Abstract
We present 10 nearly complete mitochondrial genomes of the extinct tortoise Chelonoidis alburyorum from the Bahamas. While our samples represent morphologically distinct populations from six islands, their genetic divergences were shallow and resembled those among Galápagos tortoises. Our molecular clock estimates revealed that divergence among Bahamian tortoises began ~ 1.5 mya, whereas divergence among the Galápagos tortoises (C. niger complex) began ~ 2 mya. The inter-island divergences of tortoises from within the Bahamas and within the Galápagos Islands are much younger (0.09–0.59 mya, and 0.08–1.43 mya, respectively) than the genetic differentiation between any other congeneric pair of tortoise species. The shallow mitochondrial divergences of the two radiations on the Bahamas and the Galápagos Islands suggest that each archipelago sustained only one species of tortoise, and that the taxa currently regarded as distinct species in the Galápagos should be returned to subspecies status. The extinct tortoises from the Bahamas have two well-supported clades: the first includes one sample from Great Abaco and two from Crooked Island; the second clade includes tortoises from Great Abaco, Eleuthera, Crooked Island, Mayaguana, Middle Caicos, and Grand Turk. Tortoises belonging to both clades on Great Abaco and Crooked Island suggest late Holocene inter-island transport by prehistoric humans.
Similar content being viewed by others
Introduction
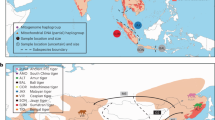
Giant tortoises (order Testudines, family Testudinidae) are charismatic reptiles, well known to naturalists, nature lovers, and the general public. Today, giant tortoises are restricted to the Galápagos Islands and the Aldabra Atoll. Little known is that giant tortoises were exterminated less than 200 years ago on the Mascarene Islands1. Unknown even to many zoologists is that large-bodied or giant tortoises roamed on many other oceanic islands during the Holocene and before2. Even in North and South America, giant tortoises occurred until the latest Pleistocene or early Holocene3,4,5,6,7,8,9,10. As a general rule, these large tortoise species disappeared after the arrival of humans, most likely as the result of unsustainable harvest2. One example is the tortoise radiation of the West Indies (Fig. 1).
Late Quaternary and Holocene records of large-bodied or giant tortoises (Chelonoidis spp.) in South America and the Caribbean. Red rectangle in inset map corresponds to enlarged map sector. Open circles: unstudied material5,6,9,10,11,21, yellow circles: samples unsuccessfully processed for aDNA, red circles: samples successfully processed for aDNA. Grey areas show the shelf to a depth of 200 m. Map was created using ArcGIS 10.4 (https://www.esri.com/en-us/arcgis/about-arcgis/overview) and Adobe Illustrator CS6 (http://www.adobe.com/products/illustrator.html).
No endemic tortoises survive today on these islands. What we know about the extinct tortoises is derived only from late Quaternary and Holocene fossils, which have been reported from the Bahamian Archipelago, Cuba, Hispaniola, Navassa, Sombrero, and Mona. These large tortoises fall into two major morphological groups, with the tortoises from the Bahamian islands (Chelonoidis alburyorum) distinguished from those on other West Indian islands, as well as the South American mainland, by lacking an epiplastral shelf11. Traditional morphological studies of West Indian tortoise fossils12,13,14,15 were greatly enhanced in 2004 by the discovery of superbly preserved tortoise fossils in Sawmill Sink, Great Abaco, Bahamas, which revealed morphological features impossible to discern in the fragmentary fossils previously available16. Furthermore, the Holocene fossils from Sawmill Sink retained enough collagen to allow radiocarbon dating and stable isotope analyses17,18. It followed, therefore, that the Sawmill Sink fossils should be assessed for ancient DNA (aDNA), which was successfully extracted and sequenced by Kehlmaier et al.19, allowing their firm phylogenetic placement in the same clade as the giant tortoises from Galápagos (Chelonoidis niger complex) and a small to medium-sized species from South America (C. chilensis).
Over the past decade, we have discovered tortoise fossils from a variety of late Quaternary localities (flooded sinkholes, dry sinkholes and caves, and archaeological sites) on 14 different islands in the Bahamian (Lucayan) Archipelago (Fig. 1). Many of these fossils are Holocene in age (< 10 ka) and retain enough collagen for radiocarbon dating and stable isotope analysis, just as had been done with the Sawmill Sink specimens11,20. In this paper, therefore, we build on the pioneering aDNA research of Kehlmaier et al.19 by expanding our coverage of tortoise aDNA across much of the island group. This information will allow us to evaluate the genetic relatedness of tortoises on different islands and different carbonate banks in the Bahamas. We also include late Quaternary material from sites in Argentina, Venezuela and the Dominican Republic (Hispaniola) with the aim to place the tortoises from the Bahamas in a comprehensive phylogenetic framework.
Materials and methods
Studied specimens
Seventeen subfossil bone samples of Chelonoidis were studied (Fig. 1), originating from the West Indies (n = 12) and mainland South America (n = 5), including material from the holotype of C. dominicensis21 from the Dominican Republic, a sample of the holotype of C. lutzae7 and three additional samples of C. cf. lutzae from Argentina, and one sample of an undescribed giant species of Chelonoidis from Venezuela6. The remaining specimens from the West Indies (C. alburyorum) originated on the Bahamian Islands (Great Abaco, Eleuthera, Long Island, Crooked Island, Mayaguana, Middle Caicos, and Grand Turk). Locality and collection details are provided in Table 1.
Processing of subfossil Chelonoidis samples
All samples were processed according to established guidelines22 in the aDNA facility of the Senckenberg Natural History Collections Dresden, which is physically separated from the main molecular genetic laboratory in another building. Negative controls (water blanks) were included during DNA extraction and library preparation and screened for evidence of contamination. Most bones were sampled using a Proxxon Micromot 50/E multitool equipped with 2–4 mm metal or stone drilling bits. Only the samples of C. lutzae/C. cf. lutzae were received as bone powder. Approximately 50 mg of bone powder of each sample was processed according to a protocol optimised for the recovery of short DNA fragments (Table S1)23. Then, up to 14 ng of DNA was converted into single-indexed, single-stranded Illumina sequencing libraries according to Gansauge and Meyer24 and Korlević et al.25, including the removal of uracil residues by uracil-DNA glycosylase (UDG) treatment. In order to increase the amount of endogenous mitochondrial DNA in the libraries, two-rounds of in-solution hybridization capture26,27 were performed in a dedicated capture-only workspace in the main laboratory using DNA baits generated from long-range PCR products of C. carbonarius, C. chilensis, C. denticulatus, and C. vicina at an equimolar rate. Details for long-range PCR, primer sequences, and PCR conditions are explained in the Supplementary Information. Sequencing was performed in-house on an Illumina MiSeq platform, generating 75 bp-long paired-end reads.
Mitogenome sequence assembly and sequence annotation
Assembly of mitogenome sequences from the enriched libraries involved adapter trimming with Skewer 0.2.228, read merging (minimum length 35 bp), quality filtering (minimum Q-score 20) and duplicate removal with BBmap-suite 37.24 (https://sourceforge.net/projects/bbmap/)29. The remaining reads were screened for contamination using FastQ Screen 0.11.430 and a set of predefined mitochondrial genomes (Table S2). The identified non-target reads were excluded from the readpools, which are subsequently referred to as readpools 1. Then, all reads mapping to a reference genome of Chelonoidis were copied into a second readpool (readpools 2). Genome assembly was achieved with MITObim31, a two-step baiting and iterative mapping approach, with an allowed mismatch value of 2 and a starting seed according to Table S1. For each sample, readpool 2 was used for the initial building of a reference genome, whereas readpool 1 was used for the actual assembly. Resulting scaffolds were visualised and checked for assembly artefacts in Tablet32. Assembly artefacts were manually removed from the assembled contigs and all positions with a coverage below threefold masked as ambiguous (N) using the maskfasta subcommand of BEDTools 2.29.233. Sequence length distribution of mapped reads was calculated with a customised awk command and Microsoft Excel.
Alignment and phylogenetic, divergence time and biogeographic analyses
The newly generated mitogenomes of Chelonoidis were merged with our previously used annotated alignment34 for tortoises, with the exception that only one sequence per Cylindraspis species was included. This alignment also contained previously obtained data for a subfossil specimen of Chelonoidis alburyorum from Sawmill Sink, Great Abaco, Bahamas19. We also included 15 recently published sequences of Chelonoidis spp. from South America and Galápagos35 and removed a GenBank sequence for a Galápagos tortoise of unknown provenance (accession number JN999704) from the original alignment34.
To briefly summarise the approach, an automated preliminary alignment was generated using Clustal W 1.436 and default parameters, as implemented in BioEdit 7.0.9.037. This alignment was adjusted manually, and sequences were annotated using MITOS38 and several published tortoise mitogenomes (GenBank/ENA accession numbers: AF069423, DQ080042, DQ080048, FJ469674, KT613185, LT599485). Each coding region was screened for internal stop codons using MEGA X39. Finally, problematic sequence features (stop codons, gene overlap, frameshifts, spacer DNA) were removed (Supplementary Information).
Our final alignment of 15,516 sites comprised 66 sequences corresponding to all extant genera and species groups of tortoises (Testudinidae). It also included one representative each of the extinct Mascarene tortoise genus Cylindraspis34. The two outgroup taxa represented the successive sister taxa of Testudinidae, Geoemydidae (Mauremys reevesii) and Emydidae (Chrysemys picta).
Phylogenetic relationships of the mitogenomes were inferred with Maximum Likelihood (ML) and Bayesian Inference (BI) approaches using RAxML 8.0.040 and MrBayes 3.2.641. The best evolutionary models and partitioning schemes (Tables S3, S4) were determined with PartitionFinder242 applying the greedy search scheme and the Bayesian Information Criterion. For ML, 20 independent searches were carried out using the GTR + G substitution model, different starting conditions, and the rapid bootstrap option. Subsequently, 1000 non-parametric thorough bootstrap replicates were calculated and the values plotted against the best tree. For BI, four parallel runs (each with eight chains) were performed with 2 million generations (burn-in 0.25; print frequency 1000; sample frequency 1000). Calculation parameters were analysed using Tracer 1.7.143. In addition, uncorrected p distances were calculated in MEGA X39 using the pairwise deletion option. Divergence times were estimated using the uncorrelated lognormal relaxed clock models implemented in BEAST 1.8444 and constrained by four fossil calibration points following Kehlmaier et al.34 (Table 2). Further details for analyses are explained in the Supplementary Information.
Results
Ten of the 17 subfossil Chelonoidis samples produced high-quality data representing nearly the entire mitochondrial genome (15,288–15,350 bp length, coverage: ninefold to 482-fold; Table S1). This is an excellent yield for aDNA from tropical environments19,45, especially when it is considered that some specimens were from open unsheltered sites (Table 1).
For the mitogenomes, only the control region and part of adjacent DNA coding for tRNAs could not be reconstructed. All successfully assembled mitogenomes belonged to extinct tortoises from the Bahamas. The holotype of Chelonoidis dominicensis from the Dominican Republic, the material from Argentina (holotype of C. lutzae, three samples of C. cf. lutzae), the single species-undetermined Chelonoidis from Venezuela, and one Chelonoidis sample from Long Island, Bahamas, did not yield sufficient endogenous DNA. Assembly details of individual samples and blanks as well as genetic diversity indices and substitution rates for selected clades are provided in the Supplementary Information.
Our phylogenetic analyses and the molecular clock calculation, including the 10 new and one previously published mitogenomes of West Indian Chelonoidis and additional data for Chelonoidis species from Galápagos and South America, produced general tree topologies consistent with our previous studies19,34. Of particular interest are the relationships of the crown clade containing C. chilensis and the Chelonoidis species from the Bahamas and Galápagos. These taxa were placed in a maximally supported clade, although the branching pattern within that clade was weakly resolved. Accordingly, the ML and BI trees suggested that C. chilensis is sister to a weakly supported clade consisting of the two island clades (Fig. 2), whereas our time tree reflected the weakly supported topology of our previous studies, with C. chilensis as sister taxon of the Galápagos tortoises (Fig. 3). Thus, the divergence time inferred for this node should be taken with caution. In contrast, the two clades containing giant tortoises from the Bahamas and Galápagos were both maximally supported (Fig. 2).
Maximum Likelihood tree for near-complete mitochondrial genomes of tortoises (15,516 bp). The tree includes living and extinct Chelonoidis species and representatives of all other extant genera and of the five recently extinct Cylindraspis species from the Mascarene Islands34. Ten of the 11 mitochondrial genomes of the Bahamian Chelonoidis specimens were produced in this study. Numbers at nodes are thorough bootstrap values and posterior probabilities from a Bayesian Inference tree; top left shows alternative Bayesian topology for the respective branches. Asterisks indicate maximum support under both tree-building approaches. Codes following species names or preceding localities are GenBank/ENA accession numbers (for remaining accession numbers, see Supplementary Information). Inset: Chelonoidis alburyorum (National Museum of The Bahamas, NMB.AB50.0008, Sawmill Sink, Great Abaco, LT59948219; photo: N. A. Albury).
Fossil-calibrated time tree for near-complete mitochondrial genomes (15,516 bp) of all extant and some extinct tortoise genera. Codes following species names or preceding localities are GenBank/ENA accession numbers (for remaining accession numbers, see Supplementary Information). Inferred mean ages and 95% Highest Posterior Density intervals are shown for each node. The red circles indicate fossil-based constraints following Kehlmaier et al.34: (A) Hadrianus majusculus, 50.3–100.5 Ma; (B) Cheirogaster maurini and Gigantochersina ammon, 33.9–66.0 Ma; (C) Cheirogaster maurini, 33.9–47.8 Ma; (D) Chelonoidis hesternus, 11.8–33.9 Ma. For details of calibration, see Supplementary Information. Inset: Holotype of Chelonoidis alburyorum keegani (Florida Museum of Natural History, UF 453000, plastron, Coralie, Grand Turk, same site as sequences LR968543 and LR968547; photo: N. A. Albury).
Our 10 new samples from the Bahamas, and the previously sequenced specimen19, represented morphologically highly divergent populations from six islands11,16. Nevertheless, the genetic divergences were shallow and resembled those among Galápagos tortoises (Fig. 2). Our molecular clock estimated a mean of 2.34 × 10–3 substitutions per site per million years (95% HPD: 2.11 × 10–3–2.78 × 10–3). Divergence among tortoises from the Bahamas commenced approximately 1.5 million years ago (mya), while divergence among tortoises from the Galápagos Islands began approximately 2 mya (Fig. 3). Two samples of the congeneric species Chelonoidis carbonarius from South America were more different genetically (with a divergence time of ~ 3.7 mya), as were two other pairs of congeneric tortoises (the extinct Cylindraspis indica from Réunion and its sister species C. inepta from Mauritius, that diverged ~ 4 mya, and the sister species C. peltastes and C. vosmaeri from Rodrigues, which diverged ~ 4.3 mya). In contrast, the divergence between two samples of Chelonoidis denticulatus was shallow and estimated to be ~ 0.6 million years old.
The inter-island divergences of tortoises from within the Bahamas and within the Galápagos Islands are much younger (0.09–0.59 mya, and 0.08–1.43 mya, respectively) than the genetic differentiation between any other congeneric pair of tortoise species (in Figs. 2 and 3 from top to bottom: Psammobates (14.6 mya), Astrochelys (20.6 mya), Pyxis (11.9 mya), Kinixys (16.1 mya), Geochelone (6.5 mya), Chelonoidis carbonarius + C. denticulatus (19.1 mya), Cylindraspis (26.8–4.0 mya), Indotestudo (9.7 mya,) Testudo (21.2–7.5 mya), and Manouria (25.0 mya). The mitochondrial divergence of subspecies of Testudo graeca (7.5 mya) also resembles or exceeds that between many sister species.
Within the extinct tortoises from the Bahamas, there are two well-supported clades (Figs. 2, 3), each of which died out from ~ 900 to 700 years ago (Table 1). One clade includes sequences from one sample from Great Abaco and two tortoises from Crooked Island; the other clade contains sequences corresponding to other tortoise specimens from Great Abaco and Crooked Island, as well as ones from Eleuthera, Mayaguana, Middle Caicos, and Grand Turk. The Galápagos Islands have six or seven clades of tortoises with genetic divergences similar to those of the two Bahamian clades.
Discussion
Our study provides evidence that the extinct tortoises from the Bahamas had diverged very little genetically, despite pronounced morphological differences11,16. For example, while all of the Bahamian tortoises were large, the one from Lost Reel Cave on Great Abaco was by far the largest, approaching if not matching in size the largest extant tortoises from the Galápagos and Aldabra. The various Bahamian forms also displayed major differences (not age-related) in the shape, rugosity, and relative size of the entoplastron, epiplastron, hypoplastron, and xiphiplastron. It was upon these differences that Steadman et al.20 tentatively recognized that as many as seven distinct species of Bahamian tortoises existed. Subsequently, two of these forms were described as subspecies of Chelonoidis alburyorum, namely C. a. sementis from Middle Caicos and C. a. keegani from Grand Turk11 (Table S5).
According to our molecular clock, the Bahamian radiation commenced approximately 1.5 mya, i.e., about 500,000 years after the onset of the radiation of the giant tortoises on the Galápagos Islands. Another recently published estimate for the age of the Galápagos tortoise radiation is slightly younger than ours (1.5 mya instead of 2.0 mya)35, resembling our molecular clock results for the Bahamas.
The Galápagos Islands formed approximately 4 mya46, whereas the age of the Bahamas, from the standpoint of being able to support terrestrial vertebrates, is estimated not to exceed 400,000 years because of interglacial flooding during marine isotope stage 1147,48,49. This situation implies for the Galápagos Islands that the current diversity of giant tortoises resulted from a single colonization event and a local radiation on the archipelago35. In contrast, the two tortoise clades from the Bahamas seem to be too old for having diverged on the islands. This suggests that the Bahamas may have been colonized twice from other landmasses. If that was the case, then the two colonizers must have been very similar genetically, given the low amount of genetic divergence of tortoises across the island group. As far as known, the extinct giant tortoises from the Greater Antilles, which would seem to be the likely source region of the Bahamian tortoises, are morphologically clearly distinct11,21. We cannot exclude, however, that these differences reflect morphological plasticity, a phenomenon well known from many other tortoise taxa50,51,52,53. Alternatively, the ancestral taxa on the Greater Antilles may still be undiscovered or known only by material too fragmentary to discern the crucial morphological characters. (The majority of tortoise fossils from the Greater Antilles are represented only by very incomplete material21). Unfortunately, the only sample from the Greater Antilles that we studied (ulna from the holotype of C. dominicensis, radiocarbon-dated to the early Holocene) did not yield aDNA sequences, so that genetic evidence must await further investigation. Nevertheless, C. dominicensis remains valuable for morphological studies, such as its possession of an epiplastral shelf, which is characteristic of the Galápagos and South American clades of Chelonoidis but not the Bahamian clade21.
An unexpected result of our study was that we found tortoises belonging to the two Bahamian clades on the same islands. One of these clades was represented only by two tortoises from Crooked Island (1702 Cave) and the previously sequenced specimen from Great Abaco (Sawmill Sink)19. The two specimens from Crooked Island are ~ 2600 years old, and therefore pre-cultural, whereas that from Abaco is ~ 950 years old (Table 1). Human arrival in the Bahamas took place ~ 1200 to 1000 years ago17,20,54,55. This situation suggests prehistoric human transport of tortoises from Crooked Island to Great Abaco, which lies on a different (and distant) bank.
The second Bahamian clade contained one specimen each from these same two islands, namely from McKay’s Bluff Cave on Crooked Island, and from Lost Reel Cave on Great Abaco, as well as specimens from Eleuthera, Mayaguana, Middle Caicos, and Grand Turk. This yields a total of six islands on six different banks (Fig. 1). Because the late Holocene specimens post-date the arrival of humans (Lucayans) in the Bahamas, the inter-island mixing of clades is strong evidence that early people were moving tortoises among islands, which could have also contributed to hybridization and an increase in morphological variation.
A similar situation was discovered recently with aDNA of the Bahamian hutia (Geocapromys ingrahami), again involving prehistoric human transport between Great Abaco and Crooked Island56. Whether tortoises or hutias, their inter-island transport by people did not prevent their eventual extinction on both Great Abaco and Crooked Island. We have no evidence that indigenous tortoises survived in the Bahamas beyond 800–700 cal BP20, which is several centuries before European contact.
In any case, compared to other tortoises (Figs. 2, 3; Table S6), the shallow mitochondrial divergences of the tortoise radiations on the Bahamas and the Galápagos Islands suggest that each archipelago harboured only one species and that the many taxa currently regarded as distinct species1,35 should be returned to subspecies status. This also is in agreement with the weak nuclear genomic divergence of Galápagos tortoises57,58.
Conspecificity is further supported when mitogenomic divergences within other Chelonoidis species are compared to those of the two island radiations. Two samples of the widely distributed South American species C. carbonarius were estimated in our molecular clock calculation to have diverged ~ 3.7 mya, and these samples differed by an uncorrected p distance of 1.7% compared to maximum values of 0.7% within the Bahamian radiation and 0.9% within the Galápagos radiation. The shallower divergence between two samples of another widely distributed South American species, C. denticulatus (~ 0.6 mya; 0.2% uncorrected p distance), is in line with a previous study59 that found the savannah species C. carbonarius more differentiated than its forest-dwelling sister species C. denticulatus.
Even though the genetic divergences among the tortoises of the Bahamas (and, for that matter, the Galápagos Islands) are small, each of the populations had a distinctive morphology with which it interacted in its environment. Both archipelagos have gradients of temperature and precipitation, yielding distinctive environments and vegetation types on different islands. Giant tortoises are known to play important roles for the vegetation structure and composition on other islands60,61, and undoubtedly once did the same on the Bahamas.
References
TTWG [Turtle Taxonomy Working Group; Rhodin, A. G. J. et al.] Turtles of the World. Annotated Checklist and Atlas of Taxonomy, Synonymy, Distribution, and Conservation Status (8th Ed.) (Chelonian Research Foundation and Turtle Conservancy, Chelonian Research Monographs 7, 2017).
TEWG [Turtle Extinctions Working Group; Rhodin, A. G. J. et al.] Turtles and Tortoises of the World During the Rise and Global Spread of Humanity: First Checklist and Review of Extinct Pleistocene and Holocene Chelonians (IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, Chelonian Research Monographs 5, 2015).
Clausen, C. J., Cohen, A. D., Emiliani, C., Holman, J. A. & Stipp, J. J. Little Salt Spring, Florida: A unique underwater site. Science 203, 609–614 (1979).
Holman, J. A. & Clausen, C. J. Fossil vertebrates associated with Paleo-Indian artifact at Little Salt Spring, Florida. J. Vertebr. Paleontol. 4, 146–154 (1984).
Cantalamessa, G. et al. A new vertebrate fossiliferous site from the Late Quaternary at San José on the north coast of Ecuador: Preliminary note. J. South Am. Earth Sci. 14, 331–334 (2001).
Aguilera Socorro, O. Tesoros paleontológicos de Venezuela. El Cuaternario del Estado Falcón (Ministerio de la Cultura, Instituto del Patrimonio Cultural, Caracas, 2006).
Zacarías, G. G., de la Fuente, M. S., Fernández, M. S. & Zurita, A. E. Nueva especie de tortuga terrestre gigante del género Chelonoidis Fitzinger, 1835 (Cryptodira: Testudinidae), del miembro inferior de la Formación Toropí/Yupoí (Pleistoceno tardío/Lujanense), Bella Vista, Corrientes, Argentina. Ameghiniana 50, 298–318 (2013).
Zacarías, G. G., de la Fuente, M. S. & Zurita, A. E. Testudinoidea Fitzinger (Testudines: Cryptodira) de la Formación Toropí/Yupoí (ca. 58–28 ka) en la Provincia de Corrientes, Argentina: Taxonomía y aspectos paleoambientales. Rev. Bras. Paleontol. 17, 389–404 (2014).
Torres Chiriboga, F. J. Histología ósea de una tortuga gigante del Pleistoceno (Testudinidae) de Ecuador continental, con comentarios del origen de las tortugas de Galápagos (Disertación previa, Pontificia Universidad Católica del Ecuador, Quito, 2016).
Cadena, E. A. & Román-Carrión, J. L. A review of the fossil record of Ecuador, with insights about its challenges and future development. Ameghiniana 55, 571–591 (2018).
Franz, R., Albury, N. A. & Steadman, D. W. Extinct tortoises from the Turks and Caicos Islands. Florida Mus. Nat. Hist. Bull. 58, 1–38 (2020).
Williams, E. E. Testudo cubensis and the evolution of Western Hemisphere tortoises. Bull. Am. Mus. Nat. Hist. 95, 1–36 (1950).
Williams, E. E. A new fossil tortoise from Mona Island, West Indies, and a tentative arrangement of the tortoises of the world. Bull. Am. Mus. Nat. Hist. 99, 545–560 (1952).
Auffenberg, W. Notes on West Indian tortoises. Herpetologica 23, 34–44 (1967).
Franz, R. & Woods, C. A. A fossil tortoise from Hispaniola. J. Herpetol. 17, 79–81 (1983).
Franz, R. & Franz, S. A new fossil land tortoise in the genus Chelonoidis (Testudines: Testudinidae) from the northern Bahamas, with an osteological assessment of other Neotropical tortoises. Florida Mus. Nat. Hist. Bull. 49, 1–44 (2009).
Steadman, D. W. et al. Exceptionally well preserved late Quaternary plant and vertebrate fossils from a blue hole on Abaco, The Bahamas. Proc. Natl. Acad. Sci. USA 104, 19897–19902 (2007).
Hastings, A. K., Krigbaum, J., Steadman, D. W. & Albury, N. A. Domination by reptiles in a terrestrial food web of the Bahamas prior to human occupation. J. Herpetol. 48, 380–388 (2014).
Kehlmaier, C. et al. Tropical ancient DNA reveals relationships of the extinct Bahamian giant tortoise Chelonoidis alburyorum. Proc. R. Soc. B 284, 20162235 (2017).
Steadman, D. W. et al. The paleoecology and extinction of endemic tortoises in the Bahamian Archipelago. Holocene 30, 420–427 (2020).
Albury, N. A., Franz, R., Rimoli, P., Lehman, P. & Rosenberger, A. L. Fossil land tortoises (Testudines: Testudinidae) from the Dominican Republic, West Indies, with a description of a new species. Am. Mus. Novit. 3904, 1–28 (2018).
Fulton, T. L. & Shapiro, B. Setting up an ancient DNA laboratory. In Ancient DNA: Methods and Protocols. Methods in Molecular Biology, Vol. 1963 (eds Shapiro, B. et al.), 1–13 (Humana Press, Totowa, 2019).
Dabney, J. et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl. Acad. Sci. USA 110, 15758–15763 (2013).
Gansauge, M.-T. & Meyer, M. Single-stranded DNA library preparation for the sequencing of ancient or damaged DNA. Nat. Protoc. 8, 737–748 (2013).
Korlević, P. et al. Reducing microbial and human contamination in DNA extractions from ancient bones and teeth. Biotechniques 58, 87–93 (2015).
Maricic, T., Whitten, M. & Pääbo, S. Multiplexed DNA sequence capture of mitochondrial genomes using PCR products. PLoS One 5, e14004 (2010).
Horn, S. Target enrichment via DNA hybridization capture. In Ancient DNA: Methods and Protocols. Methods in Molecular Biology, Vol. 840 (eds Shapiro, B. & Hofreiter, M.), 177–188 (Springer, Berlin, 2012).
Jiang, H., Lei, R., Ding, S. W. & Zhu, S. Skewer: A fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinform. 15, 182 (2014).
Bushnell, B., Rood, J. & Singer, E. BBMerge—accurate paired shotgun read merging via overlap. PLoS One 12, e0185056 (2017).
Wingett, S. W. & Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control [version 2; referees: 4 approved]. F1000Research 7, 1338 (2018).
Hahn, C., Bachmann, L. & Chevreux, B. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads—a baiting and iterative mapping approach. Nucleic Acids Res. 41, 1–9 (2013).
Milne, I. et al. Using Tablet for visual exploration of second-generation sequencing data. Brief. Bioinform. 14, 193–202 (2013).
Quinlan, A. R. & Hall, I. M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Kehlmaier, C. et al. Ancient mitogenomics clarifies radiation of extinct Mascarene giant tortoises. Sci. Rep. 9, 17487 (2019).
Poulakakis, N. et al. Colonization history of Galapagos giant tortoises: Insights from mitogenomes support the progression rule. J. Zool. Syst. Evol. Res. 58, 1262–1275 (2020).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Hall, T. A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Bernt, M. et al. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 69, 313–319 (2013).
Kumar, S., Stecher, G., Knyaz, C. & Tamura, K. MEGA X: Molecular Evolutionary Genetic Analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Ronquist, F. et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T. & Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 34, 772–773 (2016).
Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 5, 901–904 (2018).
Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012).
Woods, R. et al. Rapid size change associated with intra-island evolutionary radiation in extinct Caribbean “island shrews”. BMC Evol. Biol. 29, 106 (2020).
Geist, D., Snell, H. L., Snell, H. M., Goddard, C. & Kurz, M. Paleogeography of the Galápagos Islands and biogeographical implications. In The Galápagos: A Natural Laboratory for the Earth Sciences, Vol. 204 (eds Harpp, K., Mittelstaedt, E., d’Ozouville, N. & Graham, D.) 145–166 (American Geophysical Union, New York, 2014).
Hearty, P. J., Kindler, P., Cheng, H. & Edwards, R. A +20 m middle Pleistocene sea-level highstand (Bermuda and the Bahamas) due to partial collapse of Antarctic ice. Geology 27, 375–378 (1999).
Bowen, D. Sea level ∼400 000 years ago (MIS 11): Analogue for present and future sea-level? Clim. Past 6, 19–29 (2010).
Steadman, D. W. & Franklin, J. Origin, paleoecology, and extirpation of bluebirds and crossbills in the Bahamas across the last glacial-interglacial transition. Proc. Natl. Acad. Sci. USA 114, 9924–9929 (2017).
Fritz, U., Široký, P., Kami, H. & Wink, M. Environmentally caused dwarfism or a valid species—Is Testudo weissingeri Bour, 1996 a distinct evolutionary lineage? New evidence from mitochondrial and nuclear genomic markers. Mol. Phylogenet. Evol. 37, 389–401 (2005).
Fritz, U. et al. Phenotypic plasticity leads to incongruence between morphology-based taxonomy and genetic differentiation in western Palaearctic tortoises (Testudo graeca complex; Testudines, Testudinidae). Amphibia-Reptilia 28, 97–121 (2007).
Fritz, U. et al. Mitochondrial phylogeography and subspecies of the wide-ranging sub-Saharan leopard tortoise Stigmochelys pardalis (Testudines: Testudinidae)—a case study for the pitfalls of pseudogenes and GenBank sequences. J. Zool. Syst. Evol. Res. 48, 348–359 (2010).
Fritz, U. et al. Northern genetic richness and southern purity, but just one species in the Chelonoidis chilensis complex. Zool. Scr. 41, 220–232 (2012).
Carlson, L. A. & Keegan, W. F. Resource depletion in the prehistoric northern West Indies. In Voyages of Discovery (ed. Fitzpatrick, S. M.) 85–107 (Praeger, Westport, 2004).
Keegan, W. F. Taino Indian Myth and Practice: The Arrival of the Stranger King (University Press of Florida, Gainesville, 2007).
Oswald, J. A. et al. Ancient DNA and high-resolution chronometry reveal a long-term human role in the historical diversity and biogeography of the Bahamian hutia. Sci. Rep. 10, 1373 (2020).
Loire, E. & Galtier, N. Lacking conservation genomics in the giant Galápagos tortoise. bioRxiv 101980, 1–14 (2017).
Fontaine, M. C. A genomic perspective is needed for the re-evaluation of species boundaries, evolutionary trajectories, and conservation strategies of the Galápagos giant tortoises. PCI Evol. Biol. 100031, 1–3 (2017).
Vargas-Ramírez, M., Maran, J. & Fritz, U. Red- and yellow-footed tortoises (Chelonoidis carbonaria, C. denticulata) in South American savannahs and forests: Do their phylogeographies reflect distinct habitats? Org. Divers. Evol. 10, 161–172 (2010).
Blake, S. et al. Seed dispersal by Galápagos tortoises. J. Biogeogr. 39, 1961–1972 (2012).
Walton, R. et al. In the land of giants: Habitat use and selection of the Aldabra giant tortoise on Aldabra Atoll. Biodiv. Conserv. 28, 3183–3198 (2019).
Acknowledgements
Samples were provided by the National Museum of The Bahamas/The Antiquities Monuments and Museum and Michael Pateman (Turks and Caicos National Museum). Special thanks go to Renato Rímoli Martínez (Museo del Hombre Dominicano, Santo Domingo, Dominican Republic), Gerardo Gabriel Zacarías (Centro de Ecología Aplicada del Litoral CECOAL-CONICET, Universidad Nacional del Nordeste, Corrientes, Argentina), Verónica Todaro (Colección Paleontológica de la UNNE "Dr. Rafael Herbst," Corrientes, Argentina), and Marcelo Sánchez Villagra (Paläontologisches Museum der Universität Zürich, Switzerland) for tortoise samples from the Dominican Republic, Argentina, and Venezuela. We would have liked that their material worked better! The research of D.W.S. was supported by the National Science Foundation Grants BCS-1118369 and GSS-1461496. The research of E.G. and U.F. was partially supported by the Spanish Ministry of Science and Innovation through the project PID2019-105682RA-100/AEI/10.13039/501100011033.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
D.W.S. and U.F. conceived and designed the study. C.K. performed laboratory work and analysed most data. E.G. contributed molecular clock calculations. N.A.A., D.W.S., and R.F. contributed samples, including doing the fieldwork. C.K., D.W.S., E.G., and U.F. wrote the manuscript. All authors reviewed the manuscript and consented to its submission and publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kehlmaier, C., Albury, N.A., Steadman, D.W. et al. Ancient mitogenomics elucidates diversity of extinct West Indian tortoises. Sci Rep 11, 3224 (2021). https://doi.org/10.1038/s41598-021-82299-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82299-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.