Abstract
The aim of the present systematic review and meta-analysis was to assess the effect of the different therapeutic options for repeated embryo implantation failure (RIF) on a subsequent IVF cycle outcome. Twenty-two RCTs and nineteen observational studies were included. Pooling of results showed a beneficial effect of intrauterine PBMC infusion on both CPR (RR 2.18; 95% CI 1.58–3.00; p < 0.00001; OR 2.03; 95% CI 1.22–3.36; p = 0.006) and LBR (RR 2.41; 95% CI 1.40–4.16; p = 0.002; OR 3.73; 95% CI 1.13–12.29; p = 0.03), of subcutaneous G-CSF administration on CPR (RR 2.29; 95% CI 1.58–3.31; p < 0.0001) and of intrauterine PRP infusion on CPR (RR 2.45; 95% CI 1.55–3.86; p = 0.0001). Observational studies also demonstrated a positive effect of IVIG and intrauterine hCG infusion on both CPR and LBR and of atosiban on CPR. Studies investigating intrauterine G-CSF infusion, LMWH, intravenous intralipid, hysteroscopy, blastocyst-stage ET, ZIFT, PGT-A and AH failed to observe an impact on IVF outcome. The quality of the evidence that emerged from RCTs focused on intrauterine PBMC infusion and subcutaneous G-CSF administration was moderate. For all other therapies/interventions it varied from low to very low. In conclusion, intrauterine PBMC infusion and subcutaneous G-CSF administration are the most promising therapeutic options for RIF. However, further well conducted RCTs are necessary before their introduction into clinical practice.
Similar content being viewed by others
Introduction
Repeated embryo implantation failure (RIF) is an extremely frustrating condition for both patients and clinicians and its treatment constitutes one of the most difficult challenges in the field of in vitro fertilization (IVF). Possible causes of RIF include wrong lifestyle habits (i.e. smoking and obesity), low quality of gametes [in particular in older women], thrombophilia, uterine factors (i.e. congenital uterine anomalies, endometrial polyps, submucosal fibroids, intrauterine adhesions) and adnexal pathologies (i.e. hydrosalpinx)1,2,3. However, in the great majority of cases, the etiology remains unknown.
Diagnosis
The definition of RIF is controversial. Several experts consider the number of previous IVF-embryo transfer (ET) failures as a diagnostic criterion. ‘Three previous IVF-ET failed attempts’ is the most commonly used threshold 4. However, a minority but not negligible proportion of authors prefer a broader definition and diagnoses RIF after only two previous IVF-ET failed attempts1. Another school of thought suggests that the focus should be also on the number and quality of transferred embryos. According to Simon and Laufer, RIF can be defined as the failure to obtain a clinical pregnancy after three consecutive IVF attempts, in which one to two embryos of high-grade quality are transferred in each cycle5. Coughlan et al. proposed more stringent diagnostic criteria and defined RIF as the failure after the transfer of at least four good-quality embryos within minimum three fresh or frozen cycles under 40 years of age6. However, the definition of good quality embryos is subjective and the authors often do not refer to shared classification criteria.
Most of the previous meta-analyzes aimed at determining the efficacy of single therapeutic intervention for RIF included patients with at least two previous failed ET attempts. However, by applying these criteria, the rate of false positive RIF diagnosis is estimated to be considerable [at least 46%]7 and, as a consequence, the studied population probably included a significant proportion of patients without a real obstacle to conception but who had not yet succeeded just because of statistical misfortune. Evidence about efficacy of therapeutic interventions deriving from meta-analyzes conducted with these assumptions cannot therefore be considered completely reliable.
In the present systematic review and meta-analysis, we defined RIF as the failure to obtain a clinical pregnancy after at least three ET attempts. By using this threshold, the risk of false positive diagnosis is significantly lower7. Importantly, these diagnostic criteria also exclude elements of subjectivity and are therefore easily replicable in any clinical setting.
Therapies and interventions
Proposed therapies and interventions for RIF can be grouped in four categories:
-
1.
Uterine interventions (e.g. intentional endometrial injury; hysteroscopy; endometrial sampling for histology and microbiological investigations and endometritis treatment; atosiban administration; copper intrauterine device placement)8,9,10,11,12;
-
2.
Laboratory and procedural technologies and interventions (i.e. sequential ET [i.e. sequential ET on day 2/3 and on day 5); ET medium enriched with hyaluronic acid; autologous embryo-cumulus cells co-culture; intracytoplasmic morphologically selected sperm injection (IMSI); blastocyst stage ET; zygote intrafallopian tube transfer (ZIFT); assisted hatching (AH); preimplantation genetic testing for aneuploidies (PGT-A))13,14,15,16,17,18,19,20;
-
3.
Immunomodulatory therapies (e.g. intravenous immunoglobulin (IVIG); intrauterine peripheral blood mononuclear cell (PBMC) infusion; tacrolimus; subcutaneous or intrauterine granulocyte colony stimulating factor (G-CSF) administration; intrauterine autologous platelet-rich plasma (PRP) infusion; intravenous intralipid; intrauterine human chorionic gonadotropin (hCG) injection; low-molecular-weight heparin (LMWH); aspirin; prednisolone)21,22,23,24,25,26,27,28;
-
4.
Treatments enhancing endometrial receptivity or technologies aimed at identifying the endometrial window of implantation (WOI) (e.g. intramuscular growth hormone (GH); vaginal sildenafil; endometrial receptivity array (ERA))29,30,31,32,33.
In most cases, the abovementioned therapeutic interventions are promising. However, clinicians can hardly orient themselves toward such a plethora of options with often unproven efficacy2.
Aim
Considering the methodological weaknesses of the previous contributions and the uncertainties about the preferred treatment strategies, we conducted the present systematic review and meta-analysis with the aim to assess the effect of the different therapies and interventions for RIF on the subsequent IVF cycle outcomes.
Materials and methods
This literature overview was reported according to the PRISMA guidelines for systematic reviews34,35 and the meta-analysis was conducted according to the MOOSE guidelines36. Since published de-identified data were used, this study was exempt from institutional review board approval.
Sources and study selection
The present systematic review and meta-analysis was restricted to published research articles that investigated the effect of all proposed therapies and interventions for RIF on the subsequent IVF cycle outcomes. Primary outcomes were Live Birth Rate (LBR) per patient and Clinical Pregnancy Rate (CPR) per patient. “Live birth” was defined as the delivery of one or more living infants. “Clinical pregnancy” was defined as the presence of one or more intrauterine gestational sacs on transvaginal ultrasound or other definitive clinical signs37. Secondary outcomes were implantation rate (IR) per embryo, multiple pregnancy rate (MPR) per patient and miscarriage rate (MR) per patient. “Implantation rate” was defined as the number of gestational sacs on transvaginal ultrasound divided by the number of embryos transferred. “Multiple pregnancy” was defined as the presence of two or more intrauterine embryos on transvaginal ultrasound. “Miscarriage” was defined as fetal loss before 20 weeks’ gestation37.
We systematically searched Pubmed, MEDLINE, Embase and Scopus, from database inception to May 13th, 2020. Searches were limited to studies in humans. A first search was conducted using the following terms: ‘therapy’ OR ‘intervention’ OR ‘treatment’ AND ‘implantation failure’ OR ‘repeated implantation failure’ OR ‘recurrent implantation failure’ OR ‘RIF’. A second search was carried out by combining each therapy or intervention emerged from the first search (i.e. endometrial injury; hysteroscopy; endometrial sampling for histology and microbiological investigations and endometritis treatment; atosiban; copper intrauterine device placement; sequential embryo transfer; embryo transfer medium enriched with hyaluronic acid; autologous embryo-cumulus cells co-culture; intracytoplasmic morphologically selected sperm injection; blastocyct stage embryo transfer; zygote intrafallopian tube transfer; assisted hatching; preimplantation genetic testing for aneuploidies; intravenous immunoglobulin; intrauterine administration of peripheral blood mononuclear cell; tacrolimus; subcutaneous administration of granulocyte colony stimulating factor; intrauterine infusion of autologous platelet-rich plasma; intravenous intralipid infusion; human chorionic gonadotropin; low-molecular-weight heparin; aspirin; growth hormone; corticosteroids; vaginal sildenafil; endometrial receptivity array) AND ‘implantation failure’ OR ‘repeated implantation failure’ OR ‘recurrent implantation failure’ OR ‘RIF’.
Studies could be included only if: (1) RIF was defined as the failure to obtain a clinical pregnancy after at least three ET attempts, (2) the included patients were investigated in order to exclude possible known causes of RIF, (3) they compared IVF outcomes between treated RIF patients and untreated RIF patients.
We considered eligible for inclusion published randomized controlled trials (RCTs), cohort and case control studies. Reference lists of all pertinent articles, systematic review and meta-analysis on the argument were systematically reviewed with the aim of identifying further studies that could be evaluated for inclusion. No attempt was made to identify unpublished studies.
Two authors (A.B. and P.E.L.S.) independently screened title and abstract of all articles to exclude studies deemed irrelevant. In case of opinion discrepancy, studies were discussed with two other investigators (F.C. and A.Ba.). Reports were classified according to the study design into RCTs, case–control studies, prospective and retrospective cohort studies.
Risk of bias and quality assessment
Two authors (A.B. and E.S.) independently assessed the included studies for risks of bias using the Cochrane 'Risk of bias' assessment tool38 for randomized clinical trials (RCTs) and the ROBINS-I tool39 for observational studies.
We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, indirectness, imprecision and publication bias40. Two review authors (P.E.L.S. and A.Ba.) working independently made judgements about evidence quality [high, moderate, low or very low], with disagreements resolved by discussion. We justified, documented, and incorporated our judgements into the reporting of results for each outcome.
Data extraction and analysis
Three authors (A.B., E.S. and F.C.) independently evaluated all articles and extrapolated the data on standardized forms. A final abstraction form was compiled from the three evaluation forms, after resolution of all the discrepancies among reviewers through a discussion with the two remaining authors.
The year of publication, location, study design, study period, criteria used to define RIF, investigations performed to exclude possible known causes of RIF, investigated therapy or intervention for RIF, primary and secondary outcomes were recorded.
Study outcomes were expressed using risk ratio (RR) with 95% confidence interval (95% CI) for RCTs and odds ratio (OR) with 95% CI for observational studies.
Risk estimates greater than 1 indicate an increased risk of the defined outcome; risk estimates less than 1 indicate a decreased risk of the defined outcome. We assessed statistical significance using 95%CI: if the 95%CI did not include the neutral value 1, we considered the risk statistically significant41,42. The inconsistency of the studies' results was measured using Cochrane Q and the I2 statistic38. Negative values of I2 are set equal to 0 so that I2 lies between 0 and 100%. According to the Cochrane Handbook for Systematic Reviews of Intervention, an I2 value of 0 indicates no observed heterogeneity, whereas I2 values from 30 to 60% may represent moderate heterogeneity, I2 values from 50 to 90% may represent substantial heterogeneity, and I2 values from 75 to 100% represent considerable heterogeneity38. If the I2 values indicated moderate, substantial, or considerable heterogeneity, we conducted sensitivity analyses to verify whether any one of the included studies unduly influenced the pooled effect size.
The risk estimates were combined in a meta-analysis using a fixed effects model when the heterogeneity found among the studies was absent to moderate (0% ≤ I2 < 30%). When heterogeneity was moderate, substantial, or considerable (I2 ≥ 30%), we used the DerSimonian and Laird method43,44 for a random-effects model45. Funnel plots, which graph RR/OR on a log scale (effect) against standard error of log-RR/OR (precision), were generated and visually inspected for asymmetry to determine whether the included studies were non representative of the body of possible studies on the subject (as could result from a small-study effect or other biases, such as publication and poor-quality bias). The approach by Egger et al. was used to test the significance of funnel plot asymmetry45. All analyses were performed using Review Manager version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration).
Results
Results of search and description of studies
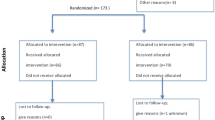
Figure 1 summarizes the process of literature identification and selection of studies. Our literature searches yielded 746 studies, from which 22 duplicates were removed. After a review of the titles and abstracts, 154 studies were identified as potentially eligible for inclusion. After a full review, we excluded 19 systematic reviews or meta-analysis2,5,22,23,37,46,47,48,49,50,51,52,53,54,55,56,57,58,59, 8 case reports60,61,62,63,64,65,66,67, 4 letters to the editor68,69,70,71 and 81 original studies [references and reasons for exclusion are reported in Table 1]. Data on the efficacy of therapies and interventions for RIF were extracted from the remaining 42 articles8,12,13,18,20,21,24,27,28,31,75,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170. Included studies investigated uterine interventions, laboratory and procedural technologies and interventions and immunomodulatory therapies. Details of the characteristics of the selected studies are shown in Table 2. Seven of the included studies were case–control studies, 12 were prospective cohort studies and 22 were RCTs. Therapies and interventions that could be pooled included subcutaneous or intrauterine G-CSF administration, sequential ET, intravenous intralipid infusion, endometrial injury, subcutaneous LMWH, hysteroscopy, PGT-A, atosiban, IVIG administration, intrauterine hCG injection, blastocyst stage ET, ZIFT, intrauterine PBMC infusion, AH and intrauterine PRP infusion.
Risk of bias and quality assessment results
Results obtained from the risk of bias assessment for RCTs and for observational studies are summarized in Fig. 2 and Table 3 respectively. The quality of the evidence for each single therapy/intervention is described in the ‘Synthesis of results’ section and summarized in Table 4.
Synthesis of results
Uterine interventions
Intentional Endometrial injury
Three RCTs8,146,164 and two observational studies152,159 evaluated the impact of an intentional injury to the endometrium during the spontaneous menstrual cycles before IVF on the outcomes of the IVF cycle.
Primary outcomes Meta-analysis of RCTs did not show significantly increased chances of pregnancy and live birth in women who underwent intentional endometrial injury (random effects model, RR 1.43; 95% CI 0.79–2.61; p = 0.24; I2 = 52% and random effects model, 1.55; 95% CI 0.81–2.94; p = 0.18; I2 = 46%, respectively)8,146,164 (Fig. 4). On the contrary, pooling of results from observational studies showed a beneficial effect of endometrial injury on pregnancy rate (fixed effects model, OR 3.03; 95% CI 1.48–6.18; p = 0.002; I2 = 0%)152,159 (Fig. 4).
Secondary outcomes Steengaard Olesen et al. observed a slight benefit of endometrial injury on implantation rate (RR 1.70; 95% CI 1.01–2.84; p = 0.04)164. Meta-analysis of RCTs did not show any impact on MR (fixed effects model, RR 1.39; 95% CI 0.55–3.53; p = 0.48; I2 = 0%)8,146,164.
Subgroup analysis Gurgan et al., performed endometrial injury on the 10th–12th day of the late follicular phase; Baum et al., on days 9–12 and 21–24 of the menstrual cycle and Steengaard Olesen et al. at menstrual cycle day 18–228,146,164.
Analyzing the results of the studies separately, no benefits were observed for the endometrial injury performed solely in the follicular phase (CPR, RR 1.65; 95% CI 0.98–2.77; p = 0.06 and LBR, RR 1.79; 95% CI 0.99–3.24; p = 0.05)146. Steengaard Olesen et al. observed an increased chance of clinical pregnancy (RR 1.72; 95% CI 1.05–2.83; p = 0.03) in treated subjects but failed to confirm this positive impact on LBR (RR 1.74; 95% CI 0.99–3.05; p = 0.05)164. Baum et al. did not observe a significant effect on both outcomes (CPR, RR 0.20; 95% CI 0.03–1.55; p = 0.12 and LBR, RR 0.11; 95% CI 0.01–1.92; p = 0.13)8. Gurgan et al. were also the only ones who performed the endometrial injury via hysteroscopy146.
Quality of the evidence We downgraded the quality of the evidence provided by RCTs by one level for risk of bias and, considering the low number of events, by one level for imprecision. The quality of the evidence provided by observational studies was downgraded by one level for risk of bias and, considering the wide confidence interval, by one level for imprecision and upgraded by one level for the large magnitude of the effect (Table 4).
Hysteroscopy
One RCT investigated whether outpatient hysteroscopy in the month before starting IVF treatment cycle could improve the outcome in women with RIF143.
Primary outcomes 144 failed to show an increase in live birth chances (RR 0.96; 95% CI 0.69–1.32; p = 0.79)143 (Fig. 4).
Quality of the evidence The data reported in the present meta-analysis were extrapolated from a sub-analysis carried out by El-Thouky et al.143. Furthermore, the number of events is low. Hence, we downgraded the quality of the evidence by one level for imprecision (Table 4).
Atosiban
One observational study12 examined the effect of atosiban administered before transfer of frozen-thawed embryo to women with RIF.
Primary outcomes Authors observed an increased CPR in treated women when compared to controls (OR 2.63; 95% CI 1.08–6.40; p = 0.03)12 (Fig. 4).
Secondary outcomes 148 showed an effect on chances of embryo implantation (OR 3.12; 95% CI 1.54–6.28; p = 0.002) and did not find any impact of miscarriage risk (OR 1.66; 95% CI 0.43–6.35; p = 0.46) of atosiban administration12.
Quality of the evidence The quality of the evidence provided by He et al. was downgraded by one level for risk of bias (Table 4).
Laboratory and procedural technologies and interventions
Sequential ET
One RCT13 and two observational studies75,144 compared sequential ET (cleavage stage ET followed by blastocyst ET) vs blastocyst stage ET in women with RIF.
Primary outcomes Meta-analysis of observational studies showed an increased chance of clinical pregnancy in women who underwent sequential ET (fixed effects model, OR 2.64; 95% CI 1.56–4.47; p = 0.0003; I2 = 0%)75,144 (Fig. 4). On the contrary, Shahrokh Tehraninejad et al. failed to show a beneficial effect (RR 1.04; 95% CI 0.67–1.63; p = 0.85)13 (Fig. 4).
Secondary outcomes Fang et al., observed a beneficial effect of sequential ET on implantation rate (OR 2.95; 95% CI 1.65–5.27; p = 0.0003) (Fang et al., 2013). Meta-analysis of observational studies75,144 and Shahrokh Tehraninejad et al. did not show an impact on MPR (fixed effects model, OR 2.38; 95% CI 0.87–6.47; p = 0.09; I2 = 36% and RR 1.13; 95% CI 0.47–2.72; p = 0.79, respectively).
Quality of the evidence We downgraded the quality of the evidence provided by Shahrokh Tehraninejad et al. by one level for risk of bias and, considering the low number of events, by one level for imprecision. The quality of the evidence provided by observational studies was downgraded by one level for risk of bias (Table 4).
PGT-A
Two RCTs20,141 and three observational studies145,160,167 investigated the potential role of PGT-A in improving IVF outcomes in women with RIF.
Primary outcomes Meta-analysis of RCTs failed to show an improvement in both clinical pregnancy and live birth chances (random effects model, RR 1.07; 95% CI 0.36–3.15; p = 0.90; I2 = 89% and RR 0.98; 95% CI 0.32–2.94; p = 0.97; I2 = 87%) in women who underwent PGT-A20,141 (Fig. 4).
Pooling of results of observational studies did not show a beneficial effect of PGT-A on both pregnancy (random effects model, OR 1.58; 95% CI 0.35–7.12; p = 0.55; I2 = 86%)145,160,167 and live birth chances (random effects model, OR 0.83; 95% CI 0.33–2.07; p = 0.69; I2 = 44%)160,167 (Fig. 4).
Secondary outcomes Rubio et al. did not observe an impact of PGT-A on chances of embryo implantation and miscarriage in women who underwent PGT-A (RR 1.71; 95% CI 0.99–2.94; p = 0.05 and RR 3.58; 95% CI 0.42–30.83; p = 0.25, respectively)20.
Quality of the evidence The evidence emerged from RCTs was downgraded by one level for risk of bias and, considering the low number of events, by one level for imprecision. For CPR, we downgraded the quality of the evidence provided by observational studies by one level for risk of bias. For LBR, we did not downgrade the quality of the evidence (Table 4).
Blastocyst-stage ET
One RCT compared blastocyst-stage ET outcomes with day 2–3 ET outcomes in women who failed to conceive after three or more day 2–3 IVF/ET cycles149.
Primary outcomes Levitas et al. failed to show a benefit of this strategy on both CPR (RR 1.68; 95% CI 0.51–5.59; p = 0.39) and LBR (RR 1.35; 95% CI 0.30–6.08; p = 0.70)149.
Secondary outcomes Authors observed a significantly increased chance of embryo implantation in treated women (RR 3.54; 95% CI 1.28–9.77; p = 0.01)149. MPR did not result significantly different between groups (RR 0.90; 95% CI 0.16–4.95; p = 0.90)149.
Quality of the evidence The quality of the evidence was downgraded by one level for risk of bias and, considering the low number of events, by one level for imprecision (Table 4).
ZIFT
Three observational studies investigated the possible beneficial effect of ZIFT in women with RIF18,150,163.
Primary outcomes Meta-analysis did not show increased chances of clinical pregnancy (random effects model, OR 2.40; 95% CI 0.52–11.05; p = 0.26; I2 = 87%)18,150,163 and live birth (random effects model, OR 3.43; 95% CI 0.03–43.80; p = 0.62; I2 = 91%) in women who underwent ZIFT (Fig. 4).
Secondary outcomes Pooling of results failed to show a benefit on embryo implantation chances (random effects model, OR 3.73; 95% CI 0.69–20.27; p = 0.13; I2 = 64%)18,150. MPR resulted significantly lower in women who underwent ZIFT (OR 0.26; 95% CI 0.07–0.91; p = 0.04)163. Shahrokh Tehraninejad et al. did not observe an impact on MR (OR 2.09; 95% CI 0.70–6.21; p = 0.19)163.
Quality of the evidence The quality of the evidence was downgraded by one level for risk of bias (Table 4).
AH
One RCT153 and one observational study158 investigated the effect of AH on IVF outcomes in women with RIF.
Primary outcomes 156 did not observe an increased chance of clinical pregnancy in women who underwent AH (RR 0.78; 95% CI 0.48–1.27; p = 0.31)153 (Fig. 4).
Primi et al., confirmed this finding (CPR, OR 1.42; 95% CI 0.45–4.48; p = 0.55) and failed to show a beneficial effect also on chances of live birth (OR 1.92; 95% CI 0.48–7.67; p = 0.36)158 (Fig. 4).
Secondary outcomes Primi et al. did not observed any difference in MPR between groups (OR, 1.49; 95% CI 0.09–24.44; p = 0.78)158.
Quality of the evidence The quality of the evidence provided by Rufas-Sapir et al. was downgraded by one level for risk of bias and, considering the low number of events, by one level for imprecision2. We downgraded the quality of the evidence emerged from the study conducted by Primi et al., by one level for risk of bias (Table 4).
Immunomodulatory therapies
G-CSF administration
Six RCTs evaluated the possible beneficial effect of the subcutaneous or intrauterine G-CSF administration140,142,147,148,161,162.
Primary outcomes Pooling of results from studies showed increased chances pregnancy in treated subjects (fixed effects model, 1.94; 95% CI 1.47–2.55; p < 0.00001; I2 = 0%)140,142,147,148,161,162. Only one study investigated the impact of intrauterine G-CSF infusion on the chances of live birth and failed to show a benefit (RR 0.84; 95% CI 0.41–1.73; p = 0.64)147.
Secondary outcomes Two trials reported implantation rate. Pooling of results showed a beneficial effect (fixed effects model, RR 2.41; 95% CI 1.38–4.22; p = 0.002; I2 = 0%)140,142. Kalem et al. did not observe any impact on MR (RR 3.20; 95% CI 0.69–14.93; p = 0.14)147.
Subgroup analysis Subcutaneous and intrauterine route of administration were analyzed separately (Fig. 3). Subcutaneous G-CSF administration resulted associated with an increased chance of clinical pregnancy (fixed effects model, RR 2.29; 95% CI 1.58–3.31; p < 0.0001; I2 = 0%) when compared with no treatment140,148,161,162 (Fig. 3). On the contrary, intrauterine administration had no impact on CPR (fixed effects model, RR 1.53; 95% CI 1.00–2.33; p = 0.05; I2 = 0%)142,147 (Fig. 3). Aleyasin et al. who investigated the subcutaneous route of administration observed a positive effect on embryo implantation chances (RR 2.94; 95% CI 1.24–5.01; p = 0.01)140. In contrast, Davari-tanha et al. who focused on intrauterine G-CSF injection did not observe any impact on IR (RR 2.28; 95% CI 0.90–5.74; p = 0.08)142.
(A) Effect of subcutaneous G-CSF administration on CPR in women with RIF (RCTs). (B) Effect of intrauterine G-CSF infusion on CPR in women with RIF (RCTs). (C) Effect of subcutaneous G-CSF administration on LBR in women with RIF (RCT). (D) Effect of intrauterine PBMC infusion on CPR in women with RIF (RCTs). (E) Effect of intrauterine PBMC infusion on LBR in women with RIF (RCT). (F) Effect of intrauterine PBMC infusion on CPR in women with RIF (observational studies). (G) Effect of intrauterine PBMC infusion on LBR in women with RIF (observational studies). RIF repeated implantation failure, G-CSF granulocyte-colony stimulating factor, PBMC peripheral blood mononuclear cells, RCT randomized clinical trial, CPR clinical pregnancy rate, LBR live birth rate.
Quality of the evidence In the majority of RCTs, the description of allocation concealment was unclear or the treatment providers were not blinded, hence we downgraded the quality of the evidence by one level for risk of bias for all outcomes. Considering the low total number of events, we also downgraded the quality of the evidence by one level for imprecision for all outcomes. For CPR evaluated in studies focused on subcutaneous G-CSF administration, we upgraded the quality of evidence by one level for the large magnitude of the effect (Table 4).
Intravenous intralipid infusion
One RCT investigated the effect of the intravenous infusion of intralipid27.
Primary outcomes Authors failed to show a benefit of the intravenous intralipid infusion on both the clinical pregnancy rate and the live birth rate (RR 1.30; 95% CI 0.80–2.10; p = 0.29 and 1.30; 95% CI 0.61–2.77, respectively) (Fig. 4).
(A) Effect of intentional endometrial injury on CPR in women with RIF (RCTs). (B) Effect of intentional endometrial injury on LBR in women with RIF (RCTs). (C) Effect of intentional endometrial injury on CPR in women with RIF (observational studies). (D) Effect of hysteroscopy on LBR in women with RIF (RCT). (E) Effect of atosiban on CPR in women with RIF (observational study). (F) Effect of sequential ET on CPR in women with RIF (RCT). (G) Effect of sequential ET on CPR in women with RIF (observational studies). (H) Effect of PGT-A on CPR in women with RIF (RCTs). (I) Effect of PGT-A on LBR in women with RIF (RCTs). (J) Effect of PGT-A on CPR in women with RIF (observational studies). (K) Effect of PGT-A on LBR in women with RIF (observational studies). (L) Effect of ZIFT on CPR in women with RIF (observational studies). (M) Effect of ZIFT on LBR in women with RIF (observational studies). (N) Effect of AH on CPR in women with RIF (RCT). (O) Effect of AH on LBR in women with RIF (observational study). (P) Effect of intravenous intralipid on CPR (RCT). (Q) Effect of intravenous intralipid on LBR in women with RIF (RCT). (R) Effect of LMWH on CPR in women with RIF (RCTs). (S) Effect of LMWH on LBR in women with RIF (RCT). (T) Effect of LMWH on CPR in women with RIF (observational study). (U) Effect of LMWH on LBR in women with RIF (observational study). (V) Effect of IVIG on CPR in women with RIF (observational study). (W) Effect of IVIG on LBR in women with RIF (observational study). (X) Effect of intrauterine hCG infusion on CPR in women with RIF (observational studies). (Y) Effect of intrauterine hCG infusion on LBR in women with RIF (observational study). (Z) Effect of intrauterine PRP infusion on CPR in women with RIF (RCT). ET embryo transfer, RIF repeated implantation failure, RCT randomized clinical trial, CPR clinical pregnancy rate, LBR live birth rate, LMWH low molecular weight heparin, PGT-A preimplantation genetic testing for aneuploidy, IVIG intravenous immunoglobulin, hCG human chorionic gonadotropin, ZIFT zygote intrafallopian transfer, AH assisted hatching, PRP platelet rich plasma.
Quality of the evidence Quality of the evidence was downgraded by one level for risk of bias and by one level for imprecision (Table 4).
LMWH
Two RCTs165,166 and one observational study31 investigated the effect of subcutaneous LMWH administration.
Primary outcomes Meta-analysis of RCTs failed to show a beneficial effect on both CPR (RR 1.39; 95% CI 0.87–2.23; p = 0.17; I2 = 4%)165,166 and LBR (RR 1.38; 95% CI 0.64–2.96; p = 0.41)165. Berker et al. also did not observe a significant increase of pregnancy and live birth chances (OR 1.42, 95% CI 0.58–3.45; p = 0.44 and OR 1.50; 95% CI 0.59–3.82; p = 0.40, respectively) (Fig. 4).
Quality of the evidence The quality of the evidence provided by RCTs was downgraded by two levels for risk of bias and by one level for imprecision. We also downgraded the level of the evidence provided by Berker et al. by one level for risk of bias (Table 4).
IVIG
One observational study21 evaluated the efficacy of IVIG in women with RIF.
Primary outcomes Chances of clinical pregnancy and live birth resulted significantly increased in treated women (OR 2.08; 95% CI 1.28–3.36; p = 0.003 and OR 1.76; 95% CI 1.08–2.89; p = 0.02, respectively)21 (Fig. 4).
Secondary outcomes Ho et al., observed an increased chance of embryo implantation (OR 1.43; 95% CI 1.06–1.94; p = 0.02) in treated subjects21.
Quality of the evidence The quality of the evidence was downgraded by one level for risk of bias (Table 4).
Intrauterine hCG injection
Two observational studies investigated the effect of intrauterine hCG injection in women with RIF28,151.
Primary outcomes Chances of clinical pregnancy (fixed effects model, OR 1.81; 95% CI 1.23–2.65; p = 0.002; I2 = 0%)28,151 and live birth (OR 1.78; 95% CI 1.02–3.09; p = 0.04)151 resulted significantly increased in treated women (Fig. 4).
Secondary ooutcomes Liu et al. showed a beneficial effect of intrauterine hCG injection on implantation rate (OR 1.71; 95% CI 1.08–2.71; p = 0.02)151.
Quality of the evidence The quality of the evidence was downgraded by one level for risk of bias (Table 4).
Intrauterine PBMC infusion
Three RCTs154,156,169 and three observational studies24,157,168 investigated the effect of intrauterine administration of autologous PBMC on IVF outcomes in women with RIF.
Primary outcomes Meta-analysis of RCTs showed a significant increase in chances of clinical pregnancy (fixed effects model, RR 2.18; 95% CI 1.58–3.00; p < 0.00001; I2 = 0%)154,156,169 and live birth (RR 2.41; 95% CI 1.40–4.16; p = 0.002)169 in treated women (Fig. 3). Pooling of results of observational studies confirmed the positive effect on both CPR (fixed effects model, OR 2.03; 95% CI 1.22–3.36; p = 0.006; I2 = 28%)24,157,168 and LBR (fixed effects model, OR 3.73; 95% CI 1.13–12.29; p = 0.03; I2 = 13%)157,168 (Fig. 3).
Secondary outcomes Meta-analysis of observational studies showed an increased chance of embryo implantation in treated women (fixed effects model, OR 4.54; 95% CI 1.82–11.35; p = 0.001; I2 = 0%)157,168.
Quality of the evidence The quality of the evidence provided by RCTs was downgraded by one level for risk of bias, by one level for imprecision and upgraded by one level for the large magnitude of the effect (Table 4). The quality of the evidence provided by observational studies was downgraded by one level for risk of bias and by one level for imprecision and upgraded by one level for the large magnitude of the effect (Table 4).
Intrauterine PRP infusion
Two RCTs155,170 investigated whether administration of intrauterine PRP could improve IVF outcomes in women with RIF.
Primary outcomes Pooling of results showed a significantly increased chance of clinical pregnancy in treated women (fixed effects model, RR 2.45; 95% CI 1.55–3.86; p = 0.0001; I2 = 0%)155,170 (Fig. 4).
Quality of the evidence The quality of the evidence was downgraded by one level for risk of bias and, considering the low number of events, by one level for imprecision (Table 4).
Discussion
In the present study, meta-analysis of RCTs showed a beneficial effect of PBMC intrauterine infusion on both LBR and CPR and of subcutaneous G-CSF administration and intrauterine PRP infusion on CPR in women with RIF. Pooling of results of observational studies also demonstrated a positive effect of IVIG and hCG intrauterine infusion on both CPR and LBR and of atosiban administration on CPR. Meta-analysis of studies investigating the possible impact of intrauterine G-CSF infusion, LMWH, hysteroscopy, blastocyst-stage ET, ZIFT, PGT-A and AH failed to observe an impact on IVF outcome. Results about the effects of sequential ET and intentional endometrial injury are conflicting. The quality of the evidence that emerged from RCTs investigating the effect of intrauterine PBMC infusion and subcutaneous G-CSF administration was moderate. For all other therapies/interventions it varied from low to very low.
Among the therapies that have been proven to be potentially effective, the intrauterine infusion of PBMC is supported by the most convincing evidence. In fact, meta-analyses of RCTs and of observational studies agree in demonstrating the positive effect on both primary outcomes and the magnitude of calculated effect estimates is considerable. Pourmoghadam et al. in an interesting meta-analysis had already shown a beneficial effect in women with at least three IVF failures171. The subsequent publication of the study conducted by Nobijari et al.156, which was the first RCT to report the chances of live birth, further strengthened the evidence. Nevertheless, data on the impact on the LBR as well as on the safety profile of this therapy should still be considered scanty.
The administration of G-CSF also emerged as a promising treatment option in women with RIF. Our findings confirmed those recently published by Kamath et al. who showed that in women with two or more IVF failures, G-CSF administration may improve CPR versus placebo47. Interestingly, we observed that of the two possible routes of administration, the only potentially effective seems to be the systemic one. Importantly, the magnitude of the effect was considerable and, as a consequence, we upgraded the quality of the evidence to moderate. Unfortunately, no data about the rate of live birth can be extracted from included studies that investigated this route of administration, which may impair the convincingness of the analysis. Reasons for discrepancies between the effects of systemic and intrauterine administration have yet to be fully elucidated. One could speculate that when administered systemically, G-CSF has a positive effect on oocyte maturation and embryonic development, while in locally endometrial cavity applications oocytes and embryos are deprived of this positive support147.
Intrauterine hCG infusion constitutes an excellent candidate to be tested in women with RIF. In fact, by acting as the homologous isomer of LH, hCG shares a common receptor with LH, namely, LHCGR, and their combination can regulate both endometrium receptivity and embryo implantation172. Importantly, in a recent meta-analysis, Gao et al., showed that infertile women who received intrauterine hCG injection before ET exhibited significantly higher rates of implantation, ongoing pregnancy and live birth and a lower rate of miscarriage172. In the present meta-analysis, pooling of results of observational studies focusing on patients with RIF showed a beneficial effect on both CPR and LBR. Unfortunately, the quality of the evidence was very low. In particular, the different volumes of culture medium (1 ml and 0.2 ml) and doses of hCG (1000 UI and 500 UI) impair the clinical homogeneity between studies and significantly limit the reliability of our results28,151.
Hypothesizing a key role of the immune response in the pathogenesis of RIF, IVIG, intravenous intralipid injection and PRP intrauterine infusion have also been proposed as possible treatments. Initial results regarding the efficacy of IVIG and PRP intrauterine injection are encouraging. However, even in these cases, the very low quality of the evidence does not allow reliable conclusions.
The decrease of the frequency and amplitude of uterine contractions obtained through the administration of atosiban, has also been theorized as a method to enhance the probability of embryo implantation and pregnancy in women with RIF. Our results were obtained from the data extrapolated from a single observational study and are in line with those of a recent meta-analysis conducted by Huang et al., who, using less stringent inclusion criteria [i.e. two or more consecutive failed IVF-ET attempts in which at least 1 ± 2 high quality embryos were transferred in each cycle], demonstrated increased chances of implantation, clinical pregnancy and live birth in women with RIF treated with atosiban28. Well conducted RCTs focusing on women with RIF diagnosed according to the criteria proposed in the present study are warranted.
Inconclusive results and demonstrations of inefficacy that emerged from the present meta-analysis are of particular importance. Over the years, we witnessed the emergence of a number of RIF treatment options of simple execution but characterized by weak rational bases. Nonetheless, their introduction into current clinical practice occurred rapidly without waiting for adequate evidence of efficacy and safety. Such conduct evidently conflicts with the principle of the traditional medical ethics summarized in the injunction “primum non nocere” and with the duty to protect patients, already psychologically frustrated, from false hopes and to avoid waste of resources.
In this perspective, the results about the effect of intentional endometrial injury deserve to be commented. The biological plausibility and relative ease of execution of this intervention attracted the attention of many clinicians around the world. Endometrial scratching is a safe procedure. However, it is somewhat painful. When performed in the luteal phase, patients reported pain scores between 3 and 7 of 10, and the procedure was discontinued due to pain in a number of cases173. Its efficacy in women with RIF is debated. Nonetheless, an online survey distributed to 189 fertility clinics across Australia, New Zealand and the UK found that 92% of clinicians recommend endometrial scratching to women with RIF173. In our study, meta-analysis of RCTs demonstrated the inefficacy of this intervention in increasing CPR and LBR. On the contrary, pooling of results of observational studies suggested a beneficial effect on CPR. These discrepancies combined with the relatively small sample size of the included studies and the statistical moderate/substantial heterogeneity do not allow conclusive interpretations.
A recent RCT showed a potentially harmful effect of the endometrial biopsy performed in the follicular phase. In fact, authors reported a higher incidence of clinical miscarriages in the context of in-cycle scratching, which led to the study premature halt174. This considered, we conducted a sub-analysis on the basis of endometrial injury timing without however observing the superiority of one strategy over the others. Importantly, a recent retrospective study questioned the existence of RIF due to endometrial effect. In a cohort of 4229 women whose endometrium was sonographically normal and who underwent up to three frozen euploid single embryo-transfers, authors found a cumulative sustained implantation rate of 95.2%. As a result, RIF incidence was estimated < 5%175.
At present, there is no evidence to support the routine use of hysteroscopy as a screening and treatment tool in the population of women with RIF and a normal uterine cavity on ultrasound or hysterosalpingogram to improve the reproductive success rate. However, available data are scanty. Notably, there is compelling rationale that hysteroscopy might be effective in women with RIF. In fact, intrauterine pathology has been reported in as many as 50% of women with RIF leading to suggest that the correction of such pathology could improve IVF outcome143. Benefit could also be due to the negotiation of the cervical canal, thus, facilitating the subsequent embryo transfer176. Hysteroscopy has also the considerable advantage of allowing targeted endometrial biopsies. In this regard, a recent interesting meta-analysis showed that chronic endometritis therapy might be beneficial in patients suffering from RIF even if, according to the authors, the body of evidence on this topic is still insufficient to recommend routine chronic endometritis screening as intervention in such patients37. Future RCTs are thus welcomed in order to test such multiple hypothetical beneficial function of hysteroscopy in women with RIF.
Notably, we also failed to show a significant impact of LMWH administration on both CPR and LBR in non-thrombophilic women with RIF. However, the reliability of the results is limited by the very low quality of the evidence. Furthermore, the absence of data regarding the undesirable effects of LMWH administration [e.g. risk of bleeding] does not allow to grasp the whole picture.
Pooling of results of studies investigating the possible role of PGT-A did not show a positive effect on both clinical pregnancy and live birth chances per patient. Future research efforts should probably test this intervention on a population of older women in whom one may suspect with higher confidence that aneuploidy constitutes the cause of RIF. In this regard, it has however to be highlighted that PGT-A cannot be expected to increase the chance of live birth per patient177. It can at most only alleviate the burden of treatment to patients by reducing the number of transfers.
Finally, as for the sequential ET, the evidence is conflicting: pooling of results of observational studies showed a significantly increased CPR while the results of the only included RCT demonstrated no benefit. Safety of this intervention is questionable. The transfer of two embryos at a distance and the transfer of the second one at the blastocyst stage may increase the risk of dizygotic and monozygotic twinning respectively41. Published data about these possible complications are reassuring but still insufficient. The potential serious obstetric and neonatal consequences and the unconvincing results on the efficacy discourage the conduct of further studies. Moreover, data demonstrating no differences in CPR for the first 6 IVF cycles deserve careful study on the role of chance and even of different multiple factors influencing CPR and LBR178.
Other treatment hypotheses might be valid and some RCTs are ongoing in order to test them. In this context, of particular relevance is the study protocol published by Lu et al.179. Authors aim to determine if prednisone can enhance live birth in women with RIF undergoing IVF. Interestingly, studies have shown that prednisone could not only suppress the inflammatory response in pre-implantation endometrium, but also stimulate the secretion of hCG and promote proliferation and invasion of trophoblast179. The efficacy of ad hoc treatments in women with known diseases and RIF also deserves to be clarified. In this context, the benefits and risks of aspirin and/or heparin in women with persistent antiphospholipid antibodies and RIF have been rather neglected until now.
Strengths and limitations
To the best of our knowledge the present meta-analysis is the first to give a comprehensive view of the efficacy of all therapies or interventions proposed in order to improve IVF outcome in women with RIF. The population was selected according to strict inclusion criteria in order to reduce as much as possible the risk of misleading conclusions due to the high incidence of false positive diagnosis and, consequently, of inappropriate treatment. Moreover, being aware in advance of the limited available evidence, we decided to include also observational studies rather than limiting our analyses to RCTs. This choice allowed us also to also report on options that could become of interest in the future, i.e. once properly tested with RCTs.
Several limitations need to be considered in the interpretation of our results. First, many of the included studies suffered from serious risk of bias. Additionally, in the majority of cases, they recruited too few women to have enough statistical power to detect clinically relevant effect sizes, as is common in our field. Second, some studies included only frozen-thawed embryo replacement cycles while others only fresh IVF cycles. Furthermore, the protocols for ovarian stimulation, endometrial preparation, luteal phase support and the proposed interventions themselves also present marked variations between studies. In most cases, a proper investigation of this clinical heterogeneity was not feasible due to the limited number of studies. Third, in the present meta-analysis we focused on patients who had been investigated as much as possible to rule out possible known causes of RIF. However, it cannot be sustained with certainty that the selected population is affected by unexplained RIF. In fact, some contributions also included women of advanced age. In this context, it is pretty impossible to exclude the embryonic cause of RIF, without the use of PGT-A. Finally, there are few data addressing the safety profile of these treatments and their effect on the development and health of conceived children. Future studies focusing on treatment-related side effects and long-term follow-up data among the offspring are needed before introducing such interventions into daily clinical practice.
Conclusion
In women with RIF, moderate quality evidence suggests that intrauterine PBMC infusion improves chances of clinical pregnancy and live birth and that subcutaneous G-CSF administration has a beneficial effect on CPR. These treatment options are the most promising among those investigated. However, prior to their introduction into routine clinical practice, high quality RCTs are needed. Trials design should include an identical placebo in the control arm to reduce performance bias and report ongoing pregnancy or live birth rate as primary outcome. The major and minor adverse effects of their administration should also be captured in any future studies.
Notably, our results should limit the use of many adjunct or add-on interventions in women with RIF whose prescription is currently extremely popular in IVF clinics around the world. In this regard, the administration of LMWH is not supported by evidence either regarding its efficacy or its safety profile. We also strongly discourage intentional endometrial injury with the aim of improving IVF outcome outside of registered experimental protocols.
RIF of unknown cause significantly hampers IVF success. An effective treatment strategy would constitute a revolution in the field. In this context, future research should focus on confirming therapeutic approaches for which robust efficacy data are already available [i.e. intrauterine PBMC infusion and subcutaneous G-CSF administration] before investigating new interventions or therapies or retest those supported by preliminary flabby evidence. Finally, regardless of the option to be tested, we plea for collaborative efforts that could allow to run large and robust RCTs. In recent years, RIF has become extremely popular with entire meetings exclusively dedicated to the argument. The time has now come for facts rather than speculations.
References
Somigliana, E. et al. Repeated implantation failure at the crossroad between statistics, clinics and over-diagnosis. Reprod. Biomed. Online. 36, 32–38 (2018).
Cakiroglu, Y. & Tiras, B. Determining diagnostic criteria and cause of recurrent implantation failure. Curr. Opin. Obstet. Gynecol. 32, 198–204 (2020).
Sheikhansari, G., Pourmoghadam, Z., Danaii, S., Mehdizadeh, A. & Yousefi, M. Etiology and management of recurrent implantation failure: A focus on intra-uterine PBMC-therapy for RIF. J. Reprod. Immunol. 139, 103121 (2020).
Polanski, L. T. et al. What exactly do we mean by “recurrent implantation failure”? A systematic review and opinion. Reprod. Biomed. Online. 28, 409–423 (2014).
Simon, A. & Laufer, N. Repeated implantation failure: Clinical approach. Fertil. Steril. 97, 1039–1043 (2012).
Coughlan, C. et al. Recurrent implantation failure: Definition and management. Reprod. Biomed. Online. 28, 14–38 (2014).
Busnelli, A. et al. How common is real repeated implantation failure? An indirect estimate of the prevalence. Reprod. Biomed. Online. 40, 91–97 (2020).
Baum, M. et al. Does local injury to the endometrium before IVF cycle really affect treatment outcome? Results of a randomized placebo controlled trial. Gynecol. Endocrinol. 28, 933–936 (2012).
Cicinelli, E. et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum. Reprod. 30, 323–330 (2015).
Gao, M., Sun, Y., Xie, H., Fang, S. & Zhao, X. Hysteroscopy prior to repeat embryo transfer may improve pregnancy outcomes for asymptomatic women with repeated implantation failure. J. Obstet. Gynaecol. Res. 41, 1569–1576 (2015).
Mao, X., Zhang, J., Chen, Q., Kuang, Y. & Zhang, S. Short-term copper intrauterine device placement improves the implantation and pregnancy rates in women with repeated implantation failure. Fertil. Steril. 108, 55–61 (2017).
He, Y. et al. Application of atosiban in frozen-thawed cycle patients with different times of embryo transfers. Gynecol. Endocrinol. 32, 811–815 (2016).
Tehraninejad, E. S. et al. The sequential embryo transfer compared to blastocyst embryo transfer in in vitro fertilization (IVF) cycle in patients with the three repeated consecutive IVF: A randomized controlled trial. Gynecol. Endocrinol. 35, 955–959 (2019).
Fu, W., Yu, M. & Zhang, X. J. Effect of hyaluronic acid-enriched transfer medium on frozen-thawed embryo transfer outcomes. J. Obstet. Gynaecol. Res. 44, 747–755 (2018).
Benkhalifa, M. et al. Autologous embryo-cumulus cells co-culture and blastocyst transfer in repeated implantation failures: A collaborative prospective randomized study. Zygote. 20, 173–180 (2012).
Delaroche, L. et al. Intracytoplasmic morphologically selected sperm injection (IMSI) after repeated IVF or ICSI failures: A prospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 167, 76–80 (2013).
Zhang, X. et al. Frozen blastocyst embryo transfer vs. frozen cleavage-stage embryo transfer in couples with recurrent implantation failure: a cohort study. Hum. Fertil. (Camb). 5, 1–6 (2019).
Levran, D., Mashiach, S., Dor, J., Levron, J. & Farhi, J. Zygote intrafallopian transfer may improve pregnancy rate in patients with repeated failure of implantation. Fertil. Steril. 69, 26–30 (1998).
Edirisinghe, W. R. et al. A study failing to determine significant benefits from assisted hatching: patients selected for advanced age, zonal thickness of embryos, and previous failed attempts. J. Assist. Reprod. Genet. 16, 294–301 (1999).
Rubio, C. et al. Preimplantation genetic screening using fluorescence in situ hybridization in patients with repetitive implantation failure and advanced maternal age: Two randomized trials. Fertil. Steril. 99, 1400–1407 (2013).
Ho, Y. K. et al. Peripheral CD56(+)CD16(+) NK cell populations in the early follicular phase are associated with successful clinical outcomes of intravenous immunoglobulin treatment in women with repeated implantation failure. Front. Endocrinol. 21, 937 (2020).
Li, J., Chen, Y., Liu, C., Hu, Y. & Li, L. Intravenous immunoglobulin treatment for repeated IVF/ICSI failure and unexplained infertility: A systematic review and a meta-analysis. Am. J. Reprod. Immunol. 70, 434–447 (2013).
Li, J., Mo, S. & Chen, Y. The effect of G-CSF on infertile women undergoing IVF treatment: A meta-analysis. Syst. Biol. Reprod. Med. 63, 239–247 (2017).
Li, S. et al. Intrauterine administration of hCG-activated autologous human peripheral blood mononuclear cells (PBMC) promotes live birth rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J. Reprod. Immunol. 119, 15–22 (2017).
Nakagawa, K. et al. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood TH1/TH2 cell ratios. Am. J. Reprod. Immunol. 73, 353–361 (2015).
Aghajanzadeh, F. et al. Using autologous intrauterine platelet-rich plasma to improve the reproductive outcomes of women with recurrent implantation failure. JBRA. Assist. Reprod. 24, 30–33 (2020).
Al-Zebeidi, J. et al. Effect of empiric intravenous intralipid therapy on pregnancy outcome in women with unexplained recurrent implantation failure undergoing intracytoplasmic sperm injection-embryo transfer cycle: A randomized controlled trial. Gynecol. Endocrinol. 36, 131–134 (2020).
Huang, P., Wei, L., Li, X. & Qin, A. Effects of intrauterine perfusion of human chorionic gonadotropin in women with different implantation failure numbers. Am. J. Reprod. Immunol. 79, 112–115 (2018).
Akhtar, M. A. et al. Aspirin and heparin as adjuvants during IVF do not improve live birth rates in unexplained implantation failure. Reprod. Biomed. Online. 26, 586–594 (2013).
Altmäe, S. et al. Effect of growth hormone on uterine receptivity in women with repeated implantation failure in an oocyte donation program: A randomized controlled trial. J. Endocr. Soc. 2, 96–105 (2017).
Berker, B. et al. The role of low-molecular-weight heparin in recurrent implantation failure: A prospective, quasi-randomized, controlled study. Fertil. Steril. 95, 2499–2502 (2011).
Ruiz-Alonso, M. et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil. Steril. 100, 818–824 (2013).
Siristatidis, C. et al. Administration of prednisolone and low molecular weight heparin in patients with repeated implantation failures: A cohort study. Gynecol. Endocrinol. 34, 136–139 (2018).
Deeks, J.J. et al. Cochrane handbook for systematic reviews of interventions, version 6.0. Cochrane Collaboration; Available at; www.training.cochrane.org/handbook.
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS. Med. 6, e1000097 (2009).
Stroup, D.F. et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 283, 2008–2012 (2000).
Vitagliano, A. et al. Endometrial scratch injury for women with one or more previous failed embryo transfers: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 110, 687–702 (2018).
Higgins, J.P.T. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sterne, J. A. et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919 (2016).
Schünemann, H.J. et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Busnelli, A. et al. Risk factors for monozygotic twinning after in vitro fertilization: A systematic review and meta-analysis. Fertil. Steril. 111, 302–317 (2019).
Busnelli, A. et al. Fertility in female cancer survivors: A systematic review and meta-analysis. Reprod. Biomed. Online. 41, 96–112 (2020).
DerSimonian, R. & Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials. 28, 105–114 (2007).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials. 7, 177–188 (1986).
Egger, M., Smith, G. D. & Phillips, A. N. Meta-analysis: Principles and procedures. BMJ 315, 1533–1537 (1997).
Kimura, F. et al. Review: Chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 45, 951–960 (2019).
Kamath, M.S., Kirubakaran, R. & Sunkara, S.K. Granulocyte-colony stimulating factor administration for subfertile women undergoing assisted reproduction. Cochrane Database Syst. Rev. 1, CD013226 (2020).
Huang, Q. Y. et al. The impact of atosiban on pregnancy outcomes in women undergoing in vitro fertilization- embryo transfer: A meta-analysis. PLoS ONE 12, e0175501 (2017).
Zhang, L. et al. Therapeutic role of granulocyte colony-stimulating factor (G-CSF) for infertile women under in vitro fertilization and embryo transfer (IVF-ET) treatment: A meta-analysis. Arch. Gynecol. Obstet. 298, 861–871 (2018).
Cao, H., You, D., Yuan, M. & Xi, M. Hysteroscopy after repeated implantation failure of assisted reproductive technology: A meta-analysis. J. Obstet. Gynaecol. Res. 44, 365–373 (2018).
Eftekhar, M., Naghshineh, E. & Khani, P. Role of granulocyte colony-stimulating factor in human reproduction. J. Res. Med. Sci. 23, 7 (2018).
Valdes, C. T., Schutt, A. & Simon, C. Implantation failure of endometrial origin: It is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil. Steril. 108, 15–18 (2017).
Zohni, K. M., Gat, I. & Librach, C. Recurrent implantation failure: A comprehensive review. Minerva Ginecol. 68, 653–667 (2016).
Moustafa, S. & Young, S.L. Diagnostic and therapeutic options in recurrent implantation failure. F1000Res 2020:9.
Vitagliano, A. et al. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: A systematic review and meta-analysis. Fertil. Steril. 110, 103-112.e1 (2018).
Zeyneloglu, H. B. & Onalan, G. Remedies for recurrent implantation failure. Semin. Reprod. Med. 32, 297–305 (2014).
Margalioth, E. J., Ben-Chetrit, A., Gal, M. & Eldar-Geva, T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum. Reprod. 21, 3036–3043 (2006).
Urman, B., Yakin, K. & Balaban, B. Recurrent implantation failure in assisted reproduction: how to counsel and manage. B. Treatment options that have not been proven to benefit the couple. Reprod. Biomed. Online. 11, 382–391 (2005).
van Hoogenhuijze, N.E., Kasius, J.C., Broekmans, F.J.M, Bosteels, J. & Torrance, H.L. Endometrial scratching prior to IVF; does it help and for whom? A systematic review and meta-analysis. Hum. Reprod. Open. 2019, hoy025 (2019).
Garcia-Grau, I. et al. Taxonomical and functional assessment of the endometrial microbiota in a context of recurrent reproductive failure: A case report. Pathogens. 8, 205 (2019).
Sfakianoudis, K. et al. Successful implantation and live birth following autologous platelet-rich plasma treatment for a patient with recurrent implantation failure and chronic endometritis. Vivo. 33, 515–521 (2019).
Orvieto, R., Brengauz, M. & Feldman, B. A novel approach to normal responder patient with repeated implantation failures: A case report. Gynecol. Endocrinol. 31, 435–437 (2015).
Aflatoonian, N., Eftekhar, M., Aflatoonian, B., Rahmani, E. & Aflatoonian, A. Surrogacy as a good option for treatment of repeated implantation failure: A case series. Iran. J. Reprod. Med. 11, 77–80 (2013).
Shen, M. S., Wang, C. W., Chen, C. H. & Tzeng, C. R. New horizon on successful management for a woman with repeated implantation failure due to unresponsive thin endometrium: use of extended estrogen supplementation. J. Obstet. Gynaecol. Res. 39, 1092–1094 (2013).
Chen, L. N. et al. Frozen embryo transfer combined with intrauterine administration of autologous peripheral blood mononuclear cells for repeated implantation failure: Report of 3 cases. Nan Fang Yi Ke Da Xue Xue Bao. 31, 724–726 (2018).
Esfandiari, N., Coogan-Prewer, J., Gotlieb, L., Claessens, E. A. & Casper, R. F. Successful pregnancy following double-frozen embryo transfer in a patient with repeated implantation failure. Fertil. Steril. 90(1199), e13–e15 (2008).
Asaad, M. & Carver-Ward, J. A. Twin pregnancy following transmyometrial-subendometrial embryo transfer for repeated implantation failure. Hum. Reprod. 12, 2824–2825 (1997).
Simón, C., Bosch, E. & Bellver, J. Reply: Endometrial scratching for women with repeated implantation failure. Hum. Reprod. 29, 2856–2857 (2014).
Nastri, C. O., Polanski, L. T., Raine-Fenning, N. & Martins, W. P. Endometrial scratching for women with repeated implantation failure. Hum. Reprod. 29, 2855–2856 (2014).
Liang, Y.L., Kuo, T.C., Hung, K.H., Chen, T.H. & Wu MH. Oxytocin antagonist for repeated implantation failure and delay of delivery. Taiwan J. Obstet. Gynecol. 48, 314–316 (2009).
Lédée-Bataille, N. et al. Controlled natural in vitro fertilization may be an alternative for patients with repeated unexplained implantation failure and a high uterine natural killer cell count. Fertil. Steril. 82, 234–236 (2004).
Ahmadi, M. et al. Sirolimus as a new drug to treat RIF patients with elevated Th17/Treg ratio: A double-blind, phase II randomized clinical trial. Int. Immunopharmacol. 74, 105730 (2019).
Ahmadi, M. et al. Effect of Intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). Biomed. Pharmacother. 92, 1095–1102 (2017).
Al-Turki, H. A. Hysteroscopy as an investigation tool in recurrent implantation failure in vitro fertilization. Saudi Med. J. 39, 243–246 (2018).
Almog, B. et al. Interval double transfer improves treatment success in patients with repeated IVF/ET failures. J. Assist. Reprod. Genet. 25, 353–357 (2008).
Aslan, D. et al. Comparison of zygote intrafallopian tube transfer and transcervical uterine embryo transfer in patients with repeated implantation failure. Eur. J. Obstet. Gynecol. Reprod. Biol. 122, 191–194 (2005).
Arefi, S. et al. Granulocyte-colony stimulating factor may improve pregnancy outcome in patients with history of unexplained recurrent implantation failure: An RCT. Int. J. Reprod. Biomed. (Yazd) 16, 299–304 (2018).
Bar, G. et al. Recurrent implantation failure: Which patients benefit from endometrial scratching prior to IVF?. Arch. Gynecol. Obstet. 301, 817–822 (2020).
Barash, A. et al. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil. Steril. 79, 1317–1322 (2003).
Barrenetxea, G. et al. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: A comparison of day 5 and day 6 transfers. Fertil. Steril. 83, 49–53 (2005).
Chao, K. H. et al. Assisted hatching increases the implantation and pregnancy rate of in vitro fertilization (IVF)-embryo transfer (ET), but not that of IVF-tubal ET in patients with repeated IVF failures. Fertil. Steril. 67, 904–908 (1997).
Debrock, S. et al. Higher implantation rate using modified quarter laser-assisted zona thinning in repeated implantation failure. Gynecol. Obstet. Invest. 67, 127–133 (2009).
Dunne, C. & Taylor, B. Does endometrial injury improve implantation of frozen-thawed embryos?. Arch. Gynecol. Obstet. 290, 575–579 (2014).
Eftekhar, M., Miraj, S., Farid Mojtahedi, M. & Neghab, N. Efficacy of Intrauterine infusion of granulocyte colony stimulating factor on patients with history of implantation failure: A randomized control trial. Int. J. Reprod. Biomed. (Yazd). 14, 687–690 (2016).
El Khattabi, L. et al. Is intracytoplasmic morphologically selected sperm injection effective in patients with infertility related to teratozoospermia or repeated implantation failure?. Fertil. Steril. 100, 62–68 (2013).
Friedler, S. et al. A randomized clinical trial comparing recombinant hyaluronan/recombinant albumin versus human tubal fluid for cleavage stage embryo transfer in patients with multiple IVF-embryo transfer failure. Hum. Reprod. 22, 2444–2448 (2007).
Fawzy, M. & El-Refaeey, A. A. Does combined prednisolone and low molecular weight heparin have a role in unexplained implantation failure?. Arch. Gynecol. Obstet. 289, 677–680 (2014).
Gatimel, N., Parinaud, J. & Leandri, R. D. Intracytoplasmic morphologically selected sperm injection (IMSI) does not improve outcome in patients with two successive IVF-ICSI failures. J. Assist. Reprod. Genet. 33, 349–355 (2016).
Gianaroli, L. et al. Preimplantation genetic diagnosis increases the implantation rate in human in vitro fertilization by avoiding the transfer of chromosomally abnormal embryos. Fertil. Steril. 68, 1128–1131 (1997).
Gibreel, A. et al. Endometrial scratching for women with previous IVF failure undergoing IVF treatment. Gynecol. Endocrinol. 31, 313–316 (2015).
Hamdi, K. et al. The role of heparin in embryo implantation in women with recurrent implantation failure in the cycles of assisted reproductive techniques (without history of thrombophilia). J. Family. Reprod. Health. 9, 59–64 (2015).
Hayashi, T. et al. Single curettage endometrial biopsy injury in the proliferative phase improves reproductive outcome of subsequent in vitro fertilization-embryo transfer cycle in infertile patients with repeated embryo implantation failure. Clin. Exp. Obstet. Gynecol. 40, 323–326 (2013).
Heilmann, L., Schorsch, M. & Hahn, T. CD3-CD56+CD16+ natural killer cells and improvement of pregnancy outcome in IVF/ICSI failure after additional IVIG-treatment. Am. J. Reprod. Immunol. 63, 263–265 (2010).
Hiraoka, K. et al. Effect of the size of zona pellucida opening by laser assisted hatching on clinical outcome of frozen cleaved embryos that were cultured to blastocyst after thawing in women with multiple implantation failures of embryo transfer: A retrospective study. J. Assist. Reprod. Genet. 25, 129–135 (2008).
Hosseini, M. A. et al. Hysteroscopy in patients with repeated implantation failure improves the outcome of assisted reproductive technology in fresh and frozen cycles. J. Obstet. Gynaecol. Res. 40, 1324–1330 (2014).
Huang, S. Y. et al. Site-specific endometrial injury improves implantation and pregnancy in patients with repeated implantation failures. Reprod. Biol. Endocrinol. 9, 140 (2011).
Inal, Z. H. O., Gorkemli, H. & Inal, H. A. The effect of local injury to the endometrium for implantation and pregnancy rates in ICSI–ET cycles with implantation failure: A randomised controlled study. Eur. J. Gen. Med. 9, 223–229 (2012).
Jayot, S. et al. Coculture of embryos on homologous endometrial cells in patients with repeated failures of implantation. Fertil. Steril. 63, 109–114 (1995).
Jelinkova, L. et al. Improved implantation rate after chemical removal of the zona pellucida. Fertil. Steril. 79, 1299–1303 (2003).
Johnston-MacAnanny, E. B. et al. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil. Steril. 93, 437–441 (2010).
Kanazawa, E. et al. Injury to the endometrium prior to the frozen-thawed embryo transfer cycle improves pregnancy rates in patients with repeated implantation failure. J. Obstet. Gynaecol. Res. 43, 128–134 (2017).
Kanyo, K. et al. The impact of laser-assisted hatching on the outcome of frozen human embryo transfer cycles. Zygote. 24, 742–747 (2016).
Karabulut, S., Aksunger, O., Korkmaz, O., Eren Gozel, H. & Keskin, I. Intracytoplasmic morphologically selected sperm injection, but for whom? Zygote. 27, 299–304 (2019).
Karacan, M. et al. Comparison of the transfer of equal numbers of blastocysts versus cleavage-stage embryos after repeated failure of in vitro fertilization cycles. J. Assist. Reprod. Genet. 31, 269–274 (2014).
Karimzadeh, M.A., Ayazi Rozbahani, M. & Tabibnejad, N. Endometrial local injury improves the pregnancy rate among recurrent implantation failure patients undergoing in vitro fertilisation/intra cytoplasmic sperm injection: a randomized clinical trial. Aust. N. Z. J. Obstet. Gynaecol. 49, 677–680 (2009).
Kitaya, K. et al. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am. J. Reprod. Immunol. 78, (2017).
Lambers, M. J. et al. Low dose aspirin in non-tubal IVF patients with previous failed conception: A prospective randomized double-blind placebo-controlled trial. Fertil. Steril. 92, 923–929 (2017).
Lee, C. I. et al. Performance of preimplantation genetic testing for aneuploidy in IVF cycles for patients with advanced maternal age, repeat implantation failure, and idiopathic recurrent miscarriage. Taiwan J. Obstet. Gynecol. 8, 239–243 (2015).
Lee, J. W. et al. Effects of laser-assisted thinning versus opening on clinical outcomes according to maternal age in patients with repeated implantation failure. Lasers Med. Sci. 34, 1889–1895 (2019).
Lodigiani, C. et al. The effect of parnaparin sodium on in vitro fertilization outcome: A prospective randomized controlled trial. Thromb. Res. 159, 116–121 (2017).
Loutradis, D. et al. A double embryo transfer on days 2 and 4 or 5 improves pregnancy outcome in patients with good embryos but repeated failures in IVF or ICSI. Clin. Exp. Obstet. Gynecol. 31, 63–66 (2004).
Lu, X., Liu, Y., Cao, X., Liu, S. Y. & Dong, X. Laser-assisted hatching and clinical outcomes in frozen-thawed cleavage-embryo transfers of patients with previous repeated failure. Lasers Med. Sci. 34, 1137–1145 (2019).
Madhavan, A., Naidu, P., Rani, K., Kaur, J. & Mahajan, N. Intrauterine autologous platelet-rich plasma therapy to improve implantation rates in patients undergoing frozen embryo transfer: A pilot study. Onco Fertil. J. 1, 83–85 (2018).
Mak, J. S. M. et al. The effect of endometrial scratch on natural-cycle cryopreserved embryo transfer outcomes: A randomized controlled study. Reprod. Biomed. Online. 35, 28–36 (2017).
Moini, A. et al. The effect of vaginal sildenafil on the outcome of assisted reproductive technology cycles in patients with repeated implantation failures: A randomized placebo-controlled trial. Int. J. Fertil. Steril. 13, 289–295 (2020).
Munné, S. et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: A multicenter randomized clinical trial. Fertil. Steril. 112, 1071-1079.e7 (2019).
Seval, M. M. et al. Does adding endometrial scratching to diagnostic hysteroscopy improve pregnancy rates in women with recurrent in-vitro fertilization failure?. Gynecol Endocrinol. 32, 957–960 (2016).
Narvekar, S. A. et al. Does local endometrial injury in the nontransfer cycle improve the IVF-ET outcome in the subsequent cycle in patients with previous unsuccessful IVF? A randomized controlled pilot study. J. Hum. Reprod. Sci. 3, 15–19 (2010).
Ng, E. H. et al. A randomized double blind comparison of atosiban in patients undergoing IVF treatment. Hum. Reprod. 29, 2687–2694 (2014).
Oliveira, J. B. et al. Pregnancy outcomes in women with repeated implantation failures after intracytoplasmic morphologically selected sperm injection (IMSI). Reprod. Biol. Endocrinol. 9, 99 (2011).
Petersen, C. G. et al. Implantation failures: success of assisted hatching with quarter-laser zona thinning. Reprod. Biomed. Online. 10, 224–229 (2005).
Qublan, H. et al. Low-molecular-weight heparin in the treatment of recurrent IVF-ET failure and thrombophilia: A prospective randomized placebo-controlled trial. Hum. Fertil. (Camb) 11, 246–253 (2008).
Rama Raju, G. A., Shashi Kumari, G., Krishna, K. M., Prakash, G. J. & Madan, K. Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Arch. Gynecol. Obstet. 274, 160–164 (2006).
Shahrokh-Tehraninejad, E. et al. A randomized trial to evaluate the effect of local endometrial injury on the clinical pregnancy rate of frozen embryo transfer cycles in patients with repeated implantation failure. J. Family Reprod. Health. 10, 108–114 (2016).
Shalom-Paz, E. et al. Can intra cytoplasmatic morphologically selected sperm injection (IMSI) technique improve outcome in patients with repeated IVF-ICSI failure? A comparative study. Gynecol. Endocrinol. 31, 247–251 (2015).
Shohayeb, A. & El-Khayat, W. Does a single endometrial biopsy regimen (S-EBR) improve ICSI outcome in patients with repeated implantation failure? A randomised controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 164, 176–179 (2012).
Singh, N., Toshyan, V., Kumar, S., Vanamail, P. & Madhu, M. Does endometrial injury enhances implantation in recurrent in-vitro fertilization failures? A prospective randomized control study from tertiary care center. J. Hum. Reprod. Sci. 8, 218–223 (2015).
Singh, N., Davis, A. A., Kumar, S. & Kriplani, A. The effect of administration of intravenous intralipid on pregnancy outcomes in women with implantation failure after IVF/ICSI with non-donor oocytes: A randomised controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 240, 45–51 (2019).
Siristatidis, C. et al. Endometrial injury for RIF patients undergoing IVF/ICSI: A prospective nonrandomized controlled trial. Gynecol. Endocrinol. 33, 297–300 (2017).
Stein, A. et al. Assisted hatching by partial zona dissection of human pre-embryos in patients with recurrent implantation failure after in vitro fertilization. Fertil. Steril. 63, 838–841 (1995).
Tan, J. et al. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J. Assist. Reprod. Genet. 35(4), 683–692 (2018).
Tersoglio, A.E. et al. Repeated implantation failure in oocyte donation. What to do to improve the endometrial receptivity? JBRA Assist. Reprod. 19, 44–52 (2015).
Tk, A. et al. Local endometrial injury in women with failed IVF undergoing a repeat cycle: A randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 214, 109–114 (2017).
Tumanyan, A., Gemilyan, M. & Hambartsoumian, E. Single and double endometrial scratching (ES) in infertile women with strict criteria of recurrent implantation failure (RIF). Gynecol. Endocrinol. 35(sup1), 11–14 (2019).
Valojerdi, M. R., Eftekhari-Yazdi, P., Karimian, L. & Ashtiani, S. K. Effect of laser zona pellucida opening on clinical outcome of assisted reproduction technology in patients with advanced female age, recurrent implantation failure, or frozen-thawed embryos. Fertil. Steril. 90, 84–91 (2008).
Volovsky, M., Healey, M., MacLachlan, V. & Vollenhoven, B. J. Should intrauterine human chorionic gonadotropin infusions ever be used prior to embryo transfer?. J. Assist. Reprod. Genet. 35, 273–278 (2018).
Yang, R. et al. The hysteroscopy and histological diagnosis and treatment value of chronic endometritis in recurrent implantation failure patients. Arch. Gynecol. Obstet. 289, 1363–1369 (2014).
Yeung, T. W. et al. The effect of endometrial injury on ongoing pregnancy rate in unselected subfertile women undergoing in vitro fertilization: a randomized controlled trial. Hum. Reprod. 29, 2474–2481 (2014).
Zhang, R. et al. Fertiloscopy improves in vitro fertilization for women with repeated implantation failure. J. Gynecol. Obstet. Hum. Reprod. 46, 743–746 (2017).
Aleyasin, A., Abediasl, Z., Nazari, A. & Sheikh, M. Granulocyte colony-stimulating factor in repeated IVF failure, a randomized trial. Reproduction 151, 637–642 (2016).
Blockeel, C. et al. Prospectively randomized controlled trial of PGS in IVF/ICSI patients with poor implantation. Reprod. Biomed. Online. 17, 848–854 (2008).
Davari-Tanha, F., Shahrokh Tehraninejad, E., Ghazi, M. & Shahraki, Z. The role of G-CSF in recurrent implantation failure: A randomized double blind placebo control trial. Int. J. Reprod. Biomed. (Yazd). 14, 737–742 (2016).
El-Toukhy, T. et al. Hysteroscopy in recurrent in-vitro fertilisation failure (TROPHY): A multicentre, randomised controlled trial. Lancet 387, 2614–2621 (2016).
Fang, C. et al. Day-2 and day-3 sequential transfer improves pregnancy rate in patients with repeated IVF-embryo transfer failure: A retrospective case-control study. Reprod. Biomed. Online. 26, 30–35 (2013).
Greco, E. et al. Comparative genomic hybridization selection of blastocysts for repeated implantation failure treatment: A pilot study. Biomed. Res. Int. 2014, 457913 (2014).
Gürgan, T. et al. Systematic and standardized hysteroscopic endometrial injury for treatment of recurrent implantation failure. Reprod. Biomed. Online. 39, 477–483 (2019).
Kalem, Z. et al. Intrauterine G-CSF Administration in Recurrent Implantation Failure (RIF): An Rct. Sci. Rep. 10, 5139 (2020).
Kim, C.H. et al. Effect of granulocyte colony-stimulating factor on pregnancy outcome following IVF/ICSI in patients with repeated implantation failure. Abstracts of the 27th Annual Meeting of ESHRE, Stockholm, Sweden, 3 July–6 July, 2011.
Levitas, E. et al. Blastocyst-stage embryo transfer in patients who failed to conceive in three or more day 2–3 embryo transfer cycles: a prospective, randomized study. Fertil. Steril. 81, 567–571 (2004).
Levran, D. et al. Prospective evaluation of blastocyst stage transfer vs. zygote intrafallopian tube transfer in patients with repeated implantation failure. Fertil. Steril. 77, 971–977 (2002).
Liu, X. et al. Intrauterine administration of human chorionic gonadotropin improves the live birth rates of patients with repeated implantation failure in frozen-thawed blastocyst transfer cycles by increasing the percentage of peripheral regulatory T cells. Arch. Gynecol. Obstet. 299, 1165–1172 (2019).
Matsumoto, Y., Kokeguchi, S. & Shiotani, M. Effects of endometrial injury on frozen-thawed blastocyst transfer in hormone replacement cycles. Reprod. Med. Biol. 16, 196–199 (2017).
Rufas-Sapir, O. et al. Is assisted hatching beneficial in patients with recurrent implantation failures?. Clin. Exp. Obstet. Gynecol. 3, 110–112 (2004).
Madkour, A. et al. Intrauterine insemination of cultured peripheral blood mononuclear cells prior to embryo transfer improves clinical outcome for patients with repeated implantation failures. Zygote. 24, 58–69 (2016).
Nazari, L., Salehpour, S., Hosseini, M.S. & Hashemi Moghanjoughi, P. The effects of autologous platelet-rich plasma in repeated implantation failure: A randomized controlled trial. Hum. Fertil. (Camb). 4, 1–5 (2019).
Nobijari, F. F. et al. Endometrium immunomodulation by intrauterine insemination administration of treated peripheral blood mononuclear cell prior frozen/thawed embryos in patients with repeated implantation failure. Zygote. 27, 214–218 (2019).
Okitsu, O. et al. Intrauterine administration of autologous peripheral blood mononuclear cells increases clinical pregnancy rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J. Reprod. Immunol. 92, 82–87 (2011).
Primi, M. P. et al. A European multicentre prospective randomized study to assess the use of assisted hatching with a diode laser and the benefit of an immunosuppressive/antibiotic treatment in different patient populations. Hum. Reprod. 19, 2325–2333 (2004).
Raziel, A. et al. Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure. Fertil. Steril. 87, 198–201 (2007).
Sato, T. et al. Preimplantation genetic testing for aneuploidy: A comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum. Reprod. 35, 255 (2020).
Scarpellini, F. & Sbracia, M. The use of G CSF for implantation failure in IVF: A clinical trial. Fertil. Steril. 96(3 Suppl. 1), S93 (2013).
Scarpellini, F. & Sbracia, M. G-CSF treatment in the implantation failure with a fixed dose of 60 mcg/day: Preliminary data of a controlled trial. Hum. Reprod. 28, 145 (2013).
Shahrokh Tehraninejad, E. et al. Zygote intrafallopian tube transfer versus intrauterine cleavage or blastocyst stage transfer after intracytoplasmic sperm injection cycles in patients with repeated implantation failure: A prospective follow-up study. J. Obstet. Gynaecol. Res. 41, 1779–1784 (2015).
Olesen, M. S. et al. Therapeutic endometrial scratching and implantation after in vitro fertilization: A multicenter randomized controlled trial. Fertil. Steril. 112, 1015–1021 (2019).
Urman, B. et al. Luteal phase empirical low molecular weight heparin administration in patients with failed ICSI embryo transfer cycles: a randomized open-labeled pilot trial. Hum. Reprod. 24, 1640–1647 (2009).
Xiong, Z. F., Dang, X. H., Li, B. & Wang, L. Y. Low-molecularweight heparin in women with repeated implantation failure. J. Pract. Obstet. Gynecol. 31, 614–617 (2015).
Yakin, K., Ata, B., Ercelen, N., Balaban, B. & Urman, B. The effect of preimplantation genetic screening on the probability of live birth in young women with recurrent implantation failure; a nonrandomized parallel group trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 140, 224–229 (2008).
Yoshioka, S. et al. Intrauterine administration of autologous peripheral blood mononuclear cells promotes implantation rates in patients with repeated failure of IVF-embryo transfer. Hum. Reprod. 21, 3290–3294 (2006).
Yu, N. et al. Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) activated by HCG improves the implantation and pregnancy rates in patients with repeated implantation failure: A prospective randomized study. Am. J. Reprod. Immunol. 76, 212–216 (2016).
Zamaniyan, M. et al. Effect of platelet-rich plasma on pregnancy outcomes in infertile women with recurrent implantation failure: A randomized controlled trial. Gynecol. Endocrinol. 2, 1–5 (2020).
Pourmoghadam, Z. et al. Efficacy of intrauterine administration of autologous peripheral blood mononuclear cells on the pregnancy outcomes in patients with recurrent implantation failure: A systematic review and meta-analysis. J. Reprod. Immunol. 137, 103077 (2020).
Gao, M. et al. Intrauterine injection of human chorionic gonadotropin before embryo transfer can improve in vitro fertilization-embryo transfer outcomes: A meta-analysis of randomized controlled trials. Fertil. Steril. 112, 89-97.e1 (2019).
Lensen, S., Sadler, L. & Farquhar, C. Endometrial scratching for subfertility: Everyone’s doing it. Hum. Reprod. 31, 1241–1244 (2016).
Mackens, S. et al. Follicular-phase endometrial scratching: a truncated randomized controlled trial. Hum. Reprod. 35, 1090–1098 (2020).
Pirtea, P. et al. Rate of true recurrent implantation failure is low: results of three successive frozen euploid single embryo transfers (Fertil, Steril, 2020).
Kamath, M.S. et al. Screening hysteroscopy in subfertile women and women undergoing assisted reproduction. Cochrane Database Syst Rev. 4, CD012856 (2019).
Mastenbroek, S. & Repping, S. Preimplantation genetic screening: back to the future. Hum. Reprod. 29, 1846–1850 (2014).
Malizia, B. A., Dodge, L. E., Penzias, A. S. & Hacker, M. R. The cumulative probability of liveborn multiples after in vitro fertilization: a cohort study of more than 10,000 women. Fertil. Steril. 99, 393–399 (2013).
Lu, Y. et al. Prednisone for patients with recurrent implantation failure: study protocol for a double-blind, multicenter, randomized, placebo-controlled trial. Trials. 21, 719 (2020).
Author information
Authors and Affiliations
Contributions
A.B., E.S. and P.E.L.S. conceived and designed the study. All authors acquired, analyzed and interpreted data. A.B. drafted the first version of the manuscript. E.S., A.Ba., F.C. and P.E.L.S. revised the first version of the manuscript. All authors approved the final manuscript version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Busnelli, A., Somigliana, E., Cirillo, F. et al. Efficacy of therapies and interventions for repeated embryo implantation failure: a systematic review and meta-analysis. Sci Rep 11, 1747 (2021). https://doi.org/10.1038/s41598-021-81439-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81439-6
This article is cited by
-
The reproductive potential of vitrified-warmed euploid embryos declines following repeated uterine transfers
Reproductive Biology and Endocrinology (2024)
-
Intravenous immunoglobulin for patients with unexplained recurrent implantation failure: a 6-year single center retrospective review of clinical outcomes
Scientific Reports (2024)
-
The efficacy of diazepam administration during embryo transfer: a retrospective multicenter cohort study on reproductive outcomes
Journal of Assisted Reproduction and Genetics (2024)
-
Immunology Updates in Recurrent Implantation Failure in In Vitro Fertilization
Current Obstetrics and Gynecology Reports (2024)
-
Efficacy of granulocyte colony-stimulating factor for infertility undergoing IVF: a systematic review and meta-analysis
Reproductive Biology and Endocrinology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.