Abstract
This paper presents a research on the kinetics and gas release laws of the cooperative spontaneous combustion of coal-polyurethane binary system by means of thermogravimetric analysis and spontaneous combustion simulation experiments. The coal-polyurethane binary system is more prone than individual samples to combustion. The polyurethane mass fraction has a great influence on the cooperative spontaneous combustion process. As the polyurethane mass fraction rises, the activation energies of oxygen absorption stage and pyrolysis stage decrease. The combustion residues of coal-polyurethane binary system scorches into lumps and the surfaces of the particles become rougher. During spontaneous combustion, the initial release temperatures of the four gases CO2, CO, C2H6 and C2H4 drop as the polyurethane mass fraction increases. The gas release amount of them increases with increasing polyurethane mass fraction. And the amount of CH4 decreases as the polyurethane mass fraction increases. The CO/CO2 ratio with temperature for different polyurethane mass fraction have the same trends. The results of this study have implications concerning polyurethane materials application and fire protection in coal mine.
Similar content being viewed by others
Introduction
In coal mine areas, spontaneous combustion of coal is one of the greatest catastrophes that disturbs safe mining1,2,3,4. And polyurethane is a widely used organic polymer material. It is a lightweight material characterized by high strength, high adhesion, safety and efficiency of use. In coal mine production, polyurethane is mainly used to strengthen broken coal and rock layers, fill large falling areas, construct sealed partition walls for goafs and block air leakages5,6,7. During the polyurethane grouting process, the reaction of isocyanate with polyhydric alcohol releases a large amount of heat, which causes the temperature to rise rapidly. This excessive temperature will cause the pyrolysis and combustion of polyurethane and coal. In recent years, there have been many fire accidents caused by the reinforcement and filling of polyurethane materials8,9,10,11. Furthermore, the oxidation and spontaneous combustion of coal may lead to the pyrolysis and combustion of the polyurethane left in goafs. And a large amount of heat and fire gases will release during these process. In coal mine production, gas monitoring of goafs is an important measure to prevent spontaneous combustion and fire accidents. The cooperative spontaneous combustion of coal-polyurethane binary system have significant impacts on the concentrations of the main fire gases in goafs, which will seriously interferes the monitoring of fire gas. In 2020, the National Coal Mine Safety Administration of China promulgated the Measures for the safety management of reactive polymer materials in coal mines to regulate the useage of polyurethane grouting materials.
Scholars in China and abroad have performed many studies on spontaneous coal combustion mechanisms and gas monitoring. The thermogravimetric analysis (TGA) method has been widely used to research the coal spontaneous combustion mechanism and division of stages therein12,13. Chen explained the mechanism of coal pyrolysis and spontaneous combustion and proposed a model for particle fragmentation and volatile diffusion during coal pyrolysis14. Deng used the thermogravimetric-Fourier transform infrared (TG-FTIR) method to research the mechanism and ignition temperature of the spontaneous combustion of different kinds of coal. The experiments were designed to investigate the change laws of the temperature, fire gas concentrations and heat intensity of spontaneous combustion15,16. In actual production, CO, CO2, C2H6, C2H4, C2H2 and other index gases are usually used to judge the situation of coal spontaneous combustion17,18. Lu conducted experimental research on the gas release laws of the spontaneous combustion of different kinds of coal based on three factors: temperature, water content and flame retardants. He used CO and C2H4 as index gases to judge the spontaneous combustion situation of coal19. Zhu researched the relationship between the oxygen consumption rate and temperature during the low-temperature spontaneous combustion stage of coal. And he obtained the critical temperatures of coal spontaneous combustion and the fire gas release laws20.
Polyurethane is an organic polymer material that contains many additives. It is flammable but will not ignite spontaneously at room temperature. There are a lot of CO, CO2 and many toxic gases, such as CO and HCN, released during the combustion of polyurethane21. Many scholars researched the mechanisms of pyrolysis and combustion and the flame retardancy modification of polyurethane22,23,24,25. Polyurethane begins to pyrolyse from 200 °C. During its pyrolysis process, first, formate groups decompose into isocyanate segments and polyols. Then, the polyols decompose into ethers and alcohols with increasing temperature. From 300 to 500 °C, the residues decompose into amines, ethers, volatiles and CO226. Oxygen has a significant influence on the pyrolysis and combustion of polyurethane23,27. The distribution of the pyrolysis products of polyurethane is determined by temperature, and the primary and secondary pyrolysis products are generated by the breaking of urethane bonds and hydrogen conversion of polyhydric alcohol28. Hobbs established a detailed model of the bond breaking and gas product generation mechanism of polyurethane pyrolysis based on TGA experiments, percolation theory, vapor pressure and partial pressure of volatiles29. Duquesne measured the types and concentrations of gases during the combustion of polyurethane and explained the fire mechanism and fire hazard of polyurethane30.
Many researches have shown that there are big differences between the pyrolysis and combustion of multiple system and single system31. During the spontaneous combustion process of coal-polyurethane binary system, there are synergistic effects, which greatly change the mechanism and gas release laws of spontaneous combustion32,33,34. However, under the existing index gas-based assessment system, there will be some serious errors in discriminating the fire situation of polyurethane-containing goafs, which will affect normal production. In this research, the dynamic mechanism and gas product release laws of the spontaneous combustion of coal-polyurethane binary system will be investigated by a combination of TGA and spontaneous combustion simulation experiments. This study is intended to provide a theoretical basis for accurately determining the spontaneous combustion situation from fire index gases.
Experimental section
Materials
The experimental coal samples were collected from Gaohe Coal Mine in Shanxi Province, China. The polyurethane samples were collected from the same coal mine. The polyurethane consisted of two components: black and white materials. The main component of the black material is polyisocyanate (PAPI). The main component of the white material is polyether polyol N-4110. Small amounts of auxiliary ingredients (catalysts, chain extenders, flame retardants and foam stabilizers) were included in the white material. According to the construction requirements, the samples were rigid polyurethane foams made of a 1:1 volume ratio mixture of black and white materials31. The coal and polyurethane samples were pulverized before the experiments and dried at 80 °C for 24 h in a nitrogen atmosphere. The proximate analysis and elemental analysis results of the samples are shown in Table 1.
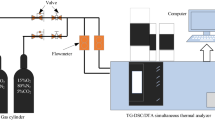
TGA experiments
The TG experiments were carried out by a NETZSCH STA-449-F3 thermal synthesis analyser. During the process of polyurethane strengthening coal rock layer, the mixing ratio of polyurethane to coal is usually lower than 1:5. For these experiments, coal was blended with polyurethane at 7 different proportions, and the samples were less than 200 mesh. The samples weighed approximately 10 mg and were heated from 30 to 800 °C at a heating rate of 2 °C/min in a synthetic air atmosphere (80 N2 and 20% O2) to simulate the spontaneous combustion process, and three repeated experiments were carried out for each sample. The samples and experimental parameters are described in Table 2.
Spontaneous combustion simulation experiments
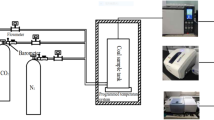
In this paper, the gas release laws of coal-polyurethane binary system were researched by spontaneous combustion simulation experiments. The experimental system is shown in Fig. 1. The components and concentrations of gas produced were analysed by a XIDONG GC-4000A GC instrument (China). Seven kinds of samples of polyurethane blended with coal, with mass fraction of polyurethane of 0.00%, 3.85%, 4.76%, 6.25%, 9.09%, 16.67% and 100.00%, were selected for these experiments. Every sample was composed of 4 kinds of particles with diameters of 1 mm, 1–3 mm, 3–5 mm and 5–7 mm according to the ratio 1:1:1:1. During the experiments, the samples were heated from 20 to 500 °C with a heating rate of 2 °C/min. Each time, a 200 g sample was used, and each group of samples was analysed three times.
Results and discussion
TGA
In this paper, the double extrapolation method was used to divide the different stages of the spontaneous combustion of blended samples35. The TG and derivative TG (DTG) results for each sample are shown in Fig. 2, and the characteristic parameters of the spontaneous combustion process of each sample are shown in Table 3.
Coal is a mixture with complex physical structures and chemical compositions, and it contains a variety of organic and inorganic substances. The spontaneous combustion processes of coal are very complex. Generally, the spontaneous combustion of coal can be divided into five stages: (1) evaporation stage, (2) physical oxygen absorption stage, (3)chemical oxygen absorption stage, (4) pyrolysis stage and (5) burning stage36. In the evaporation stage, the moisture in the coal is evaporated by heating. During the physical oxygen absorption stage, the coal contacts air and absorbs oxygen at low temperature. During the chemical oxygen absorption stage, the active groups (–CH) in coal lose hydrogen and form carbon radicals (C·), and macromolecular carbon radicals (R·) and carbon radicals (C·) absorb oxygen to form peroxides (–O–O–). Then, the peroxides (–O–O–) decompose to form hydroxyl radicals (·OH) and ether oxygen radicals (–C–H·). Hydroxyl radicals (·OH) react with aliphatic hydrocarbons and oxygen-containing functional groups in the coal, generating free radicals and oxygen-containing free radicals. Ether oxygen radicals (–C–H·) react to form carbonyl (–C=O) and hydrocarbon-like gases and carbon radicals (C·). These new carbon radicals (C·) continue to undergo the abovementioned reactions and form a chain reaction cycle. During these processes, the reactions release heat continuously, and heat accumulation leads to a temperature increase; then, the reaction rate gradually accelerates37. In the chemical oxygen absorption stage of coal, coal pyrolysis and oxygen absorption exist together at the same time. The alkyl side chains, oxygen-containing functional groups and small-molecule groups in the coal break from the main structure of the coal, and the products escape as gaseous volatiles. At the same time, some cyclic macromolecular structures in the coal begin to break. The growth rate of oxygen-absorption groups accelerates, and the amount of oxygen absorbed is enhanced. In this stage, the rate of weight gain caused by oxygen absorption of coal is faster than the rate of pyrolysis of coal, and the overall weight of the coal increases. During the pyrolysis stage, the decomposition rate of ring-structure macromolecules in coal accelerates. The number of active structures on the surface of the coal increases rapidly, and the coal oxidation and decomposition rates accelerate. Then, a large amount of CO, CO2 and heat are released. During the combustion stage, the temperature reaches the ignition temperature of coal. The aromatic ring structures in the coal begin to pyrolyse, and the fixed carbon in the coal starts to burn. The pyrolysis of coal produces tars, volatiles burn violently, and large amounts of CO and CO2 are generated. At this time, the weight loss rate and heat release rate reach their peak values35,36,38.
The combustion of polyurethane can be divided into five stages, namely, the evaporation stage, pyrolysis stage, pre-charring stage, charring stage and combustion stage. During the evaporation stage, small molecules such as water and organic solvents absorbed by the polyurethane escape. During the pyrolysis stage, the C–O bonds of the amino acid methyl ester groups in the polyurethane main chains break and decompose into dicyanate and polyhydric alcohol. With increasing temperature, these compounds decompose to form amines, olefins and CO2. During the pre-charring stage, the main reactions are the pyrolysis of isocyanate, polyol and diinide, which release ammonia, CO2 and CO and produce aromatic esters that are difficult to decompose27,39,40,41. During the charring stage, the residual polyhydric alcohols, aromatic esters, cyanates and macromolecular organics are completely pyrolysed and form HCN, CO, CO2 and carbides. During the burning stage, the fixed carbon begins to burn and release large amounts of CO and CO228,42,43.
The spontaneous combustion of coal-polyurethane binary system can be divided into five stages. The mass fraction of polyurethane has a great influence on the early stage of spontaneous combustion. The spontaneous combustion processes of blended samples with a mass fraction of polyurethane than 6.25% can be divided into the same stages as those of coal. When the mass fraction of polyurethane is higher than 6.25%, the spontaneous combustion process can be divided into five stages: the evaporation stage, the oxygen absorption–evaporation stage, the oxygen absorption-pyrolysis stage, the pyrolysis stage and the combustion stage. The evaporation stage of polyurethane is from 70.18 to 258.50 °C, and the physical and chemical oxygen absorption stages of coal are from 154.20 to 326.20 °C. The temperature ranges for the oxygen absorption of coal and the evaporation of polyurethane overlap. When the mass fraction of polyurethane is high enough, the combustion process of blended samples manifests as an oxygen absorption–evaporation stage. When the mass fraction of polyurethane is 3.85%3.85%, the temperature range of the physical oxygen absorption stage is the longest, 104.2–240.2 °C. This is because when the mass fraction of polyurethane is low, the evaporation of polyurethane and the physical oxygen absorption of coal inhibit each other. As a result, this stage occurs over a wider range of temperatures. As the mass fraction of polyurethane increases, the physical oxygen absorption stage becomes shorter and is replaced by an evaporation–oxygen absorption stage. This is because as the mass fraction of polyurethane increases, the evaporation of polyurethane is enhanced, corresponding to an evaporation–oxygen absorption stage. The temperature range of the pyrolysis of polyurethane is from 258.5 to 284.18 °C, and the temperature ranges of the oxygen absorption of coal and the pyrolysis of polyurethane also overlap. When the mass fraction of polyurethane increases, an oxygen absorption–pyrolysis stage appears. During this stage, the coal undergoes chemical oxygen absorption, and the polyurethane undergoes pyrolysis. The initial pyrolysis and ignition temperatures of blended samples decrease with increasing mass fraction of polyurethane because a large amount of heat and free radicals are quickly produced in polyurethane pyrolysis, which accelerates coal pyrolysis and combustion.
Kinetic analysis of spontaneous combustion of blended samples
The reaction rate during the TG process can be expressed as follows:
where \(g\left( \alpha \right)\) is the reaction mechanism integral function, \(\alpha\) is the loss fraction, %, \(t\) is the time, s, \(k\) is the temperature-dependent constant, \(f(\alpha )\) is the reaction mechanism differential function, and \(W_{0}\), \(W_{t}\) and \(W_{\infty }\) are the initial weight, the weight at time \(t\) and the weight at the end of combustion, respectively.
According to the Arrhenius equation:
where \(A\), \(E\), \(T\) and \(R\) are the pre-exponential factor, the apparent active energy, kJ/mol, temperature, K and the universal gas constant, respectively. By using the Coats–Redfern method to approximate the integration results, the following transformation can be obtained:
In the above formula, \(\beta\) is the heating rate, K/min. Let \(y = \ln \left[ {\frac{g\left( \alpha \right)}{{T^{2} }}} \right]\); its value can be obtained from the experimental TGA data. Let \(x = \frac{1}{T}\); \(y\) is linear with \(x\). The slope of the equation is \(k = - \frac{E}{R}\), and the intercept is \(b = \ln \frac{AR}{{\beta E}}\). It can be calculated that \(E = - k \cdot R\) and \(A = \frac{{e^{b} \times U \times E}}{R}\). Table 4 shows the calculation formulas of common spontaneous combustion dynamics models44,45,46.
In this paper, the Malek method is used to infer the kinetic mechanism function for the spontaneous combustion of different samples46,47. This method defines the function as follows:
The experimental data \(\alpha_{i}\), \(T_{i}\), \(\left( {\frac{d\alpha }{{dt}}} \right)_{i}\)\(\left( {i = 1,2, \cdots ,j} \right)\), \(\alpha_{0.5}\), \(T_{0.5}\) and \(\left( {\frac{d\alpha }{{dt}}} \right)_{0.5}\) can be inserted into function (5). The curve of \(y\left( \alpha \right) - \alpha\) can be regarded as the experimental curve. If the experimental curve overlaps with the standard curve, the function corresponding to the standard curve is determined to be the most probable dynamic mechanism function.
In this paper, the most probable mechanism functions in different stages of the spontaneous combustion process of blended samples are judged by the Malek method. The stages of spontaneous combustion for the samples and their corresponding reaction model number, curve thermodynamic equation, activation energy and pre-exponential factor are shown in Table 5.
Coal, polyurethane and blended samples have different kinetic functions at different stages of spontaneous combustion. The dominating chemical reactions during each stage of the spontaneous combustion process of different samples are different. During the evaporation stage, the moisture and organic solvents in the samples are heated and volatilized. There are almost no chemical reactions. Only moisture is present in coal, and the activation energy of the coal evaporation stage is the lowest, 27.66 kJ/mol. Polyurethane contains moisture and organic solvents, and the activation energy of the polyurethane evaporation stage is the greatest, 55.67 kJ/mol. The activation energy of the evaporation stage of blended samples increases as the mass fraction of polyurethane rises. During the physical oxygen absorption and evaporation–oxygen absorption stages, the TG curves change little and fluctuate greatly. Therefore, the activation energy cannot be calculated accurately. The activation energies of the chemical oxygen absorption stage and oxygen absorption-pyrolysis stage are significantly influenced by the mass fraction of polyurethane. When the mass fraction of polyurethane is less than 6.25%, the chemical oxygen-absorption stage corresponds to weight gain, and the activation energy of these blended samples is higher than that of coal. When the mass fraction of polyurethane is low, the oxygen absorption of coal dominates and competes with the pyrolysis of polyurethane. These two reactions inhibit each other, which leads to an increase in activation energy. When the mass fraction of polyurethane is higher than 6.25%, the processes in blended samples manifest as weight loss, and the activation energy of these processes is lower than that of coal. When the mass fraction of polyurethane is high, the pyrolysis of polyurethane dominates, and heat and free radicals are released. This reaction replaces chemical oxygen absorption to produce heat and free radicals. As a result, the activation energy of these processes decreases. The activation energy change law of the pyrolysis stage is the same as that of the oxygen absorption-pyrolysis stage. When the mass fraction of polyurethane is lower than 6.25%, the activation energy is higher than that of coal. When the mass fraction of polyurethane is higher than 6.25%, the activation energy is lower than that of coal. Combustion is a chemical reaction process controlled jointly by energy and free radicals. When the mass fraction of polyurethane is low, a small amount of polyurethane pyrolyses and oxidizes to produce a small amount of heat and free radicals, which inhibits the pyrolysis of coal, and the activation energy of these processes increases. When the mass fraction of polyurethane is higher than 6.25%, the pyrolysis and oxidation of polyurethane release large amounts of heat and radicals, which promote the pyrolysis of coal, and the activation energy of these processes decreases. The activation energies of the burning stages of blended samples and coal are nearly the same. Because the pyrolysis products of polyurethane and coal are both fixed carbon, during these stages, there are few differences among the reactions of coal, polyurethane and blended samples, and the activation energies of different samples are nearly equal.
Morphology changes in samples after spontaneous combustion
The morphological structure and coking state of blended samples before and after the spontaneous combustion experiments changed significantly, as shown in Fig. 3. Scanning electron microscopy (SEM) was used to observe the surface structures of the mixed samples. The surface structures of the blended samples are shown in Fig. 4.
After spontaneous combustion, the surface gloss of coal disappears, and the sample particles are loose and do not undergo coking. After spontaneous combustion, the morphologies of blended samples change with mass fraction of polyurethane. Some loose porous particles, which are the residues of polyurethane melting and burning, appear in the blended samples. As the mass fraction of polyurethane increases, the number of porous particles increases, and the residues become coked. The loose porous particles in the residues after spontaneous combustion are the carbon skeletons of polyurethane after the charring process. Polyurethane is a noncrystalline polymer with a complex composition and no fixed melting temperature. During combustion, its pyrolysis, melting, charring and burning processes overlap with each other.
The surface microscopic morphologies of coal particles after spontaneous combustion are shown in Fig. 4a. The surfaces of the coal particles are flat and smooth, and there are a few cracks and almost no small particles attached to the surface. The surface microscopic morphology of the sample with a mass fraction of polyurethane of 3.85% (sample PU-C-1) after spontaneous combustion is shown in Fig. 4b. The surface is rough, with many regular extended textured features that are the traces left by the melting and burning of polyurethane. The surface microscopic morphology of the sample with a mass fraction of polyurethane of 6.25% (sample PU-C-3) is shown in Fig. 4c. The surface structure is much rougher than that of coal, and there are many irregular depressions and protrusions. The pyrolysis and combustion of polyurethane particles release a large amount of heat, which accelerates the pyrolysis and combustion of the surface of the coal particles in contact with them. Then, some depressions form on the surface of the particles. The protrusions on the surface of the particles are caused by the combustion residues of polyurethane left on them after cooling. The surface microscopic morphology of the sample with a mass fraction of polyurethane of 16.67% (sample PU-C-5) is shown in Fig. 4d. Many combustion residues are stuck to the coal surface, making the surface of the coal much rougher, and there are more cracks and small particles on the surface. Therefore, blending with polyurethane changes the surface morphology of coal after spontaneous combustion. It makes the surface of coal particles rougher, increases their specific surface area and promotes their combustion.
Gas release laws during spontaneous combustion
The fire gas release curves of coal, polyurethane and blended samples determined during spontaneous combustion simulation experiments are shown in Fig. 5.
Release laws of carbon oxide gases
-
1.
Release laws of CO2. During the spontaneous combustion of coal, the initial concentration of CO2 is 865.47 ppm. From 20 to 320 °C, the CO2 concentration rises slowly. At 360 °C, the CO2 concentration begins to increase rapidly and reaches a maximum of 18,594.00 ppm at 500 °C. Before 360 °C, the main reaction of coal is oxygen absorption, and its pyrolysis is relatively weak, which leads to a lower yield of CO2. When the temperature reaches 360 °C, the pyrolysis of coal accelerates, and more carbonyl and hydroxyl groups in the coal break to produce CO2.
During the combustion of polyurethane, the initial concentration of CO2 is 3167.29 ppm, which is higher than that of coal. From 20 °C, the CO2 concentration increases rapidly and reaches 21,830 ppm at 140℃. From 140 to 280 °C, the CO2 concentration remains stable. From 280 to 400 °C, the CO2 concentration rises rapidly again and reaches a maximum at 400 °C of 48,611.64 ppm. From 400 to 500 °C, the CO2 concentration drops to 37,491.30 ppm. In the polyurethane synthesis process, isocyanate and polyhydric alcohol react to form CO2, which is stored in the pores of the polyurethane. After spontaneous combustion starts, the moisture, organic solvent and residual CO2 in the polyurethane desorb, and the CO2 concentration increases. From 140 to 280 °C, the main reaction is the depolymerization of polyurethane, and almost no CO2 is generated. At 280 °C, the pre-charring stage of polyurethane begins, and the concentration of CO2 rises rapidly. When the temperature reaches 400 °C, the main reaction is the combustion of the residual fixed carbon from polyurethane pyrolysis. Due to the insufficient oxygen supply, the combustion is incomplete, and the CO2 concentration drops.
During the spontaneous combustion of blended samples, the CO2 concentration is significantly affected by the mass fraction of polyurethane. When the temperature is higher than 120 °C, the CO2 concentration at a given temperature of the blended samples increases with the mass fraction of polyurethane. The CO2 concentration change laws of blended samples with a mass fraction of polyurethane higher than 6.25% are similar to that of polyurethane; the CO2 concentration increases significantly after 200 °C and remains stable from 200 to 280 °C. From 280 to 460 °C, the CO2 concentration increases rapidly. After 460 °C, the CO2 concentration drops or remains stable. When the mass fraction of polyurethane is less than 6.25%, the CO2 concentration change law is similar to that of coal. When coal is blended with polyurethane, the CO2 concentration rises during spontaneous combustion compared to that of coal. This is because the pyrolysis of polyurethane produces large amounts of free radicals and CO2, and the activation energy of each stage of the combustion of polyurethane is lower than that of coal. The pyrolysis of polyurethane in the blended samples provides free radicals for the pyrolysis and combustion of coal. With increasing mass fraction of polyurethane, the combustion of blended samples accelerates, and the CO2 concentration rises.
-
2.
Release laws of CO. During the spontaneous combustion of coal, the CO concentration increases with increasing temperature. At 200 °C, the CO concentration exceeds 24 ppm. From 320 to 500 °C, the CO concentration rises rapidly and reaches a maximum of 3848.60 ppm. When the temperature is lower than 320 °C, the main reaction is the oxygen absorption of coal, and the pyrolysis of coal is weak. After 320 °C, the pyrolysis of coal is enhanced. During this stage, there are two main reactions. One reaction is the cleavage of carboxyl groups and carbonyl groups, and the other is CO2 reacting with coal to produce CO. The CO concentration increases rapidly.
During the combustion of polyurethane, the CO concentration increases with increasing temperature. At 180 °C, the CO concentration exceeds 24 ppm. From 280 to 500 °C, the CO concentration increases rapidly and reaches a maximum of 8356.60 ppm. The pre-charring stage of polyurethane starts at 320 °C. During this stage, isocyanate, amides and oxygen-containing functional groups break to produce CO. At the same time, the volatiles generated by the pyrolysis of polyurethane burn and release CO. As the temperature increases, the production rate of CO accelerates.
During the spontaneous combustion of blended samples, the mass fraction of polyurethane has a significant influence on the CO concentration. As the mass fraction of polyurethane increases, the temperature at which the CO concentration abruptly starts to rapidly increase becomes lower, and the CO concentration at a given temperature is higher. The temperature at which the CO concentration abruptly changes in sample PU-C-5 is 160 °C. The CO concentration in the spontaneous combustion of blended samples with a mass fraction of polyurethane less than 6.25% is higher than that of coal and lower than that of polyurethane. When the mass fraction of polyurethane is higher than 6.25%, the CO concentration of blended samples is higher than those of coal and polyurethane, and the maximal CO concentration of sample PU-C-5 is 12,767.66 ppm. There are synergistic effects during the spontaneous combustion of blended samples. The pyrolysis and combustion processes of polyurethane provide heat and free radicals to coal, which accelerates the pyrolysis and combustion of coal. The pyrolysis and oxidation of polyurethane consume more oxygen than the corresponding reactions of coal. The incomplete combustion of coal-polyurethane binary system accelerates the generation of CO. Moreover, the polyurethane in blended samples melts as the temperature rises, and the combustion of blended samples scorches the particles of coal and polyurethane into lumps. The cracks in blended samples enable air flow, which promotes the burning of the surface of sample blocks. The combustion inside the sample blocks is incomplete, and the CO concentration increases. Therefore, the spontaneous combustion of coal-polyurethane binary system promotes the formation of CO.
The curves of the CO/CO2 ratio during the spontaneous combustion of blended samples are shown in Fig. 5f. The CO/CO2 ratio curves of coal and blended samples are almost the same, and the CO/CO2 ratio curve of polyurethane is lower than those of other samples. CO2 production during the spontaneous combustion of polyurethane is much higher than that during the spontaneous combustion of other samples. The main reactant in coal samples and blended samples is coal. Therefore, the CO/CO2 ratios of coal and blended samples are similar at the same temperature. From 20 to 350 °C, the CO/CO2 ratio rises with increasing temperature. From 350 to 420 °C, the CO/CO2 ratio decreases slightly. From 420 to 500 °C, the ratio increases again. This trend occurs because from 350 to 420 °C, the pyrolysis and violent oxidation of coal release a large amount of CO2, and the CO/CO2 ratio decreases. As pyrolysis and oxidation proceed, the samples begin to burn, and the oxygen supply is insufficient. Then, the generation of CO increases, the generation of CO2 decreases, and the CO/CO2 ratio drops. The CO/CO2 ratios of coal and blended samples are independent of the mass fraction of polyurethane. The CO/CO2 ratio has a good correlation with spontaneous combustion temperature, meaning that this parameter can describe the situation of spontaneous combustion more accurately than the existing index gas method.
Release laws of hydrocarbon gases
-
1.
Release laws of CH4. During the spontaneous combustion of coal, the CH4 concentration first increases and then decreases. From 20 to 400 °C, the CH4 concentration rises with increasing temperature and reaches a maximum of 7928.51 ppm at 400 °C. Then, from 400 to 500 °C, the CH4 concentration drops rapidly. The coal used in the experiments is bituminous coal, which has a high degree of coalification, and a certain amount of CH4 exists in the pores of coal. During the spontaneous combustion process, as the temperature rises, CH4 desorbs from the coal. At 400 °C, the desorption of CH4 ends, CH4 begins to oxidize, and the CH4 concentration decreases.
During the combustion of polyurethane, the CH4 concentration increases with increasing temperature. At 500 °C, the CH4 concentration reaches 1395.18 ppm. There is no CH4 in the pores of polyurethane. As the temperature rises, the polyurethane begins to pyrolyse, and the methyl groups on the macromolecular branches in polyurethane break to form CH4. Then, the CH4 concentration increases.
During the spontaneous combustion of blended samples, the CH4 concentration at a given temperature decreases with increasing mass fraction of polyurethane. The CH4 concentration change laws of blended samples with a mass fraction of polyurethane is less than 9.09% are similar to that of coal. At 400 °C, the CH4 concentration reaches a maximum and then begins to decrease. The higher the mass fraction of polyurethane is, the lower the maximum CH4 concentration is. The change trend of the CH4 concentration of blended samples with a mass fraction of polyurethane of 16.67% is close to that of polyurethane. During the spontaneous combustion process, the coal samples remain loose. As the temperature rises, the polyurethane in the blended samples melts and scorches with coal, and the particles of coal are coked into lumps. The cracks between particles are blocked, which inhibits the desorption of CH4. Therefore, coal-polyurethane binary system inhibits the generation and desorption of CH4.
-
2.
Release laws of C2H6. During the spontaneous combustion of coal, C2H6 begins to release at 260 °C, and the concentration of C2H6 rises with increasing temperature. At 500 °C, the C2H6 concentration reaches a maximum of 51.06 ppm.
During the combustion of polyurethane, C2H6 begins to release at 340 °C. As the temperature increases, the C2H6 concentration rises rapidly. At 500 °C, it reaches a maximum of 623.65 ppm.
During the spontaneous combustion of blended samples, C2H6 begins to release at 260 °C. The C2H6 concentration of different blended samples at a given temperature rises with increasing mass fraction of polyurethane. The ethyl groups in the macromolecular structure of coal and polyurethane break to produce ethane. Some small molecular structures in coal gradually decompose to ethane after 200 °C. Polyurethane is pyrolysed into isocyanate and polyhydric alcohol first and then further cracked into small molecular substances after 300 °C. These small molecules pyrolyse produce C2H6. During the combustion of polyurethane, the temperature at which C2H6 begins to release is higher, and more C2H6 is produced. From 300 to 500 °C, the activation energy of the spontaneous combustion of blended samples decreases as the mass fraction of polyurethane increases, and the pyrolysis of samples is easier. Therefore, v promotes the formation of C2H6.
-
3.
Release laws of C2H4. During the spontaneous combustion of coal, the concentration of C2H4 rises with increasing temperature. At 500 °C, the concentration of C2H4 reaches a maximum of 22.36 ppm.
During the combustion of polyurethane, C2H4 begins to release at 200 °C. The C2H4 concentration rises with increasing temperature and reaches a maximum of 255.54 ppm at 500 °C.
During the spontaneous combustion of blended samples, the temperature of at which C2H4 is first released drops as the mass fraction of polyurethane increases. The C2H4 concentration at a given temperature rises with increasing mass fraction of polyurethane. At 300 °C, the unsaturated macromolecular bonds in the coal decompose to produce ethylene. Polyurethane contains more unsaturated bonds, and the pyrolysis temperature of polyurethane is lower than that of coal. From 300 to 500 °C, the activation energy of the spontaneous combustion of blended samples drops as the mass fraction of polyurethane increases, and the samples are easier to pyrolyse and burn. Therefore, coal-polyurethane binary system promotes the formation of C2H4.
In summary, during the spontaneous combustion of coal-polyurethane binary system, the initial release temperatures of CO2, CO, C2H6 and C2H4 decrease as the mass fraction of polyurethane increases. The concentration of these gases at a given temperature rises with increasing mass fraction of polyurethane, and the CH4 concentration drops as the mass fraction of polyurethane increases. Polyurethane promotes the generation of fire gases during the spontaneous combustion of coal, and it makes the release temperature of fire gases drop and their concentration increase. Therefore, when spontaneous combustion occurs in polyurethane-containing coal seams or goafs, there will be some errors in assessments of the spontaneous combustion temperature and situation based on the index gases of CO2, CO, C2H6 and C2H4. The ratios of CO/CO2 during the spontaneous combustion of coal samples and blended samples have the same change trends with respect to temperature. Using the CO/CO2 ratio as the judgement index can effectively reduce the error.
Conclusions
-
1.
The mass fraction of polyurethane changes the oxygen absorption stage and pyrolysis stage of spontaneous combustion processes of coal-polyurethane binary system. When the mass fraction of polyurethane is lower than 6.25%, polyurethane inhibits the oxygen absorption and pyrolysis of coal, and the activation energies of the oxygen absorption stage and pyrolysis stage are higher than those of coal. When the mass fraction of polyurethane is higher than 6.25%, polyurethane promotes the spontaneous combustion of coal. The pyrolysis temperature and ignition temperature of blended samples decrease as the mass fraction of polyurethane rises. The activation energies of the oxygen absorption-pyrolysis stage and pyrolysis stage decrease as the mass fraction of polyurethane rises, which makes spontaneous combustion easier.
-
2.
During spontaneous combustion, the sample particles of coal-polyurethane binary system scorch into lumps. The higher the mass fraction of polyurethane is, the larger the coking lumps are. SEM observation shows that the surface of blended sample particles is covered with polyurethane combustion residues. When the mass fraction of polyurethane is low, obvious textured features indicating polyurethane residues on the coal particles are present. When the mass fraction of polyurethane is high, the surface of the coal particles is rough and irregular. Polyurethane increases the specific surface area of samples and promotes spontaneous combustion.
-
3.
During the spontaneous combustion of coal-polyurethane binary system, the mass fraction of polyurethane has a significant influence on index gas release laws. The initial release temperature of the index gases CO, CO2, C2H6 and C2H4 drops as the mass fraction of polyurethane increases. The CH4 release amount drops as the mass fraction of polyurethane increases. The trends of the CO/CO2 ratio with respect to temperature of blended samples of different mass fraction of polyurethanes are almost the same.
References
Saikia, B. K., Saikia, J., Rabha, S., Silva, L. F. O. & Finkelman, R. Ambient nanoparticles/nanominerals and hazardous elements from coal combustion activity: Implications on energy challenges and health hazards. Geosci. Front. 9, 863–875 (2018).
Sehn, J. L. et al. Nanomineralogy in the real world: A perspective on nanoparticles in the environmental impacts of coal fire. Chemosphere 147(3), 439–443 (2016).
Oliveira, M. L. S. et al. Multifaceted processes controlling the distribution of hazardous compounds in the spontaneous combustion of coal and the effect of these compounds on human health. Environ. Res. 160, 562–567 (2018).
Silva, L. F. O. et al. Applied investigation on the interaction of hazardous elements binding on ultrafine and nanoparticles in Chinese anthracite-derived fly ash. Sci. Total Environ. 419, 250–264 (2012).
Xu, M. et al. Research on application of polyurethane grouting material for mining. Mater. Rev. 28(17), 96–100 (2014).
Chen, X., Liu, H. N., Liu, Z. S. & Hu, Y. Research progress of polyurethane for consolidating coal and rock mass. Clean Coal Tech. 17(04), 108–112 (2011).
Wu, J., Yan, H., Wang, J., Wu, Y. & Zhou, C. Flame retardant polyurethane elastomer nanocomposite applied to coal mines as air-leak sealant. J. Appl. Polym. Sci. 129(6), 3390–3395 (2013).
Zhou, P. & Li, T. Study on exotherm problem of polyurethane hole-sealing materials for coal gas drainage. Chem. Propull. Polym. Mater. 13(4), 79–83 (2015).
Dong, J., Liu, G. & Liu, Z. Exothermic reaction controlling technology and curing characteristics of polymer foam materials. J. China Coal Soc. 35(3), 377–380 (2010).
Xu, Y. Causes and control of disaster by the heat from polyurethane materials used in mine 1–3 (China Coal Research Institute, Beijing, 2019).
Yang, J. J. & Li, X. C. Mechanism analysis and preventive measures of the fire accidents by Polymer chemical grouting material used in mining. Saf. Coal Mines 42(08), 178–180 (2011).
Kok, M. V. Coal pyrolysis: Thermogravimetric study and kinetic analysis. Energy Sources 25(10), 1007–1014 (2003).
Wang, J. et al. Kinetics modeling of low-rank coal pyrolysis based on a three-Gaussian distributed activation energy model (DAEM) reaction model. Energy Fuels 30(11), 9693–9702 (2016).
Chen, Y. & He, R. Fragmentation and diffusion model for coal pyrolysis. J. Anal. Appl. Pyrol. 90(1), 72–79 (2011).
Deng, J., Xiao, Y., Li, Q., Lu, J. & Wen, H. Experimental studies of spontaneous combustion and anaerobic cooling of coal. Fuel 157, 261–269 (2015).
Deng, J., Wang, K., Zhang, Y. & Yang, H. Study on the kinetics and reactivity at the ignition temperature of Jurassic coal in North Shaanxi. J. Therm. Anal. Calorim. 118(1), 417–423 (2014).
Ray, S. K., Singh, R. P., Sahay, N. & Varma, N. K. Assessing the status of sealed fire in underground coal mines. J. Sci. Ind. Res. (India) 63(7), 579–591 (2004).
Singh, A. K., Singh, R. V. K., Singh, M. P., Chandra, H. & Shukla, N. K. Mine fire gas indices and their application to Indian underground coal mine fires. Int. J. Coal Geol. 69(3), 192–204 (2007).
Lu, P., Liao, G. X., Sun, J. H. & Li, P. D. Experimental research on index gas of the coal spontaneous at low-temperature stage. J. Loss Prev. Process Ind. 17(3), 243–247 (2004).
Zhu, J., He, N. & Li, D. The relationship between oxygen consumption rate and temperature during coal spontaneous combustion. Saf. Sci. 50, 842–845 (2012).
Tesser, R., Di Serio, M., Sclafani, A. & Santacesaria, E. Modeling of polyurethane foam formation. J. Appl. Polym. Sci. 92, 1875–1886 (2004).
Li, M., Jiang, L., He, J. J. & Sun, J. H. Kinetic triplet determination and modified mechanism function construction for thermo-oxidative degradation of waste polyurethane foam using conventional methods and distributed activation energy model method. Energy 175, 1–13 (2019).
Branca, C., Di Blasi, C., Casu, A., Morone, V. & Costa, C. Reaction kinetics and morphological changes of a rigid polyurethane foam during combustion. Thermochim. Acta. 399(1–2), 127–137 (2003).
Thirumal, M., Khastgir, D., Nando, G. B., Naik, Y. P. & Singha, N. K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 95(6), 1138–1145 (2010).
Singh, H. & Jain, A. K. Ignition, combustion, toxicity, and fire retardancy of polyurethane foams: A comprehensive review. J. Appl. Polym. Sci. 111(2), 1115–1143 (2009).
Jiao, L., Xiao, H., Wang, Q. & Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 98(12), 2687–2696 (2013).
Yoshitake, N. & Furukawa, M. Thermal degradation mechanism of α, γ-diphenyl alkyl allophanate as a model polyurethane by pyrolysis-high-resolution gas chromatography/FT-IR. J. Anal. Appl. Pyrol. 33(3), 269–281 (1995).
Zhang, Y., Xia, Z., Huang, H. & Chen, H. Thermal degradation of polyurethane based on IPDI. J. Anal. Appl. Pyrol. 84(1), 89–94 (2009).
Hobbs, M. L., Erickson, K. L. & Chu, T. Y. Modeling decomposition of unconfined rigid polyurethane foam. Polym. Degrad. Stab. 69, 47–66 (2000).
Duquesne, S. et al. Analysis of fire gases released from polyurethane and fire-retarded polyurethane coatings. J. Fire Sci. 18, 456–482 (2000).
Wang, H., Tian, Y., Chen, X. & Li, J. Kinetic and synergetic effect analysis of the co-combustion of coal blended with polyurethane materials. ACS Omega 5, 26005–26014 (2020).
Qu, X. et al. Synergetic effect on the combustion of lignite blended with humus: Thermochemical characterization and kinetics. Appl. Therm. Eng. 152, 137–146 (2019).
Onay, O. & Koca, H. Determination of synergetic effect in co-pyrolysis of lignite and waste tyre. Fuel 150(1), 169–174 (2015).
Haykiri-Acma, H. & Yaman, S. Combinations of synergistic interactions and additive behavior during the co-oxidation of chars from lignite and biomass. Fuel Process. Technol. 89(2), 176–182 (2008).
Lei, Y. & Liang, D. Pyrolysis and Smoldering Propurties of Polyurethane Foam Materials 27–41 (Sun Yatsen University Press, Sun Yat, 2013).
Qu, L. A study on the prediction method of coal spontaneous combustion development period based on critical temperature. Environ. Sci. Pollut. Res. 25(35), 35748–35760 (2018).
Wang, D. M., Xin, H. H., Qi, X. Y., Dou, G. L. & Zhong, X. X. Mechanism and relationships of elementary reactions in spontaneous combustion of coal: The coal oxidation kinetics theory and application. Meitan Xuebao J. China Coal Soc. 39(8), 1667–1674 (2014).
Jiang, Y. et al. Pyrolysis behaviors and product distribution of Shenmu coal at high heating rate: A study using TG-FTIR and Py-GC/MS. Energy Convers. Manag. 179, 72–80 (2019).
Hobbs, M. L., Erickson, K. L. & Chu, T. Y. Modeling decomposition of unconfined rigid polyurethane foam. Polym. Degrad. Stab. 69(6), 47–66 (2000).
Yuan, K., Jiang, Z., Li, S. & Zhou, Y. The thermal degradation of polyurethane. Polym. Bull. 6(12), 22–26 (2005).
Branca, C., Di Blasi, C., Casu, A., Morone, V. & Costa, C. Reaction kinetics and morphological changes of a rigid polyurethane foam during combustion. Thermochim. Acta 399(1–2), 127–137 (2003).
Wang, H., Tian, Y. & Zhang, L. Experimental study of the characteristic parameters of the combustion of the wood of ancient buildings. J. Fire Sci. 37(2), 117–136 (2019).
Tang, Z. et al. Thermal degradation behavior of rigid polyurethane foams prepared with different fire retardant concentrations and blowing agents. Polymer 43(24), 6471–6479 (2002).
Liu, L., Wang, Z., Che, K. & Qin, Y. Research on the release of gases during the bituminous coal combustion under low oxygen atmosphere by TG–FTIR. J. Energy Inst. 91, 323–330 (2018).
Jiang, L. et al. Pyrolytic behavior of waste extruded polystyrene and rigid polyurethane by multi kinetics methods and Py-GC/MS. Fuel 222, 11–20 (2018).
Hu, R. et al. Thermal Analysis Kinetics 28–127 (Science Press, Beijing, 2001).
Li, W. Fundamental Study on the Thermokinetics Process of Coal Oxidation Under Oxygen-Depleted Atmosphere 67–98 (Xi’an University of Science and Technology, Shaanxi, 2018).
Acknowledgements
The authors gratefully acknowledge the support of the National Nature Science Foundation of China (51874313) and the National Key Research and Development Program of China (2018YFC0808101).
Author information
Authors and Affiliations
Contributions
H.W. designed the research and reviewed the manuscript; Y.T. wrote the original draft and analysed the results; J.L. and X.C. conducted the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Tian, Y., Li, J. et al. Experimental study on thermal effect and gas release laws of coal-polyurethane cooperative spontaneous combustion. Sci Rep 11, 1994 (2021). https://doi.org/10.1038/s41598-021-81537-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81537-5
This article is cited by
-
Thermal Kinetics of Coal Spontaneous Combustion Based on Multiphase Fully Coupled Fluid–Mechanical Porous Media Model
Natural Resources Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.