Abstract
Synthetic estrogens such as ethinylestradiol (EE2) are persistent micropollutants that are not effectively removed from wastewater by conventional treatments. These contaminants are released into waterbodies, where they disrupt endocrine systems of organisms and cause harmful effects such as feminization, infertility, reproduction problems and genital malformations. The consequences of this pollution for key marine ecosystems such as coral reefs and their associated microbiomes are underexplored. We evaluated the effects of EE2 concentrations of 100 ng L−1 and 100 µg L−1 on the coral metaorganism Mussismilia harttii. The results indicated no effects on visible bleaching or Fv/Fm ratios in the corals during a 17-day microcosm experiment. However, next-generation sequencing of 16S rDNA revealed a statistically significant effect of high EE2 concentrations on OTU richness, and shifts in specific microbial groups after treatments with or without EE2. These groups might be bioindicators of early shifts in the metaorganism composition caused by EE2 contamination.
Similar content being viewed by others
Introduction
Estrogenic hormones are part of a group of endocrine-disrupting compounds (EDCs) that can alter the endocrine system of animals1,2,3. 17α-ethinylestradiol (EE2) is a synthetic hormone used in birth-control pills and in hormone-replacement drugs in the menopausal period, or to treat other hormonal deficiencies4. EE2 is derived from the main endogenous human estrogen, estradiol (E2), but is 10 to 50 times more powerful5,6. Its longer half-life makes EE2 more available for bioaccumulation by other animals7,8,9,10. This compound is released into the environment through human excreta, and is persistent4,11.
Studies have shown that EE2 can cause estrogenic effects in very low concentrations (1 to 5 ng L−1), with a predicted no-effect concentration of 0.35 ng L−112. However, EE2 concentrations of 0.001 to 0.042 µg L−113 or higher, of 0.831 μg L−114 have been reported in some wastewater-treatment plants and their receiving water bodies, and may come into direct contact with wildlife2,7,15,16,17. The presence of estrogens in water bodies is responsible for female infertility, feminization of male fish, and sexual dysfunction of many aquatic species2,3,18,19,20. These compounds also affect the microbiome associated with different organisms, such as the insect Megaselia scalaris, which had its development impacted by a hormone diet (including EE2), with a significant effect on microbial groups compared with control samples and the appearance of Mycobacterium, Sphingobacterium, Nocardioides, Acinetobacter and other genera21. Mosquitoes exposed to hormone treatment in environmentally important concentrations showed a significant difference in their microbiome compared to untreated samples22,23. In one study, Microbacterium laevaniformans was the most abundant species in hormone-treated samples, whereas Wolbachia pipientis was most abundant in the control23. Continuous use of human oral contraceptive pills was found to alter the normal vaginal microbiome and increase yeast colonization24. These examples from humans and insects are used as references due to the lack of information regarding the effects of EE2 on the coral-associated microbiome. The investigation of the impacts of EE2 on other animal models and their associated microbiomes will add to the current body of information regarding the importance of this pollutant in potentially symbiotic microbial populations.
The effects of estrogens on corals have been examined in a few studies focusing on their metabolism, reproduction and physiology25,26,27,28,29. Sex steroids are biologically active in invertebrates, including corals, although their mechanisms of action remain unclear26,30. It is suggested that estrogens stimulate the process of gamete release and coral spawning, and that their eggs contain estrogenic compounds that will aid in their final maturation27,31. It is also speculated that differences in estrogen concentration will regulate the reproductive processes of corals32. Tarrant et al.33 found that corals have the capacity to take up estrogens from the water and accumulate these compounds in their tissue, although the consequences of this bioaccumulation are unknown.
Corals are metaorganisms, i.e., a biological unit that includes the host and its associated microbiome, whether stable or transitory34. Coral-associated microbes are diverse, complex, dynamic, and essential for the functioning and balance of the coral reef ecosystem35,36,37,38. These microorganisms have several functions that aid in coral fitness36,37,39,40, such as nitrogen fixation41, ammonia metabolism42, removal of nitrogenous waste42, production of antibiotics, physical occupation of coral space, and competition with other microorganisms, in order to protect the host from invasion of opportunistic pathogens37,43,44.
The coral-associated microbial community is sensitive to environmental changes and can quickly respond and adapt to new environmental conditions, which is important in maintaining the homeostasis of the metaorganism37,42,44. Different environmental stressors, such as thermal stress38,45,46, oil47,48 the presence of pathogens40,49,50 or ocean acidification51,52 can cause shifts in the coral microbiome, which may change their diversity, abundance and functionality for long periods of time53. For example, exposure of the coral Acropora muricata to relatively high levels of nickel and copper resulted in coral bleaching. Copper pollution also modified the coral microbiome (eukaryotes and prokaryotes)54. One study showed that the bacterial core associated with corals can respond to a gradient of anthropogenic pollution55. Different stressors, such as climate change, pollution and overfishing are correlated with an increase in the richness and diversity of specific coral-associated microbes, including the commonly found association of higher abundances of members of Vibrionaceae and Altermonadaceae with stressed and diseased corals56,57,58. Further, coral micro-organisms are able to degrade or neutralize toxic substances that threaten coral health, suggesting that the microbiome can protect coral health and mitigate the effects of pollution47,59. However, no reports about the effects of EDCs on the health of corals and their microbiome are currently available.
Here, we evaluated the effects of EE2 on the coral Mussismilia harttii in a microcosm system, and, for the first time, the impacts of an EDC on a coral-associated microbiome.
Results
Visual health status and F v/F m
No significant variations in water-quality parameters (temperature, salinity and pH) (Supplementary Table S1) or visual changes (Fig. S1) were observed during the experiment. These observations agreed with lack of change in the Fv/Fm ratios. At the beginning of the experiment (sampling time T0), Fv/Fm = 0.569 in the control samples, 0.553 in the 100 ng L−1 and 0.540 in the 100 µg L−1 samples. The ratio of Fv/Fm in all samples did not change over time, remaining at about 0.537, above the healthy Fv/Fm value (> 0.5) (Fig. 1). After normalization of the Fv/Fm data, no significant difference was apparent among the samples (either in the sampling times or in treatments with or without EE2).
Coral microbiome data analyses
To investigate the microbial profile of corals exposed to two different EE2 concentrations and in a control treatment (i.e., without EE2), the 16S rRNA gene was sequenced and analyzed. A total of 2,603,433 sequences, ranging from 162,262 to 11,409 sequences per sample, were obtained. A large number of sequences were assigned to mitochondrial DNA from the host coral and were removed from the dataset. Quality trimming and data normalization yielded 104,640 sequences, corresponding to 2180 sequences per sample. This sequencing depth was enough to evaluate the total microbiome patterns, as shown in the rarefaction curve (Fig. S2), establishing that most of the microbiome was covered by the sequencing. The diversity and richness of the bacterial community were calculated based on the numbers of OTUs, using the Chao1 and Shannon indexes. The numbers of OTUs of the samples varied over the experiment; at the end, the samples from the 100 µg L−1 EE2 treatment had the most OTUs, followed by the control and 100 ng L−1 EE2 (Fig. S3A). A two-way ANOVA of the OTUs indicated a significant influence of EE2 (p = 0.02), with the number of OTUs in the 100 µg L−1 EE2 treatment higher than in the control. The 100-ng treatment did not differ from the other treatments, nor did time have a significant influence. The Shannon index showed no significant difference between any factors (Fig. S3B).
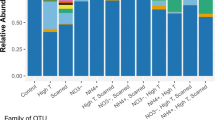
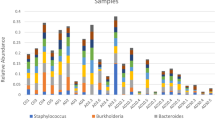
NMDS ordination of the OTU results demonstrated that, despite a slight trend toward treatment-based clustering at the last sampling time, the total coral-associated microbiome was not significantly affected by EE2 contamination, and was clustered only by time (days 0, 3, 9 and 17, i.e. T0, T3, T9 and T17 respectively) (p < 0.0001, pseudo F = 1.7321), regardless of the treatment (Fig. 2). Taxonomic investigation showed that Proteobacteria was the most abundant phylum in the coral samples, with or without estrogen. This group comprised about 60% of the bacterial community in all samples, followed by unclassified Bacteria, Firmicutes and Bacteroidetes. Additionally, Acidobacteria showed significant differences between times T0 and T17, as did Deinococcus-Thermus, which also showed interaction among factors, according to ANOVA followed by a Tukey test (p < 0.05) (Fig. S4). At the class level, Alphaproteobacteria predominated, comprising about 40% of the total microbiome. Betaproteobacteria showed a significant reduction in relative abundance compared to the control with 100 μg of EE2 (Two-Way ANOVA, p < 0.05) and Gammaproteobacteria and Acidobacteria groups GP4 and GP10 showed significant differences as a function of the incubation time (Two-Way ANOVA, p < 0.05) (Fig. 3A). At the genus level, taxonomic diversity and relative abundance were similar (with few differences) among all samples. Rhizobiales_unclassified, Verrucomicrobiaceae_unclassified, Sphingomonadaceae_unclassified, Erythrobacter, and Blastopirellula differed significantly among the different collection times; the last genus showed interaction among the factors (Fig. 3B). Most genera were unclassified, related to Rhodobacteriaceae, Bacteria, Gammaproteobacteria, Alphaproteobacteria, Ruegeria, Clostridiales and Pseudobacteriovorax.
(A) Taxonomic classification and relative abundance of the bacterial classes from Mussismilia harttii treatments: control (without EE2), 100 ng L−1 and 100 µg L−1 of EE2 (n = 4). + indicates significant difference between times (days; T3 and T9) and treatment control with 100 µg L−1 of EE2; *indicates significant difference between times (days; T3, T9 and T17 in Gammaproteobacteria) and T3 and T17 in Acidobacteria. (B) Taxonomic classification and relative abundance of bacterial genera from M. harttii treatments: control (without EE2), 100 ng L−1 and 100 µg L−1 of EE2 (n = 4). + indicates significant difference between times (days; T0 and T17) and interaction among factors; *indicates significant difference between times. The third day (T3) was significantly different in Sphingomonadaceae_unclassified; times T0 and T17 in Verrucomicrobiaceae_unclassified; times T3 and T9 in Erythrobacter; and in Rhizobiales_unclassified, times T0 and T17 differed significantly, as did T9 and T17.
An Indicator Species Analysis (ISA) was performed with the 115 most abundant OTUs (all OTUs with more than 0.1% of the global relative abundance), to assess the impacts of EE2 contamination on specific organisms. We found 14 OTUs that were significant for at least one treatment. OTU8-Proteobacteria, OTU59-Bacteria, OTU62-Clostridiales, OTU68-Methyloceanibacter, and OTU79-Rhodobacteraceae were more abundant in the EE2 treatments than in the control. OTU30-Rhodobacteraceae, OTU33-Actinobacteria, OTU96-Pseudomonas and OTU109-Acinetobacter were more abundant in the control than in 100 µg L−1 EE2 (Fig. 4).
Discussion
Marine pollution and environmental stressors threaten coral reefs and wildlife worldwide60,61. Coral reefs in coastal areas are widely exposed to contaminated seawater from untreated effluents, exploratory marine activities, metals, human actions, climate change and spills of oil and other substances62,63,64,65. Corals and other cnidarians can be exposed to synthetic compounds such as estrogens by absorbing dissolved products, ingesting food, or contacting sediments or suspended solids66. They are especially sensitive to lipophilic compounds because their lipid-rich tissues facilitate absorption67. Ethinylestradiol, a synthetic estrogen of the endocrine-disruptor group, is persistent in the marine ecosystem, is difficult to degrade, and can accumulate in animal tissues68. For this reason, it is important to determine how EE2 pollution could affect coral reefs. This study pioneered in investigating the specific effects of estrogenic pollution on the health of corals and their associated microbiome.
Our experiment was carried out in a microcosm system, which proved to be efficient for testing the selected conditions. Physical–chemical measurements (temperature, pH and salinity) did not vary significantly and were not affected by the estrogen contamination, even at the highest concentration (100 µg L−1). Our results showed that over the 17 days of the experiment, the coral health status did not change visually, with no bleaching or tissue loss compared to the control and to the status at the beginning of the experiment. This agreed with the Fv/Fm measurements, which showed a mean of 0.537 in all treatments (including the control), which is above the level for healthy conditions (> 0.5)69 and is similar to the level found by Santos and colleagues (2014)46 in control samples. An Fv/Fm ratio of around 0.600 was observed in M. harttii at 25 °C; when this coral was exposed to a higher temperature (27.3 °C) and contamination by 3.8 µg L−1 of copper, the ratio decreased significantly, negatively affecting the coral70. The impacts of EE2 on coral-associated algae have not yet been investigated. Our results for Fv/Fm showed that in the concentrations used (100 ng and 100 µg L−1), EE2 did not affect the algal photosynthetic efficiency, suggesting a lack of a major impact on the metaorganism health. The use of pulse-amplitude-modulated (PAM) fluorometry is an indirect method used as a proxy for coral health, based on the photosynthetic efficiency of Symbiodiniaceae40,47,71,72,73.
To investigate whether EE2 pollution could affect the microbiome, the microbial profile of the coral samples was analyzed. The diversity and richness indexes varied among samples and sampling times (T0, T3, T9 and T17). The richness of OTUs from the 100 µg L−1 EE2 treatment differed significantly from the other samples (control and supplemented with 100 ng L−1 EE2), suggesting that higher concentrations of estrogens, on the order of µg L−1, can affect the coral-associated microbiome. The number of OTUs in all samples fluctuated during the experiment. The number of OTUs from the control samples increased until a slight decrease on the last day. The OTU numbers increased in samples with 100 ng L−1 EE2 in the first 3 days and then decreased, finishing the experiment with the lowest number; whereas samples with 100 µg L−1 EE2 showed the opposite behavior, with a decrease in the first 3 days and then an increase in OTU numbers until the end of the experiment, when the number of OTUs reached its highest level (Fig. S3a). A possible reason for this increase in the number of OTUs is that certain bacteria can use EE2 as a carbon source, and therefore the presence of EE2 can create new ecological niches, supporting a richer microbial community.
Microbial indicators of pollutants have been described in aquatic ecosystems, such as mangroves76,77 and seawater78,79. Because of their plasticity and rapid responses, microorganisms are early indicators of environmental changes and may be indicators of environmental quality80,81. The coral-microbiome association could be a key factor in the functioning and organization of marine ecosystems82. In some cases, these microbes contribute to the metaorganisms’ resilience to impacts though their rapid response to environmental disturbances, helping to mitigate stress45. In the present study, EE2 contamination (100 ng or 100 µg L−1) did not cause significant changes in the total microbial diversity, as shown in the NMDS analysis. A slight trend toward correlation between replicates of the same treatments was observed by day 17. The dominant major microbial groups observed in our samples are similar to those previously reported as associated with Mussismilia harttii46,47,74,75.
Although the taxonomic results did not indicate that EE2 impacted the total coral microbiome, the Indicator Species Analysis (ISA) showed that certain bacterial groups were enriched in the presence or absence of EE2 contamination. OTU8 (Proteobacteria) was enriched about sevenfold in 100 µg L−1 EE2-contaminated samples but nearly absent in other conditions. OTU17-Microbulbifer and OTU59-Bacteria behaved similarly, with fourfold higher abundances in 100 µg L−1 EE2-contaminated samples than in the control and 100 ng L−1 EE2. OTU79-Rhodobacteraceae, a family that includes estrogen degraders83,84,85,86, was most abundant in samples contaminated with 100 µg L−1 EE2. OTU68-Methyloceanibacter was also significantly more abundant in EE2-contaminated samples, which may be related to these microorganisms’ ability to degrade steroids and also to produce the enzyme cholesterol oxidase87. A recent study found a strong correlation between estrogen metabolism and members of Clostridia88, which could explain the statistical predominance of OTU62-Clostridiales in 100 µg L−1 EE2-exposed corals. The high amount of estrogen in this treatment could be selecting microbial groups that can use estrogens as an energy/carbon source. Likewise, OTU96-Pseudomonas, OTU109-Acinetobacter, OTU30-Rhodobacteraceae and OTU43-Verrucomicrobiaceae were significantly sensitive to EE2 contamination at concentrations of 100 ng L−1 and 100 µg L−1, and were positively correlated with control samples (without EE2). These groups can be considered indicators of an absence of EE2 contamination at these concentrations, and should be further investigated as potential Beneficial Microorganisms for Corals (BMCs)37 to mitigate the harmful effects of EE2.
Although the physiological effects of coral exposure to estrogen are still underexplored, estrogens may be active in invertebrate gametogenesis, increasing their concentration in seawater and in coral eggs during spawning27. Corals are able to take up estrogens from water at low concentrations, on the order of pg L−1, and the true effects and mechanisms of action of these micropollutants are unknown8,28,30. It is suggested that steroidal estrogens may influence and aid in coral reproduction26, and these estrogens have been designated as important bioregulators of the metabolism of scleractinian corals31. Although no harmful effects were observed in our experiment, the effects of endocrine disruptors may appear after long-term exposure to low concentrations5, and therefore longer-term experiments may be necessary to elucidate their effects. It was possible, however, to identify specific coral-associated microbial indicators of EE2 contamination. Also, it is important to consider other responses from corals, to elucidate the impact of EE2 on their health, such as reproductive ability, lipid and protein contents, calcification and respiration rate. These results suggest that specific levels of EE2 contamination might affect the microbiome of the coral metaorganism within short periods of time, and that these groups are early indicators of this contamination.
Material and methods
Coral sample
Mussismilia harttii corals were collected from Santa Cruz Cabrália, Coroa Vermelha Reefs, Brazil in January 2017, under permit number 56537-1 from the Brazilian Environmental Agency (ICMBio/SISBIO). Pieces of this endemic Brazilian coral were collected haphazardly, in triplicate, with a hammer and chisel by SCUBA diving at 8 m depth at three stations (16° 20′ 57.99′′ S; 038° 58′ 45.00′′ W; 16° 20′ 39.30′′ S; 038° 58′ 38.10′′ W; 16° 22′ 2.20′′ S; 039° 00′ 15.63′′ W), and were immediately stored in individual clean plastic bags until arrival in Rio de Janeiro for microbiome analysis. The microcosm experiment was performed in the Marine Aquarium of Rio de Janeiro (AquaRio) in March 2017, and the data were analyzed in the Molecular Microbial Ecology Laboratory (Federal University of Rio de Janeiro).
Microcosm experimental design
The microcosm was constructed of 12 1.2-L aquariums, with individual sumps (10 L) used for seawater circulation and oxygenation, in a recirculating system. Each sump had a submersible pump (Mini A, Sarlo Better, São Caetano do Sul, Brazil) connected to a hose to recirculate the water between the aquarium and its dedicated sump at a turnover of 170 L h−1 (Fig. 5). Each treatment consisted of four replicate aquariums, each containing four randomly distributed M. harttii polyp branches (~ 6 cm). M. harttii has large separate trumpet-like polyps, which facilitates treating them individually. An air-bubbling system was used to provide a turbulent flow, suitable for the corals, in each replicate. After 7 days of acclimatization, the treatments were started, consisting of 4 replicates supplemented with 100 ng L−1 of EE2, 4 with 100 μg L−1 of EE2, and 4 control aquariums (without hormone). The replicate aquarium treatments were randomly distributed in a controlled-temperature water bath, operated at 25 °C for the 17-day experiment. The water-bath temperature was controlled with Full Gauge MT-518ri thermostats (Canoas, Brazil). The aquariums were filled with seawater collected 10 km offshore from Rio de Janeiro, and 30% of the water was changed with unfiltered seawater from sumps every 2 days, with complementary addition of EE2 to maintain the experimental concentration when applicable. The microcosm was artificially illuminated with two actinic blue tubes (T5 24″, 24 W) and six fluorescent Power Compact bulbs (FLC, 20 W) in a 12-h photoperiod regime, resulting in an irradiance of 400 μmol photons m–2 s−1. The corals were not fed during the experiment. Figure 5 shows a schematic representation of the microcosm.
(A) Schematic representation in 3D modeling of the microcosm, using the software SketchUp. The letters indicate the parts of the structure. A. Water-bath; B. Treatment sumps; C. Aquarium supply hose; D. Circulation pump; E. Illumination system (2 fluorescent lamps and 2 blue lamps); F. Aquarium; G. Four fragments of Mussismilia harttii; H. Aquarium supply hose; I. Air entrance hose; J. Overflow. (B) Timeline showing an overview of the experiment.
Physical–chemical parameters
Temperature, pH and salinity were measured every 2 days in each aquarium, using a YSI 550A probe.
Evaluation of coral health parameters
Coral holobiont health was assessed based on the maximum quantum yield of the zooxanthellae-associated Photosystem II (Fv/Fm)89 and the apparent health status during the experiment, including by photographs. The color of the coral tissue was observed visually, by comparing photographs taken at T0, T3, T9 and T17. The Coral Health Chart (University of Queensland) was used to compare the photographs of corals in the different treatments with or without EE2 during the experiment90.
The photosynthetic parameters were evaluated throughout the experiment, using a Diving-PAM pulse amplitude modulated underwater fluorimeter (Heinz Walz GmbH, Germany), with the following configuration: Measuring Light Intensity (MI) = 5; Saturation Pulse Intensity (SI) = 8; Saturation Pulse Width (SW) = 0.8; Gain (G) = 1; Damping (D) = 1. The measurement was performed using a Fiber Quantum Sensor (diameter 1 mm) connected to the Diving-PAM after 20 min of dark acclimatization. The results were analyzed by two-way ANOVA, using Past 3.25 software91.
Coral microbiome investigation
Polyps of Mussismilia harttii were collected from each aquarium at times (days) T0, T3, T9 and T17 for analysis of the bacterial community and the EE2 impact on the corals microbiome. The coral polyps were fragmented and stored in cryotubes, flash-frozen in liquid nitrogen. Subsequently, the samples were macerated using a mortar and pestle, and the total DNA was extracted from 0.5 g of sample, using a Qiagen DNeasy Power Soil kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The DNA was quantified with a Qubit 2.0 Fluorometer High-Sensitivity DNA Kit (Invitrogen, USA) and stored at − 80 °C.
The V4 variable region of the 16S rRNA from the M. harttii samples was amplified using 515F/806R primers92. About 10 ng of DNA was used for PCR amplification and paired-end sequencing by a single-step 30-cycle PCR, using a HotStarTaq Plus Master Mix Kit (Qiagen, USA). The PCR conditions were: 94 °C for 3 min, followed by 28 cycles at 94 °C for 30 s, 53 °C for 40 s and 72 °C for 1 min, with a final elongation step at 72 °C for 5 min. The samples were sequenced at the Argonne National Laboratory (http://ngs.igsb.anl.gov, Lemont, IL, USA) through the Next Generation Sequencing Core on an Illumina Miseq (Illumina, San Diego, CA, USA), following the manufacturer’s guidelines.
The raw sequences were processed using Mothur v.1.39.1 software93 in order to assess the total bacterial diversity of the samples. Paired-end sequences were joined using the make.contigs command with checkorient = t. The sequences were then screened with the screen.seqs command, removing those outside the size range of 245–300 nucleotides and/or with any ambiguity and/or with homopolymers longer than 8. Sequences were then aligned against a pre-processed version of the Silva NR database (passed through a virtual PCR with the same primers used to amplify the samples). The resulting alignment was submitted to screen.seqs and filter.seqs to remove sequences with poor alignment and uninformative columns of the alignment. Then, sequences were pre-clustered using the pre.cluster command with parameter diffs = 2. Chimeric sequences were detected using the chimera.uchime command, using the sequences themselves as a reference, with the option derreplicate = t. Sequences were classified using the Greengenes database (version from August 2013), employing an 80% confidence threshold, and those classified as chloroplasts, mitochondria, Archaea, Eukarya, or not assigned to any kingdom were removed. The remaining high-quality sequences were clustered into operational taxonomic units (OTUs) using dist.seqs followed by the cluster command, with a 3% sequence dissimilarity cutoff, and all singletons were removed. Last, the samples were randomly normalized to the same number of sequences (2180). The OTU distribution in each sample was used to determine the bacterial community diversity and richness, as well as to analyze the microbial structure, using Non-metric multidimensional scaling (NMDS) ordination with Bray–Curtis distance. A two-way permutational multivariate analysis of variance (two-way PERMANOVA) was performed to test for the impact of time and EE2 contamination on OTU distribution. All statistical tests were done using PAST 3.25 software91. A blocked Indicator Species Analysis (ISA) was performed to determine microbial indicator groups. The OTUs that were significantly impacted (p < 0.05) and had an indicator value > 60 are shown. This analysis was conducted with PC-ORD 6.0 software94. The data generated were deposited in the NCBI Sequence Read Archive (SRA) and are available under accession number PRJNA543294.
References
Ghiselli, G. & Jardim, W. F. Interferentes endócrinos no meio ambiente. Quím. Nova 30, 695–706 (2007).
Vilela, C. L. S., Bassin, J. P. & Peixoto, R. S. Water contamination by endocrine disruptors: Impacts, microbiological aspects and trends for environmental protection. Environ. Poll. 235, 546–559 (2018).
Muller, M. et al. Occurrence of estrogens in sewage sludge and their fate during plant-scale anaerobic digestion. Chemosphere 81, 65–71 (2010).
Mills, M. R. et al. Removal of ecotoxicity of 17α-ethinylestradiol using TAML/peroxide water treatment. Sci. Rep. 5, 1–10 (2015).
Laurenson, J. P., Bloom, R. A., Page, S. & Sadrieh, N. Ethinylestradiol and other human pharmaceutical estrogens in the aquatic environment: A review of recent risk assessment data. AAPS J. 16, 299–310 (2014).
Luzio, A., Santos, D., Fontaínhas-Fernandes, A. A., Monteiro, S. M. & Coimbra, A. M. Effects of 17α-ethinylestradiol at different water temperatures on zebrafish sex differentiation and gonad development. Aquat. Toxicol. 174, 22–35 (2016).
Blewett, T., MacLatchy, D. L. & Wood, C. M. The effects of temperature and salinity on 17-α-ethynylestradiol uptake and its relationship to oxygen consumption in the model euryhaline teleost (Fundulus heteroclitus). Aquat. Toxicol. 127, 61–71 (2013).
Atkinson, S., Atkinson, M. J. & Tarrant, A. M. Estrogens from sewage in coastal marine environments. Environ. Health Persp. 111, 531–535 (2003).
Segner, H. et al. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: Report from the European IDEA project. Ecotoxicol. Environ. Safe. 54, 302–314 (2003).
Tyler, C. R., Jobling, S. & Sumpter, J. P. Endocrine disruption in wildlife: A critical review of the evidence. Crit. Rev. Toxicol. 28, 319–361 (1998).
Johnson, A. C., Belfroid, A. & Di Corcia, A. Estimating steroid oestrogen inputs into activated sludge treatment works and observations on their removal from the effluent. Sci. Total Environ. 256, 163–173 (2000).
Cadwell, D. J. et al. Derivation of an aquatic predicted no-effect concentration for the synthetic hormone, 17α-ethinyl estradiol. Environ. Sci. Technol. 10, 272–283 (2008).
Ternes, T. A. et al. Behavior and occurrence of estrogens in municipal sewage treatment plants—I. Investigations in Germany, Canada and Brazil. Sci. Total Environ. 225, 81–90 (1999).
Kolpin, D. W. et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 36, 1202–1211 (2002).
Huang, Y., Wang, X. L., Zhang, J. W. & Wu, K. S. Impact of endocrine-disrupting chemicals on reproductive function in zebrafish (Danio rerio). Reprod. Domest. Anim. 50, 1–6 (2009).
Länge, R. et al. Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 20, 1216–1227 (2001).
Parrott, J. L. & Blunt, B. R. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environ. Toxicol. 20, 131–141 (2005).
Bloom, M. S., Micu, R. & Neamtiu, I. Female infertility and “emerging” organic pollutants of concern. Curr. Epidemiol. Rep. 3, 39–50. https://doi.org/10.1007/s40471-016-0060-1 (2016).
Nash, J. P. et al. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ. Health Persp. 112, 1725–1733 (2004).
Wu, C., Huang, X., Lin, J. & Liu, J. Occurrence and fate of selected endocrine-disrupting chemicals in water and sediment from an urban lake. Arch. Environ. Contam. Toxicol. 68, 225–236 (2014).
Pennington, M. J. et al. Effects of contaminants of emerging concern on Megaselia scalaris (Lowe, Diptera: Phoridae) and its microbial community. Sci. Rep. 7, 1–12 (2017).
Pennington, M. J., Prager, S. M., Walton, W. E. & Trumble, J. T. Culex quinquefasciatus larval microbiomes vary with instar and exposure to common wastewater contaminants. Sci. Rep. 6, 1–9 (2016).
Pennington, M. J., Rivas, N. G., Prager, S. M., Walton, W. E. & Trumble, J. T. Pharmaceuticals and personal care products alter the holobiome and development of a medically important mosquito. Environ. Pollut. 203, 199–207 (2015).
Fosch, S. E. et al. Contraception: Influence on vaginal microbiota and identification of vaginal lactobacilli using MALDI-TOF MS and 16S rDNA sequencing. Open Microbiol. J. 12, 218–229 (2018).
Tarrant, A. M., Blomquist, C. H., Lima, P. H., Atkinson, M. J. & Atkinson, S. Metabolism of estrogens and androgens by scleractinian corals. Comp. Biochem. Phys. B. 136, 473–485 (2003).
Tarrant, A. M., Atkinson, M. J. & Atkinson, S. Effects of steroidal estrogens on coral growth and reproduction. Mar. Ecol. Prog. Ser. 269, 121–129 (2004).
Atkinson, S. & Atkinson, M. J. Detection of estradiol-17β during a mass coral spawn. Coral Reefs 11, 33–35 (1992).
Atkinson, S. Uptake of estrone from the water column by a coral community. Mar. Biol. 139, 321–325 (2001).
Rougée, L. R., Richmond, R. H. & Collier, A. C. Molecular reproductive characteristics of the reef coral Pocillopora damicornis. Comp. Biochem. Phys. A 189, 38–44 (2015).
Blomquist, C. H., Lima, P. H., Tarrant, A. M., Atkinson, M. J. & Atkinson, S. 17β-Hydroxysteroid dehydrogenase (17β-HSD) in scleractinian corals and zooxanthellae. Comp. Biochem. Phys. B 143, 397–403 (2006).
Tarrant, A. M., Atkinson, S. & Atkinson, M. J. Estrone and estradiol-17β concentration in tissue of the scleractinian coral, Montipora verrucosa. Comp. Biochem. Phys. A 122, 85–92 (1999).
Twan, W. et al. Hormones and reproduction in scleractinian corals. Comp. Biochem. Phys. A 144, 247–253 (2006).
Tarrant, A., Atkinson, M. & Atkinson, S. Uptake of estrone from the water column by a coral community. Mar. Biol. 139, 321–325 (2001).
Bosch, T. C. G. & McFall-Ngai, M. J. Metaorganisms as the new frontier. Zoology 114, 185–190 (2011).
Rosenberg, E., Koren, O., Reshef, L. & Efrony, R. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 5, 355–362 (2007).
Rosenberg, E. Coral microbiology. Microb. Biotechnol. 2, 147 (2009).
Peixoto, R. S., Rosado, P. M., Leite, D. C., Rosado, A. S. & Bourne, D. G. Beneficial microorganisms for corals (BMC): Proposed mechanisms for coral health and resilience. Front. Mar. Sci. 8, 1–16 (2017).
Ziegler, M., Seneca, F. O., Yum, L. K., Palumbi, S. R. & Voolstra, C. R. Patterns of coral heat tolerance. Nature Comm. 1, 1–8 (2017).
Peixoto, R. S., Sweet, M. & Bourne, D. G. Customized medicine for corals. Front. Mar. Sci. 6, 686 (2019).
Rosado, P. M. et al. Marine probiotics: Increasing coral resistance to bleaching through microbiome manipulation. ISME J. 13, 921–936 (2019).
Lesser, M. P., Mazel, C. H., Gorbunov, M. Y. & Falkowski, P. G. Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science 305, 997–1000 (2004).
Wegley, L., Edwards, R., Rodriguez-Brito, B., Liu, H. & Rohwer, F. Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environ. Microbiol. 9, 2707–2719 (2007).
Reshef, L., Koren, O., Loya, Y., Zilber-Rosenberg, I. & Rosenberg, E. The coral probiotic hypothesis. Environ. Microbiol. 8, 2068–2073 (2006).
Ritchie, K. B. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Prog. Ser. 322, 1–14 (2006).
Bourne, D. G., Morrow, K. M. & Webster, N. S. Insights into the coral microbiome: Underpinning the health and resilience of reef ecosystems. Annu. Rev. Microbiol. 70, 317–340 (2016).
Santos, H. F. et al. Climate change affects key nitrogen-fixing bacterial populations on coral reefs. ISME J. 8, 2272–2279 (2014).
Santos, H. F. et al. Impact of oil spills on coral reefs can be reduced by bioremediation using probiotic microbiota. Sci. Rep. 5, 1–11 (2015).
Röthig, T., Yum, L. K., Kremb, S. G., Roik, A. & Voolstra, C. R. Microbial community composition of deep-sea corals from the Red Sea provides insight into functional adaption to a unique environment. Sci. Rep. 7, 44714 (2017).
Meyer, J. L., Paul, V. J. & Teplitski, M. Community shifts in the surface microbiomes of the coral Porites astreoides with unusual lesions. PLoS ONE 9, e100316 (2014).
Sweet, M. J. & Bulling, M. T. On the importance of the microbiome and pathobiome in coral health and disease. Front. Mar. Sci. 4, 1–11 (2017).
Grottoli, A. G. et al. Coral physiology and microbiome dynamics under combined warming and ocean acidification. PLoS ONE 13, e0191156 (2018).
Webster, N. S. et al. Host-associated coral reef microbes respond to the cumulative pressures of ocean warming and ocean acidification. Sci. Rep. 6, 19324 (2016).
Ainsworth, T. D., Thurber, R. V. & Gates, R. D. The future of coral reefs: A microbial perspective. Trends Ecol. Evol. 25, 233–240 (2009).
Gissi, F. et al. The effect of dissolved nickel and copper on the adult coral Acropora muricata and its microbiome. Environ. Pollut. 250, 792–806 (2019).
Leite, D. C. et al. Coral bacterial-core abundance and network complexity as proxies for anthropogenic pollution. Front. Microbiol. 9, 833 (2018).
Vega Thurber, R. et al. Metagenomic analysis of stressed coral holobionts. Environ. Microbiol. 11, 2148–2163 (2009).
Meron, D. et al. The impact of reduced pH on the microbial community of the coral Acropora eurystoma. ISME J. 5, 51–60 (2011).
McDevitt-Irwin, J. M., Baum, J. K., Garren, M. & Vega Thurber, R. L. Responses of coral-associated bacterial communities to local and global stressors. Front. Mar. Sci. 4, 262 (2017).
Al-Dahash, L. M. & Mahmoud, H. M. Harboring oil-degrading bacteria: A potential mechanism of adaptation and survival in corals inhabiting oil-contaminated reefs. Mar. Pollut. Bull. 72, 364–374 (2013).
Wenger, A. S., Fabricius, K. E., Jones, G. P. & Brodie, J. E. Effects of sedimentation, eutrophication, and chemical pollution on coral reef fishes. In Ecology of Fishes on Coral Reefs (ed. Mora, C.) 145–153 (Cambridge University Press, Cambridge, 2015).
Zaneveld, J. R. et al. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat. Commun. 7, 1–12 (2016).
Marangoni, L. F. et al. Copper effects on biomarkers associated with photosynthesis, oxidative status and calcification in the Brazilian coral Mussismilia harttii (Scleractinia, Mussidae). Mar. Environ. Res. 130, 248–257 (2017).
Ferrigno, F. et al. Corals in high diversity reefs resist human impact. Ecol. Indic. 70, 106–113 (2016).
Hughes, T. P. et al. Climate change, human impacts, and the resilience of coral reefs. Science 80, 929–933 (2003).
Pastorok, R. & Bilyard, G. Effects of sewage pollution on coral-reef communities. Mar. Ecol. Prog. Ser. 21, 175–189 (1985).
Tarrant, A. M. Hormonal signaling in cnidarians: Do we understand the pathways well enough to know whether they are being disrupted?. Ecotoxicology 16, 5–13 (2007).
Peters, E. C., Gassman, N. J., Firman, J. C., Richmond, R. H. & Power, E. A. Ecotoxicology of tropical marine ecosystems. Environ. Toxicol. Chem. 16, 12–40 (1997).
Holert, J. et al. Metagenomes reveal global distribution of bacterial steroid catabolism in natural, engineered, and host environments. MBio 9, e02345-e2417 (2018).
Winter, A. P. M., Chaloub, R. M. & Duarte, G. A. S. Photosynthetic responses of corals Mussismilia harttii (Verrill, 1867) from turbid waters to changes in temperature and presence/absence of light. Braz. J. Oceanogr. 64, 203–216 (2016).
Fonseca, J. S., Marangoni, L. F. B., Marques, J. A. & Bianchini, A. Effects of increasing temperature alone and combined with copper exposure on biochemical and physiological parameters in the zooxanthellate scleractinian coral Mussismilia harttii. Aquat. Toxicol. 190, 121–132 (2017).
Ralph, P. J., Schreiber, U., Gademann, R., Kühl, M. & Larkum, A. W. D. Coral photobiology studied with a new imaging pulse amplitude modulated fluorometer. J. Phycol. 41, 335–342 (2005).
Sato, Y., Bourne, D. G. & Willis, B. L. Effects of temperature and light on the progression of black band disease on the reef coral, Montiporahispida. Coral Reefs 30, 753–761 (2011).
Wiedenmann, J. et al. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 3, 160–164 (2013).
Fernando, S. C. et al. Microbiota of the major south atlantic reef building. Microb. Ecol. 69, 267–280 (2015).
De Castro, A. P., Dias, S. A. & Reis, A. M. M. Bacterial community associated with healthy and diseased reef coral Mussismilia hispida from eastern Brazil. Microb. Ecol. 59, 658–667 (2010).
Santos, H. F. et al. Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: Bacterial proxies for oil pollution. PLoS ONE 6, e14693 (2011).
Santos, H. F., Cury, J. C., Carmo, F. L., Rosado, A. S. & Peixoto, R. S. 18S rDNA sequences from microeukaryotes reveal oil indicators in mangrove sediment. PLoS ONE 5, e12437 (2010).
Oleynik, G. N., Yurishinets, V. I. & Starosila, Y. V. Bacterioplankton and bacteriobenthos as biological indicators of the aquatic ecosystems’ state (a review). Hydrobiol. J. 47, 37–48 (2011).
Jain, A., Singh, B. N., Singh, S. P., Singh, H. B. & Singh, S. Exploring biodiversity as bioindicators for water pollution. Natl. Conf. Biodivers. Dev. Poverty Alleviation, 50–56 (2010).
Bloem, J. & Breure, A. M. Microbial indicators. In Bioindicators and Biomonitors (eds Markert, B. A. et al.) 257–282 (Elsevier, Amsterdam, 2003).
Parmar, T. K., Rawtani, D. & Agrawal, Y. K. Bioindicators: The natural indicator of environmental pollution. Front. Life Sci. 9, 110–118 (2016).
Wilkins, L. G. E. et al. Host-associated microbiomes drive structure and function of marine ecosystems. PLoS Biol. 17, e3000533 (2019).
Kurisu, F., Ogura, M., Saitoh, S., Yamazoe, A. & Yagi, O. Degradation of natural estrogen and identification of the metabolites produced by soil isolates of Rhodococcus sp. and Sphingomonas sp.. J. Biosci. Bioeng. 109, 576–582 (2010).
Wang, Y. et al. Degradation of 17 β-estradiol and products by a mixed culture of Rhodococcus equi DSSKP-R-001 and Comamonas testosteroni QYY20150409. Biotechnol. Biotechnol. Equip. 33, 268–277 (2019).
Yoshimoto, T. et al. Isolates from activated sludge in wastewater treatment plants. Appl. Environ. Microbiol. 70, 5283–5289 (2004).
Zhao, H. et al. Genome analysis of Rhodococcus sp. DSSKP-R-001: A highly effective β-estradiol-degrading bacterium. Int. J. Genomics 2018, 3505428 (2018).
Edet, U. O. & Antai, S. P. Correlation and distribution of xenobiotics genes and metabolic activities with level of total petroleum hydrocarbon in soil, sediment and estuary water in the Niger Delta Region of Nigeria. Asian J. Biotechnol. Genet. Eng. 1(1), 1–11 (2018).
Parida, S. & Sharma, D. The microbiome-estrogen connection and breast cancer risk. Cells 8, 1642 (2019).
Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence: A Signature of Photosynthesis (eds Papageorgiou, G. C. & Govindjee, C.) 279–319 (Springer, Berlin, 2004).
Siebeck, U. E., Marshall, N. J., Klüter, A. & Hoegh-Guldberg, O. Monitoring coral bleaching using a colour reference card. Coral Reefs 25, 453–460 (2006).
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: Paleontological statistics software package. Palaeontol. Electron. 4, 1–9 (2001).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U.S.A. 108, 4516–4522 (2011).
Schloss, P. D. et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
McCune, B. & Mefford, M. J. PC-ORD v. 6.0. MjM Software, Gleneden Beach (2010).
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Instituto Museu Aquário Marinho do Rio de Janeiro-AquaRio (IMAM/AquaRio)—Rio de Janeiro Marine Aquarium Research Center, Rio de Janeiro,
Brazil.
Author information
Authors and Affiliations
Contributions
C.L.S.V., H.D.M.V. and R.S.P. designed the experiments and wrote the manuscript. G.A.S.D. designed the microcosm system and C.L.S.V., H.D.M.V. and E.P.S. conducted the experiment and analyses. C.T.C.C.R. conducted the bioinformatics analysis. All authors revised and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vilela, C.L.S., Villela, H.D.M., Duarte, G.A.S. et al. Estrogen induces shift in abundances of specific groups of the coral microbiome. Sci Rep 11, 2767 (2021). https://doi.org/10.1038/s41598-021-82387-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82387-x
This article is cited by
-
Probiotics reshape the coral microbiome in situ without detectable off-target effects in the surrounding environment
Communications Biology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.