Abstract
Heat release of coal combustion in an oxygen-lean and multi-gas environment is a common phenomenon, coalfield fires caused by it can lead to serious environmental destruction and loss of coal resources. Simultaneous thermal analysis experiments for Bulianta (BLT, high-volatile bituminous coal) and Yuwu coal (YW, anthracite) in 21vol.%O2/79vol.%N2 and 15vol.%O2/5vol.%CO2/80vol.%N2 were carried out to study the law of heat release. Based on the TG-DTG-DSC curves, the combustion characteristic parameters were analyzed. Decreasing O2 concentration caused a significant reduction of local reactivity and further the decreasing maximum heat release rate for low-rank coal, while increasing CO2 concentration caused a significant thermal lag effect and further the increasing maximum heat release rate for high-rank coal. The relationship between the heat release rate and the reaction rate constant was quantitatively analyzed. At the increasing stage of the heat release rate, the heat release rate of the two coals increased conforming to ExpGro1 exponential model. At the decreasing stage of the heat release rate, the heat release rate of YW coal decreased exponentially with the reaction rate constant, while the heat release rate of BLT coal decreased linearly. Regardless of the atmospheres, the conversion rates corresponding to maximum heat release rate of BLT and YW coal were about 0.80 and 0.50, respectively, indicating that the coal rank played a dominant role. The results are helpful to understand the heat release process of coal oxygen-lean combustion in O2/CO2/N2.
Similar content being viewed by others
Introduction
Coal is an important energy source to meet the power demand as well as to promote the economy development because of its abundant reserves1,2,3. Coalfield fires triggered by spontaneous coal combustion also occur continuously when mining, and are considered a global crisis, which not only causes serious environmental destruction and loss of coal resources, but also poses a serious threat to human safety and health4,5,6,7. Most coalfield fires occur in an oxygen-lean (oxygen concentration lower than air) and multi-gas environment due to insufficient oxygen supply and combustion product gases8. The development and expansion of coalfield fires closely relate to the heat accumulation of coal combustion. Obtaining the law of heat release during coal oxygen-lean combustion in a multi-gas atmosphere will be beneficial to understand and reveal the dynamic spread of a coalfield fire.
At present, most scholars have carried out a lot of research on the heat release of coal combustion under conventional air combustion. Pan et al.2 studied the heat release of the oxidation characteristics of pulverized coal under conventional air combustion using a C600 microcalorimeter. The results showed that the oxidative heat evolution of pulverized coal has obvious stage characteristics of first absorbing heat and then releasing heat. Other scholars9,10,11,12 also came to a conclusion consistent with the above. Deng et al.13 investigated the gas production and thermal behavior of weathered coal and fresh coal. They found that, a significant difference existed in the thermal energy release between weathered coal and fresh coal at different oxidation stages. Su et al.14 studied the main characteristic behaviors (temperature gradient, oxygen consumption, oxidation kinetics, gaseous products and heat release) of coal combustion, and divided the evolution process into five stages. Further, heat release during the last three stages was classified into three heat levels. Zhao et al.15 divided the high-temperature oxidation process into four stages using thermogravimetric differential scanning calorimetry (TG-DSC), including water evaporation and gas desorption, oxygen absorption and weight gain, thermal decomposition and combustion, and obtained detailed heat release characteristics. Wang et al.16 studied the thermal behaviors and kinetic characteristics of coal oxygen-lean combustion at high temperature by TG-DSC synchronous thermal analysis. The results showed that the centralized weight loss and exothermic processes became dispersed in an oxygen-lean atmosphere, and the effects of oxygen concentration enhanced when it was lower than 13 vol.%. There was a linear relationship between mass and heat release, and the relationship between mass and heat release changed in stages with oxygen concentration.
Some studies analyzed the oxygen-lean combustion behaviors of coal in a O2/N2/CO2 atmosphere. Ren et al.12 performed coal oxidation and combustion heat behavior analysis experiment in O2/N2/CO2 and O2/N2 atmospheres (O2 concentration of 21%, 14%, 8%, CO2 concentration of 0%, 39%, 46%, 52%). The results illustrated that the increase of CO2 concentration or the decrease of O2 concentration had a delay effect on the TG and DSC curve. Su et al.17 studied the dynamical oxygen-lean combustion behaviors of two coal samples in 21vol.%O2/79vol.%N2 and 15vol.%O2/5vol.%CO2/80vol.%N2 atmospheres by conducting simultaneous thermal analysis. The results showed that there was an ignition delay phenomenon for coal lean-oxygen combustion in the O2/CO2/N2 atmosphere, and apparent activation energy increased at III stage and decreased at IV stage in the O2/CO2/N2 atmosphere, compared with in the O2/N2 atmosphere. However, they did not further study the heat release of coal oxygen-lean combustion in the O2/CO2/N2 atmosphere. In addition, some studies studied the combustion behavior of coal in the N2/CO2/O2, O2/H2O/CO2 and O2/N2/H2O atmospheres18,19,20. Simultaneously, they did not analyze the heat release of coal oxygen-lean combustion, and the effect of multi-gas atmosphere on the heat release of coal oxygen-lean combustion.
Heat release is the basis of coalfield fire spreading. However, there are currently few studies on the heat release of coal oxygen-lean combustion in a multi-gas environment. The purpose of this work is to analyze the law of heat release during coal oxygen-lean combustion in a O2/CO2/N2 atmosphere. Simultaneous thermal analysis experiments were carried out for two coal samples in 21vol.%O2/79vol.%N2 and 15vol.%O2/5vol.%CO2/80vol.%N2, respectively. Based on the TG-DTG-DSC curves, the combustion characteristic parameters were discussed, and the kinetic parameters were obtained. Furthermore, the relationship between the exothermic rate and the reaction rate constant was proposed. This work can provide theoretical support for revealing the spread of coalfield fires.
Experiments and methods
Preparation of coal samples
Two fresh coal samples were selected from the Bulianta colliery in Inner Mongolia and the Yuwu colliery in Shanxi, China, denoted as BLT and YW, respectively. The reason for choosing these two kinds of coal is that they belong to different rank coals and can show good experimental results. BLT coal belongs to high-volatile bituminous coal, which has a higher volatile matter (31.66%), lower fixed carbon (43.30%) and higher ash content (16.16%) than that of YW coal. YW coal belongs to anthracite. Coal samples were crushed in the laboratory, then sieved through 0.60 mm, 0.45 mm and 0.30 mm gauze. The particle size between 0.30 and 0.45 mm were selected as the experimental coal samples. The proximate analysis and ultimate analysis had been carried out in our previous research17, as shown in Table 1.
TG-DTG‑DSC experiment
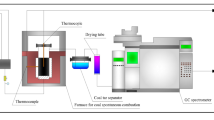
A synchronous thermal analyzer (NETZSCH STA 449 F3) was utilized. According to the detection of gas environment and gas concentration in most coal fire areas in China by scholars, coalfield fires are mostly in an oxygen-lean and multi-gas environment, wherein include an oxygen-lean environment with 15vol.%O221. In order to study the dynamical oxygen-lean combustion behaviors of coal in a multi-gas environment, we have chosen 21vol.%O2/79vol.%N2 and 15vol.%O2/5vol.%CO2/80vol.%N2 atmospheres to conduct simultaneous thermal analysis experiments of two coal samples in our previous research17. We continued to choose the above two atmospheres to carry out research in this work, the purpose is to study the law of heat release during coal oxygen-lean combustion in a multi-gas environment based on traditional air combustion. Two atmosphere gases were placed in two cylinders respectively. The coal sample was put in a container. Gases passed into the container from two inlets, one of which located the bottom with a gas flow rate of 50 ml/min and another one located the middle with a gas flow rate of 20 ml/min. Two kinds of coal samples, with a mass of about 13 mg were heated from room temperature to 1100 °C, at three heating rates of 10 °C/min, 15 °C/min, and 20 °C/min, respectively, as seen in Table 2. Based on the synchronous thermal analyzer, the schematic diagram of the experimental system is shown in Fig. 1.
Combustion kinetic theory
The coal combustion kinetic equation can be expressed as follows22
where, k(T) is the reaction rate constant. α corresponds to the conversion of coal, its expression is as follows
where, Wi means the coal mass corresponding to the time of i.
The reaction rate constant of coal combustion can be expressed as follows23.
where, A corresponds to the pre-exponential factor (min−1); E corresponds to the apparent activation energy (kJ/mol), R corresponds to the universal gas constant.
The kinetics equation of non-isothermal reaction can be expressed as follows24
where, β corresponds to the heating rate for non-isothermal experiments.
Due to the high accuracy, the Kissinger–Akahira–Sunose (KAS) method was utilized to calculate the apparent activation energy. Its expression is as follows
Based on the plot of ln(β/Tα2) versus 1000/T, activation energies were calculated from the slope of the linear regression lines, pre-exponential factors were estimated from the intercepts.
Results and discussions
The influence of the O2/CO2/N2 atmosphere on the combustion characteristic parameters
Figure 2 gives the calculation method of combustion characteristic parameters, including ignition temperature (Ti), maximum combustion rate (vp) and the temperature corresponding to maximum combustion rate (Tp), maximum heat release rate (vh) and the temperature corresponding to maximum heat release rate (Th), and burnout temperature (Tf). The vertical line passing through the DTG curve point (Tp, vp) intersects the TG curve at point A, and the tangent line passing through point A intersects the straight line when the TG curve begins to descend at point B. The abscissa of point B corresponds the Ti. Similarly, the abscissa of point C corresponds the Tf. (Tp, vp) is valley point on the DTG curve. (Th, vh) is the valley point on the DSC curve. The results obtained are shown in Table 3. When the heating rate was constant, the values of Ti, Tp, Th and Tf in the O2/CO2/N2 atmosphere visibly increased compared with that in the O2/N2 atmosphere. This indicated that a delay of ignition, heat release and burnout existed during coal oxygen-lean combustion in the O2/CO2/N2 atmosphere. This result was consistent with the literature17,18,25,26.
In the O2/CO2/N2 atmosphere, for BLT coal sample, the values of vp decreased by 0.73%/min, 1.53%/min, and 3.87%/min at 10 °C/min, 15 °C/min, and 20 °C/min, respectively, and the values of vh decreased by 2.55 mW/mg, 4.18 mW/mg, and 16.51 mW/mg at 10 °C/min, 15 °C/min, and 20 °C/min, respectively, compared with that in the O2/N2 atmosphere, because the decreasing O2 concentration leaded to a reduction of local reactivity27. For YW coal sample, the values of vp decreased by 1.45%/min, 0.48%/min, and -0.09%/min at 10 °C/min, 15 °C/min, and 20 °C/min, respectively, and the values of vh increased by -3.52 mW/mg, 1.43 mW/mg, and 4.25 mW/mg at 10 °C/min, 15 °C/min, and 20 °C/min, respectively. The reason was that coal absorbed enough oxygen at low heating rate and O2 played a leading role on the decreasing vp and vh. Decreasing O2 concentration leaded to a reduction of local reactivity and further the decreasing maximum heat release rate27. At high heating rate, coal absorbed less oxygen and CO2 played a leading role on the increasing vh. Increasing CO2 concentration leaded to a thermal lag effect and further the increasing maximum heat release rate28. It can be seen that the influence of the O2/CO2/N2 atmosphere on the maximum heat release rate was restricted by the coal rank. The low-rank coal burned faster due to its low carbon content, and O2 had a significant impact on the maximum heat release rate. The high-rank coal contained more carbon and burned slowly, and CO2 had a significant impact on the maximum heat release rate.
The influence of the O2/CO2/N2 atmosphere on the kinetic parameters by KAS method
Figure 3 shows the changes in the values of apparent activation energy and correlation coefficients (R2) by KAS method, in the two atmospheres. For BLT coal, as the conversion rate increased, the values of apparent activation energy all first decreased, then increased, and finally decreased. In order to divide the low-temperature oxidation and the combustion stages, the corresponding conversion rate at the Ti in two atmospheres was calculated respectively. The results showed that, in the O2/N2 atmosphere, the conversion rate at the Ti were 0.17, 0.12, 0.14, at 10 °C/min, 15 °C/min, and 20 °C/min, respectively. In the O2/CO2/N2 atmosphere, the conversion rate at the Ti were 0.15, 0.15, 0.11, at 10 °C/min, 15 °C/min, and 20 °C/min, respectively. Therefore, In the range of 0.5–0.15 conversion rate, three groups of experiments for BLT 1, BLT 4 and BLT 5 were in the low-temperature oxidation process, three groups of experiments for BLT 2, BLT 3 and BLT 6 were in the initial stage of ignition. Since the R2 was lower than 0.80, the values of apparent activation energy were not accurate and cannot be compared. When the conversion rate was higher than 0.15, the coal sample was ignited, and the values of apparent activation energy values in the two atmospheres appeared a sudden increase. During the combustion process, the values of apparent activation energy in the O2/CO2/N2 atmosphere were approximately 33–58% lower than that in the O2/N2 atmosphere. This was because the heat released by coal combustion accumulated more easily in the O2/CO2/N2 atmosphere than in the O2/N2 atmosphere, as a result of the reduction of 6 vol.% O2 and the addition of 5 vol.% CO2 (low heat conduction coefficient).
For YW coal, as the conversion rate increased, the values of apparent activation energy in the two atmospheres kept decreasing. When the conversion rate was 0.05, it was in the low-temperature oxidation process, and the values of apparent activation energy in the O2/CO2/N2 atmosphere were approximately 40% higher than that the O2/N2 atmosphere. This has been confirmed in the research of others17,29. When the conversion rate was higher than 0.05, the coal was ignited, and the values of apparent activation energy in the two atmospheres were close. The influence of the atmosphere was no longer obvious.
In addition, the R2 in the two atmospheres was greater than 0.99. However, for BLT coal, a decrease behavior was showed in the conversion rates ranges of 0.10–0.25 in O2/N2 and 0.15–0.45 in O2/CO2/N2, respectively. The reason was that the precipitation of the remaining volatiles was promoted by the heat release of separated volatiles combustion, and the precipitation and combustion of volatile was significantly deferred in the O2/CO2/N2 atmosphere compared with that in the O2/N2 atmosphere27,30,31, because of the slightly lower diffusivity of volatiles in CO2 than in N2 and the lower mass flux of oxygen to the volatiles flame28,32,33,34.
The influence of the O2/CO2/N2 atmosphere on the heat release
The reaction rate between oxygen and coal is the key factor influencing the heat release rate35. Studying the relationship between the heat release rate and reaction rate is beneficial to understand in the heat release process during coal oxygen-lean combustion in the O2/CO2/N2 atmosphere, which can provide a theoretical foundation for revealing the law of coalfield fire spreading. Since the value of the reaction rate constant can directly reflect the reaction rate, the reaction rate constant was used instead of the reaction rate in this study. According to our previous research17, the kinetic mechanism functions of BLT and YW coal were Jander (Diffusional (3-D)) and three-level chemical reaction, respectively. The values of pre-exponential factor were calculated though Eq. (5), and then the values of reaction rate constant were obtained though Eq. (3).
Figures 4 and 5 show the DSC-k(T) curves of BLT and YW coal, respectively. The conversion rate corresponding to the maximum heat release rate was taken as a segment point, and the DSC-k(T) curves were divided into two stages: the increasing stage and the decreasing stage of the heat release rate. The conversion rate corresponding to the maximum heat release rate of BLT and YW coal was always about 0.80 and 0.50, respectively., indicating that the conversion rate corresponding to the maximum heat release rate was only related to the coal rank, and not corrected to the atmosphere.
At the increasing stage of the heat release rate, the heat release rate of two coals increased exponentially with the increasing reaction rate constant. At the decreasing stage of the heat release rate, the heat release rate of YW coal decreased exponentially with the increasing reaction rate constant, whereas the heat release rate of BLT coal decreased linearly with the increasing reaction rate constant. This was because BLT has higher volatile content and lower fixed carbon content than YW coal, the more active nature resulted in a slow decrease in the heat release rate. ExpGro1 exponential model (see Eq. (6)) was selected to fit the DSC-k(T) curves at the increasing stage of heat release rate for the two coal samples. The model showed a high degree of fit, with the R2 for both BLT and YW coal sample above 0.94. Therefore, the relationship between the heat release rate and reaction rate constant for both BLT and YW coal sample can be effectively expressed by the model. The relationship between the heat release rate and the reaction rate constant is approximately as Eq. (7). This formula reflects the characteristic that the heat release rate varies exponentially with the reaction rate. In the follow-up study, when the reaction rate and the most probable mechanism function of coal are known, this formula can be used to carry out dynamic simulation of heat release during coal oxygen-lean combustion in the O2/CO2/N2 atmosphere.
where, y0 is the offset. A1 is the amplitude, t1 is the width.
In order to quantitatively analyze the relationship between y0, A1 and t1 and heating rate, Figs. 6 and 7 show the changes in y0, A1 and t1 with the heating rate, respectively. There was a linear relationship between y0, A1, t1 and heating rate for YW coal. For BLT coal, y0, A1 and t1 were basically linear with the heating rate in the O2/N2 atmosphere, whereas there was a non-linear relationship between y0, A1, t1 and heating rate in the O2/CO2/N2 atmosphere. Furthermore, y0−β, y0−A1 and y0−t1 curves were fitted respectively. The reaction rate constant was calculated using Eq. (1). On the whole, there was a following relationship between the heat release rate and reaction rate of coal, as follows
where, a1 and b1 are constants related to y0, a2 and b2 are constants related to A1, a3 and b3 are constants related to t1, as seen in Table 4.
Conclusions
In this work, simultaneous thermal analysis experiments for BLT coal (high-volatile bituminous coal) and YW coal (anthracite) in the 21%O2/79%N2 and 15%O2/5%CO2/80%N2 atmospheres were carried out. Based on the TG-DTG-DSC curves, the combustion characteristic parameters were discussed, the values of apparent activation energy were obtained using KAS method, and the relationship between the heat release rate and reaction rate constant was quantitatively analyzed. The following conclusions can be drawn:
-
1.
A delay of ignition and heat release existed during the coal oxygen-lean combustion in O2/CO2/N2. Decreasing O2 concentration caused a significant reduction of local reactivity and further the decreasing maximum heat release rate for low-rank coal, while increasing CO2 concentration caused a significant thermal lag effect and further the increasing maximum heat release rate for high-rank coal.
-
2.
During the combustion process, the values of apparent activation energy in the O2/CO2/N2 atmosphere were approximately 33–58% lower than that in the O2/N2 atmosphere for BLT coal, while the values of apparent activation energy in the two atmospheres for YW coal were close. For BLT coal, the values of correlation coefficients were less than 0.80 in the conversion rates ranges of 0.10–0.25 in O2/N2 and 0.15–0.45 O2/CO2/N2, respectively, which was because that the precipitation of the remaining volatiles was promoted by the heat release of separated volatiles combustion, and the precipitation and combustion of volatile was significantly deferred in the O2/CO2/N2 atmosphere compared with that in the O2/N2 atmosphere due to the slightly lower diffusivity of volatiles in CO2 than in N2 and the lower mass flux of oxygen to the volatiles flame.
-
3.
Regardless of the atmospheres, the conversion rates corresponding to maximum heat release rate of BLT and YW coal were about 0.80 and 0.50, respectively, indicating that the coal rank played a dominant role. At the increasing stage of the heat release rate, the heat release rate of the two coals increased conforming to ExpGro1 exponential model. At the decreasing stage of the heat release rate, the heat release rate of YW coal decreased exponentially with the reaction rate constant, while the heat release rate of BLT coal decreased linearly.
References
Zhu, J., Ouyang, Z. & Lu, Q. Numerical simulation on pulverized coal combustion and NOx emissions in high temperature air from circulating fluidized bed. J. Therm. Sci. 22, 261–268 (2013).
Pan, R. et al. Thermal evolution of the oxidation characteristics of pulverized coal with different particle sizes and heating rates. Thermochimica Acta. 685, 178516 (2020).
Edenhofer, O. King coal and the queen of subsidies. Science 349, 1286–1287 (2015).
Carras, J. N. & Young, B. C. Self-heating of coal and related materials: models, application and test methods. Prog Energy Combust. 20, 1–15 (1994).
Fierro V et al. Prevention of spontaneous combustion in coal stockpiles: experimental results in coal storage yard. Fuel Process Technol. 59, 23-34 (1999).
Green, U., Aizenshtat, Z., Metzger, L. & Cohen, H. Field and laboratory simulation study of hot spots in stockpiled bituminous coal. Energy Fuels. 26, 7230–7235 (2012).
He-tao, Su., Zhou, F.-B., Shi, B.-b, Qi, H.-N. & Deng, J.-C. Causes and detection of coalfield fires, control techniques, and heat energy recovery: a review. Int. J. Miner. Metall. Mater. 27, 275–291 (2020).
Stracher GB. Modern and ancient coal fires in the powder river basin, wyoming and montana. Coal Peat Fires: A Global Perspective. 71–89 (2019).
Zhang, Y., Wang, A. & Shu, P. Heat release characteristic of key functional groups during low-temperature oxidation of coal. J. Combust. Sci. Technol. 193, 2692–2703 (2020).
Ren, L.-F. et al. Low-temperature exothermic oxidation characteristics and spontaneous combustion risk of pulverised coal. Fuel 252, 238–245 (2019).
Deng, J. et al. Effect of oxygen concentration on low-temperature exothermic oxidation of pulverized coal. Thermochim. Acta 667, 102–110 (2018).
Ren, L.-F. et al. Inhibiting effect of CO2 on the oxidative combustion thermodynamics of coal. RSC Adv. 9, 41126–41134 (2019).
Deng, J. et al. Gases and thermal behavior during high-temperature oxidation of weathered coal. J. Therm. Anal. Calorim. 138, 1573–1582 (2019).
Su, H., Ji, H. & Chen, X. Model simplification of coal combustion kinetics: a case study of Weihuliang coal in Urumchi, China. Combust Theory Model. 23, 1071–1089 (2019).
Zhao, J. et al. Correlation analysis of the functional groups and exothermic characteristics of bituminous coal molecules during high-temperature oxidation. Energy 181, 136–147 (2019).
Wang, C.-P., Duan, X.-D., Xiao, Y., Li, Q.-W. & Deng, J. Thermokinetic characteristics of coal combustion under high temperatures and oxygen-limited atmospheres. Combust. Sci. Technol. 194, 1282–1300 (2020).
Hetao, S. et al. Simultaneous thermal analysis on the dynamical oxygen-lean combustion behaviors of coal in a O2/N2/CO2 atmosphere. J. Energy Inst. 96, 128–139 (2021).
Tolvanen, H. & Raiko, R. An experimental study and numerical modeling of combusting two coal chars in a drop-tube reactor: a comparison between N2/O2, CO2/O2 and N2/CO2/O2 atmospheres. Fuel 124, 190–201 (2014).
Bu, C. et al. The effect of H2O on the oxy-fuel combustion of a bituminous coal char particle in a fluidized bed: experiment and modeling. Combust. Flame 218, 42–56 (2020).
Lei, M., Zou, C., Xu, X. & Wang, C. Effect of CO2 and H2O on the combustion characteristics and ash formation of pulverized coal in oxy-fuel conditions. Appl. Therm. Eng. 133, 308–315 (2018).
China University of Mining and Technology Institute of Ventilation and Fire Protection, China Coal Pingshuo Coal Industry Co., Ltd. Key technologies and applications for detection and rapid control of underground coal fire development areas. Xuzhou: Institute of Ventilation and Fire Protection, China University of Mining and Technology (2010).
Keuleers, R. R., Janssens, J. F. & Desseyn, H. O. Comparison of some methods for apparent activation energy determination of thermal decomposition reactions by thermogravimetry. Thermochim. Acta 385, 127–142 (2002).
Jones, J. C., Chiz, P. S., Koh, R. & Matthew, J. Kinetic parameters of oxidation of bituminous coals from heat-release rate measurements. Fuel 75, 1755–1757 (1996).
Jones, J. C., Henderson, K. P., Littlefair, J. & Rennie, S. Kinetic parameters of oxidation of coals by heat-release measurement and their relevance to self-heating tests. Fuel 77, 19–22 (1998).
Barzegar, R., Yozgatligil, A. & Atimtay, A. T. Combustion characteristics of Turkish lignites at oxygen-enriched and oxy-fuel combustion conditions. J. Energy Inst. 92, 1440–1450 (2019).
Li, Q., Zhao, C., Chen, X., Wu, W. & Li, Y. Comparison of pulverized coal combustion in air and in O2/CO2 mixtures by thermo-gravimetric analysis. J. Anal. Appl. Pyrol. 85, 521–528 (2009).
Shaddix, C. R. & Molina, A. Particle imaging of ignition and devolatilization of pulverized coal during oxy-fuel combustion. Proc. Combust. Inst. 32, 2091–2098 (2009).
Huangfu, W.-H. et al. Effects of oxygen concentrations and heating rates on non-isothermal combustion properties of jet coal in East China. Procedia Engineering. 211, 262–270 (2018).
Deng, J. et al. Low-temperature oxidation and reactivity of coal in O2/N2 and O2/CO2 atmospheres, a case of carboniferous–permian coal in Shaanxi, China. Environ. Earth Sci. 78, 234 (2019).
Riaza, J. et al. Single particle ignition and combustion of anthracite, semi-anthracite and bituminous coals in air and simulated oxy-fuel conditions. Combust. Flame 161, 1096–1108 (2014).
Khatami, R. & Levendis, Y. A. An overview of coal rank influence on ignition and combustion phenomena at the particle level. Combust. Flame 164, 1–13 (2016).
Khatami, R., Stivers, C., Joshi, K., Levendis, Y. A. & Sarofim, A. F. Combustion behavior of single particles from three different coal ranks and from sugar cane bagasse in O2/N2 and O2/CO2 atmospheres. Combust. Flame 159, 1253–1271 (2011).
Qi, S., Wang, Z., Costa, M., He, Y. & Cen, K. Ignition and combustion of single pulverized biomass and coal particles in N2/O2 and CO2/O2 environments. Fuel. 283, 118956 (2021).
Yang, Xu., Li, S., Gao, Qi., Yao, Q. & Liu, J. Characterization on ignition and volatile combustion of dispersed coal particle streams: in-situ diagnostics and transient modelling. Energy Fuels. 32, 9850–9858 (2018).
Lü, H.-F. et al. Inhibiting effects of 1-butyl-3-methyl imidazole tetrafluoroborate on coal spontaneous combustion under different oxygen concentrations. Energy. 186, 115907 (2019).
Acknowledgements
This work is supported by the National Natural Science Foundation of China [52004257], the 111 Project [B17041], and the Fundamental Research Funds for the Central Universities [2652018098].
Author information
Authors and Affiliations
Contributions
J.S. Mainly responsible for doing experiments and writing articles; H.S. Mainly responsible for designing experimental schemes and guiding the completion of experiments; Y.L. Mainly responsible for assisting in designing experimental schemes; Z.H. Mainly responsible for assisting the completion of the experiment; Y.W. Mainly responsible for writing thesis; L.G. Mainly responsible for revising thesis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, J., Su, H., Li, Y. et al. Quantitative analysis of heat release during coal oxygen-lean combustion in a O2/CO2/N2 atmosphere by TG-DTG-DSC. Sci Rep 12, 6690 (2022). https://doi.org/10.1038/s41598-022-10752-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10752-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.