with the problem of different comparators. However, it does not allow the evaluation of co-factors and we would not be able to explore our hypothesis.
Conclusion
Antihyperglycemic medications that lead to better overall metabolic profile were associated with decreased all-cause and cardiovascular mortality in patients with type 2 diabetes. Our results were based on indirect comparisons of study-level information and were not confirmed in meta-regression analyses, so this topic must be further studied. Although this data must be considered preliminary, it is relevant to patient and clinicians in the choice of antihyperglycemic treatment in type 2 diabetes.
Data availability
All relevant data are within the paper and the supplementary material.
References
Lee, A. K. et al. The Association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care 41, 104–111. https://doi.org/10.2337/dc17-1669 (2018).
Aucott, L. S. et al. Patterns of weight change after the diagnosis of type 2 diabetes in Scotland and their relationship with glycaemic control, mortality and cardiovascular outcomes: a retrospective cohort study. BMJ Open 6, e010836. https://doi.org/10.1136/bmjopen-2015-010836 (2016).
Kanters, S. D., Banga, J. D., Stolk, R. P. & Algra, A. Incidence and determinants of mortality and cardiovascular events in diabetes mellitus: a meta-analysis. Vasc. Med. 4, 67–75. https://doi.org/10.1177/1358836x9900400203 (1999).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139. https://doi.org/10.1056/NEJMoa0808431 (2009).
Gerstein, H. C. et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559. https://doi.org/10.1056/NEJMoa0802743 (2008).
Patel, A. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572. https://doi.org/10.1056/NEJMoa0802987 (2008).
Turner, R. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 854–865 (1998).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322. https://doi.org/10.1056/NEJMoa1603827 (2016).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128. https://doi.org/10.1056/NEJMoa1504720 (2015).
Neal, B. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657. https://doi.org/10.1056/NEJMoa1611925 (2017).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844. https://doi.org/10.1056/NEJMoa1607141 (2016).
12Association, A. D. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2020 (2020). doi:10.2337/dc20-S009
Pfeffer, M. A. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257. https://doi.org/10.1056/NEJMoa1509225 (2015).
Ratner, R. et al. Cardiovascular safety of exenatide BID: an integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc. Diabetol. 10, 22. https://doi.org/10.1186/1475-2840-10-22 (2011).
Davies, M. J. , & Management of Hyperglycemia in Type 2 Diabetes, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41(2669–2701), 2018. https://doi.org/10.2337/dci18-0033 (2018).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. https://doi.org/10.1136/bmj.b2535 (2009).
17Higgins, J. P. T. & Green, S. Cochrane handbook for systematic reviews of interventions. In The Cochrane Collaboration (2011).
Higgins, J. P. et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. https://doi.org/10.1136/bmj.d5928 (2011).
19GRADEpro GDT: GRADEpro Guideline Development Tool [Software] (2015). https://gradepro.org.
Turner, R. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 837–853 (1998).
Begg, C. B. & Berlin, J. A. Publication bias: a problem in interpreting medical data. J. R. Stat. Soc. Ser. A (Stat. Soc.) 151, 419–463. https://doi.org/10.2307/2982993 (1988).
Sterne, J. A. C., Egger, M. & Smith, G. D. Investigating and dealing with publication and other biases in meta-analysis. BMJ 323, 101–105 (2001).
23Borenstein, M., Hedges, L. V., Higgins, J. P. T. & Rothstein, H. R. In Introduction to Meta‐Analysis Vol. 1 Ch. 21 (Wiley, 2009).
Kahn, S. E. et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443. https://doi.org/10.1056/NEJMoa066224 (2006).
Pratley, R. et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet https://doi.org/10.1016/s0140-6736(19)31271-1 (2019).
Holman, R. R. et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N. Engl. J. Med. 361, 1736–1747. https://doi.org/10.1056/NEJMoa0905479 (2009).
Gilbert, R. E. & Krum, H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 385, 2107–2117. https://doi.org/10.1016/s0140-6736(14)61402-1 (2015).
Hanefeld, M., Frier, B. M. & Pistrosch, F. Hypoglycemia and cardiovascular risk: is there a major link?. Diabetes Care 39(Suppl 2), S205-209. https://doi.org/10.2337/dcS15-3014 (2016).
Kernan, W. N. et al. Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 374, 1321–1331. https://doi.org/10.1056/NEJMoa1506930 (2016).
Rundek, T. et al. Insulin resistance and risk of ischemic stroke among nondiabetic individuals from the northern Manhattan study. Arch. Neurol. 67, 1195–1200. https://doi.org/10.1001/archneurol.2010.235 (2010).
Palmer, S. C. et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA 316, 313–324. https://doi.org/10.1001/jama.2016.9400 (2016).
Zheng, S. L. et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA 319, 1580–1591. https://doi.org/10.1001/jama.2018.3024 (2018).
Wu, S. et al. The cardiovascular effect of incretin-based therapies among type 2 diabetes: a systematic review and network meta-analysis. Expert Opin. Drug. Saf. 17, 243–249. https://doi.org/10.1080/14740338.2018.1424826 (2018).
Liu, J. et al. Incretin based treatments and mortality in patients with type 2 diabetes: systematic review and meta-analysis. BMJ 357, j2499. https://doi.org/10.1136/bmj.j2499 (2017).
Tang, H. et al. Meta-analysis of effects of sodium-glucose cotransporter 2 inhibitors on cardiovascular outcomes and all-cause mortality among patients with type 2 diabetes mellitus. Am. J. Cardiol. 118, 1774–1780. https://doi.org/10.1016/j.amjcard.2016.08.061 (2016).
Boussageon, R. et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS Med 9, e1001204. https://doi.org/10.1371/journal.pmed.1001204 (2012).
Rawshani, A. et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 379, 633–644. https://doi.org/10.1056/NEJMoa1800256 (2018).
Gregg, E. W. et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 4, 913–921. https://doi.org/10.1016/s2213-8587(16)30162-0 (2016).
Inzucchi, S. E. et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 41, 356–363. https://doi.org/10.2337/dc17-1096 (2018).
Pc, L., Aj, S., Kr, A. & Dr, J. A comparison of summary patient-level covariates in meta-regression with individual patient data meta-analysis. J. Clin. Epidemiol. https://doi.org/10.1016/s0895-4356(01)00414-0 (2002).
Dormandy, J. A. et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the proactive study (prospective pioglitazone clinical trial in macrovascular events): a randomised controlled trial. Lancet 366, 1279–1289. https://doi.org/10.1016/s0140-6736(05)67528-9 (2005).
Mazzone, T. et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA 296, 2572–2581. https://doi.org/10.1001/jama.296.21.joc60158 (2006).
Nauck, M. A., Meininger, G., Sheng, D., Terranella, L. & Stein, P. P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes. Metab. 9, 194–205. https://doi.org/10.1111/j.1463-1326.2006.00704.x (2007).
Dargie, H. J. et al. A randomized, placebo-controlled trial assessing the effects of rosiglitazone on echocardiographic function and cardiac status in type 2 diabetic patients with New York Heart Association Functional Class I or II Heart Failure. J. Am. Coll. Cardiol. 49, 1696–1704. https://doi.org/10.1016/j.jacc.2006.10.077 (2007).
Chan, J. C. et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes. Metab. 10, 545–555. https://doi.org/10.1111/j.1463-1326.2008.00914.x (2008).
Home, P. D. et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373, 2125–2135. https://doi.org/10.1016/s0140-6736(09)60953-3 (2009).
Kooy, A. et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch. Intern. Med. 169, 616–625. https://doi.org/10.1001/archinternmed.2009.20 (2009).
Bertrand, O. F. et al. Cardiometabolic effects of rosiglitazone in patients with type 2 diabetes and coronary artery bypass grafts: a randomized placebo-controlled clinical trial. Atherosclerosis 211, 565–573. https://doi.org/10.1016/j.atherosclerosis.2010.06.005 (2010).
Gaziano, J. M. et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 33, 1503–1508. https://doi.org/10.2337/dc09-2009 (2010).
Giles, T. D., Elkayam, U., Bhattacharya, M., Perez, A. & Miller, A. B. Comparison of pioglitazone vs glyburide in early heart failure: insights from a randomized controlled study of patients with type 2 diabetes and mild cardiac disease. Congest. Heart Fail. 16, 111–117. https://doi.org/10.1111/j.1751-7133.2010.00154.x (2010).
Matthews, D. R. et al. Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes. Metab. 12, 780–789. https://doi.org/10.1111/j.1463-1326.2010.01233.x (2010).
Gallwitz, B. et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet 379, 2270–2278. https://doi.org/10.1016/s0140-6736(12)60479-6 (2012).
Gallwitz, B. et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 380, 475–483. https://doi.org/10.1016/s0140-6736(12)60691-6 (2012).
Garber, A. J. et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 379, 1498–1507. https://doi.org/10.1016/s0140-6736(12)60205-0 (2012).
Gerstein, H. C. et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 367, 319–328. https://doi.org/10.1056/NEJMoa1203858 (2012).
Zinman, B. et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care 35, 2464–2471. https://doi.org/10.2337/dc12-1205 (2012).
Cefalu, W. T. et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382, 941–950. https://doi.org/10.1016/s0140-6736(13)60683-2 (2013).
Hong, J. et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 36, 1304–1311. https://doi.org/10.2337/dc12-0719 (2013).
Scirica, B. M. et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326. https://doi.org/10.1056/NEJMoa1307684 (2013).
White, W. B. et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369, 1327–1335. https://doi.org/10.1056/NEJMoa1305889 (2013).
Ridderstrale, M. et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2, 691–700. https://doi.org/10.1016/s2213-8587(14)70120-2 (2014).
Blonde, L. et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet 385, 2057–2066. https://doi.org/10.1016/s0140-6736(15)60936-9 (2015).
Giorgino, F., Benroubi, M., Sun, J. H., Zimmermann, A. G. & Pechtner, V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (award-2). Diabetes Care 38, 2241–2249. https://doi.org/10.2337/dc14-1625 (2015).
Green, J. B. et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 373, 232–242. https://doi.org/10.1056/NEJMoa1501352 (2015).
Holman, R. R. et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 377, 1228–1239. https://doi.org/10.1056/NEJMoa1612917 (2017).
Marso, S. P. et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N. Engl. J. Med. 377, 723–732. https://doi.org/10.1056/NEJMoa1615692 (2017).
Vaccaro, O. et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol. 10, 15–20. https://doi.org/10.1016/s2213-8587(17)30317-0 (2017).
Hernandez, A. F. et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392, 1519–1529. https://doi.org/10.1016/s0140-6736(18)32261-x (2018).
Rosenstock, J. et al. Effect of Linagliptin vs Placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 321, 69–79. https://doi.org/10.1001/jama.2018.18269 (2019).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet https://doi.org/10.1016/s0140-6736(19)31149-3 (2019).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa1901118 (2019).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306. https://doi.org/10.1056/NEJMoa1811744 (2019).
Pieber, T. R. et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 7, 528–539. https://doi.org/10.1016/s2213-8587(19)30194-9 (2019).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357. https://doi.org/10.1056/NEJMoa1812389 (2019).
Rosenstock, J. et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA https://doi.org/10.1001/jama.2019.13772 (2019).
Acknowledgments
This manuscript is dedicated to the memory of our dear mentor and coauthor Jorge Luiz Gross, who died in May 2017.
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundo de Incentivo à Pesquisa e Eventos – Hospital de Clínicas de Porto Alegre (FIPE-HCPA), CAPES-PROEX and PRONEX-FAPERGS. Leitao CB is recipient of a PQ scholarship form CNPq. Support for the publication fee was provided. CNPq, FIPE-HCPA, CAPES and FAPERGS had no role in the design and conduct of the study; extraction, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
DVR was responsible for study design, data acquisition, analysis, interpretation, and drafting of the manuscript. CV contributed to study design, reference selection, and data acquisition. LCP contributed to study design, data analysis and interpretation, and drafting of the manuscript. FG contributed to study design, data analysis and interpretation, and drafting of the manuscript. CBL contributed to study design, data analysis and interpretation, and drafting of the manuscript. JLG contributed to study design, data analysis and interpretation, and drafting of the manuscript. All authors have read and approved the final manuscript. DVR is the guarantor of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). JLG reports grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico, during the conduct of the study; grants and other from Eli Lilly, grants from Bristol-Myers Squibb, grants and other from Boehringer Ingelheim, grants from GlaxoSmithKline, grants and other from Novo Nordisk, grants from Janssen, all outside the submitted work; FG reports being from advisory board from Aegerion, receiving travel grants and personal fees (speaker) from Novo Nordisk, travel grants from Sanofi Aventis, travel grants from Abott, all outside the submitted work; CBL was a recipient of a scholarship grant from CNPq; no other relationships or activities that could appear to have influenced the submitted work are reported.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rados, D.V., Viecceli, C., Pinto, L.C. et al. Metabolic effects of antihyperglycemic agents and mortality: meta-analysis of randomized controlled trials. Sci Rep 10, 12837 (2020). https://doi.org/10.1038/s41598-020-69738-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69738-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.