Abstract
This meta-analysis analyzed the clinical pregnancy outcomes of repeated implantation failure (RIF) patients treated with immunomodulatory therapies. Publications (published by August 16, 2021) were identified by searching the PubMed, Embase, and Web of Science databases. The quality of the studies was evaluated with the Cochrane bias risk assessment tool, and a network meta-analysis was performed with Stata 14.0. The outcomes were clinical pregnancy rate (CPR), live birth rate (LBR), and implantation rate (IR). The results of our network meta-analysis of 16 RCTs (including 2,008 participants) show that PBMCs, PRP, and SC-GCSF can significantly improve the CPR compared with LMWH (PBMCs: OR 2.15; 95% CI 1.21–3.83; PRP: OR 2.38; 95% CI 1.08–5.24; SC-GCSF: OR 2.46; 95% CI 1.05–5.72). The LBR of PRP was significantly higher than those of IU-GCSF (OR 3.81; 95% CI 1.22–11.86), LMWH (OR 4.38; 95% CI 1.50–12.90), and intralipid (OR 3.85; 95% CI 1.03–14.29), and the LBR of PBMCs was also significantly better than that of LMWH (OR 2.35; 95% CI 1.14–4.85). Furthermore, PRP treatment significantly improved the IR compared with LMWH treatment (OR 2.81; 95% CI 1.07–7.4). The limited evidence from existing RCTs suggests that PBMCs and PRP are the best therapeutic options for RIF patients. However, owing to the quantity limitation, more top-quality research is required to obtain additional high-level evidence.
Similar content being viewed by others
Introduction
Repeated implantation failure (RIF) is the inability to achieve a clinical pregnancy after multiple cycles of in vitro fertilization and the cumulative transfer of multiple high-quality embryos in patients using assisted reproductive techniques1. Different academic organizations and researchers have attempted to propose clear diagnostic criteria; however, because of the complexity of the causes of RIF and the high diversity of affected patients, no consensus has been generated to date. The current widely used definition of RIF, proposed by Coughlan et al.2,3, is a lack of successful clinical pregnancy in a woman under the age of 40 years after the transfer of at least four good-quality embryos over a minimum of three fresh or frozen cycles. Implantation is a very complicated process, and there are numerous factors, of either maternal or embryonic origin, that contribute to RIF. The embryo, as a homozygous hemizygous antigen, is subject to a variety of factors for its successful implantation1,4. After an embryo is transferred into the uterine cavity, the endometrium must be acceptable for embryo synchronization, and the maternal immune system must tolerate the continued presence of the paternal alloantigen during the pregnancy5. Many potential factors, such as uterine abnormalities, hormonal or metabolic disorders, infections, immunological factors, thrombophilias, severe male factors, or an abnormal immunological response, can contribute to defective maternal–fetal immunotolerance and impaired endometrium receptivity.
There are a variety of immune cells in the endometrium, including natural killer (NK) cells, macrophages (Mφ), dendritic cells (DCs), and T cells, all of which play a role in regulating endometrial receptivity and embryo implantation6. In addition, immune-related cytokines in the intima, including interleukin (IL)-6, IL-10, IL-15, IL-17, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), and nuclear factor kappa B (NF-κB), are also involved in determining the success of embryo implantation and development7,8. In order to restore the underlying immunological imbalance, some immunomodulatory therapies have been introduced to enhance clinical outcomes in women with unexplained RIF9,10,11. These immunomodulatory therapies include low-molecular-weight heparin (LMWH), intravenous immunoglobulin (IVIG), intrauterine (IU) human chorionic gonadotropin (hCG), subcutaneous (SC) or IU infusion of granulocyte colony-stimulating factor (GCSF), peripheral blood mononuclear cells (PBMCs), and intrauterine autologous platelet-rich plasma (PRP)12,13,14,15. However, there is conflicting evidence supporting the efficacy of these treatments, and the comparable efficacy of these immunomodulatory therapies in the rescue of RIF has not been determined.
Therefore, our network meta-analysis study compared the efficacy of the most widely used immunomodulatory therapies for RIF treatment to provide an evidence basis for theclinical application.
Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (Supplementary Material 1).
Search strategy
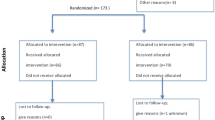
Publications were identified for inclusion in this meta-analysis by searching the PubMed, Embase, and Web of Science (all databases) databases (see screening flow chart in Fig. 1). The last search date was August 16, 2021, and the search language was limited to English. The following terms were applied for this search: “repeated implantation failure,” “recurrent implantation failure,” “intravenous immunoglobulin,” “PBMC,” “G-CSF,” “IVIG,” “PRP,” “intralipid,” “glucocorticoid,” “hCG,” “LMWH,” and “aspirin” (See detailed retrieval strategies in Supplementary Material 2).
Selection criteria
Two authors independently screened the literature compiled in EndNote software. Any disagreements between the two authors regarding the inclusion of a publication were resolved by discussion with the third author to reach a consensus. Strict literature inclusion and exclusion criteria were established. The selected publications were required to meet the following criteria: (1) The study was a randomized controlled trial in which the experimental group was treated with an immunomodulatory therapy and the control group was given the standard care/placebo/no immunomodulatory therapy; (2) The study participants had two or more episodes of implantation failure; and (3) The study included at least one of three defined outcome metrics (clinical pregnancy rate [CPR], live birth rate [LBR], and implantation rate [IR]).
Research was excluded if it met any of the following conditions: (1) the data were incomplete or unable to be used for statistical analysis; or (2) the publication was a non-authoritative document, such as a review, letter, conference abstract, or review.
Data extraction
Two authors independently derived the relevant data from the qualified literature. The extracted content included: first author, publication year, research type, total number of included participants, mean participant age, RIF inclusion criteria, and outcome indicators. The Cochrane bias risk assessment tool was utilized to access the quality of the identified randomized control trials. If the opinions of the two authors differed, the third author would make a judgment.
Statistical analysis
Stata 14.0 was used to conduct the network meta-analysis under the consistency model. The odds ratio (OR) and 95% confidence interval (CI) were calculated for dichotomous outcomes. A paired meta-analysis was performed using a fixed-effects model based on the main results. I2 was used to assess the heterogeneity, and I2 ≥ 50% was taken to indicate statistical heterogeneity. When there was no closed triangle or quadratic loop connecting the three arms, the inconsistency between direct and indirect comparisons was assessed using a node-splitting method. The surface under the cumulative ranking (SUCRA) was used to evaluate the likelihood that each intervention was the most beneficial or safest treatment. A greater SUCRA value was taken to indicate a higher treatment efficacy. A comparison-correction funnel chart was used to assess the publication bias. P > 0.05 was taken to indicate no statistical inconsistency.
Results
Baseline characteristics of the included studies
Overall, 3,350 documents were identified by applying our search criteria. Of these, 901 duplicate articles were eliminated, and 2,379 publications were eliminated after examining the title and abstract. After reading the full text of the remaining 70 publications, 16 studies that met our requirements were finally included in this meta-analysis (Fig. 1, Supplementary Material 3). Among these 16 studies, which included 2,008 participants16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31, three examined LMWH, six investigated GCSF, four trialed PBMCs, one tested hCG, one studied intralipid, and two assessed PRP. Because the identified studies examining glucocorticoid and IVIG did not meet our selection criteria, so no studies on these therapies were included in our meta-analysis. The mean age of the study population ranged from 30.51 to 37.8.
The baseline characteristics of the involved studies were presented in Table 1. An assessment of the quality of these selected studies, as determined using the Cochrane risk of bias tool, was presented in Fig. 2. The network of eligible comparisons for each outcome was shown in Fig. 3. There was no closed loop between interventions, which suggested that all of these pairwise comparisons were indirect. Therefore, the statistical analysis was performed directly under the consistency model.
CPR network meta-analysis
The results of the CPR network meta-analysis were indicated in Fig. 4. PBMC, PRP, SC-GCSF, and hCG administration could all significantly increase the CPR as compared with the control (PBMCs: OR 2.44; 95% CI 1.67–3.57; PRP: OR 2.70; 95% CI 1.41–5.26; SC-GCSF: OR 2.78; 95% CI 1.35–5.88; hCG: OR 2.44; 95% CI 1.20–4.98). Additionally, PBMCs, PRP, and SC-GCSF could also significantly increase the CPR as compared with LMWH (PBMCs: OR 2.15; 95% CI 1.21–3.83; PRP: OR 2.38; 95% CI 1.08–5.24; SC-GCSF: OR 2.46; 95% CI 1.05–5.72).
LBR network meta-analysis
Nine of the included studies reported data on the LBR. The network meta-analysis outcomes implied that the administration of PBMCs and PRP led to a higher LBR in comparison with the control group (PBMCs: OR 2.86; 95% CI 1.64–5.00; PRP: OR 5.26; 95% CI 2.00–14.29) (Fig. 5). The effect of PRP on the LBR was significantly better than those of IU-GCSF (OR 3.81; 95% CI 1.22–11.86), LMWH (OR 4.38; 95% CI 1.50–12.90), or intralipid (OR 3.85; 95% CI 1.03–14.29), and the efficacy of PBMCs for improving the LBR was also significantly better than that of LMWH (OR 2.35; 95% CI 1.14–4.85).
IR network meta-analysis
We conducted a network meta-analysis on the nine studies that reported IR data. The results showed that IU-GCSF, PBMCs, PRP, SC-GCSF, and hCG were each significantly associated with a higher IR as compared with the control group (IU-GCSF: OR 3.57; 95% CI 1.16–11.1; PBMCs: OR 2.56; 95% CI 1.28–5.26; PRP: OR 3.23; 95% CI 1.43–7.69; SC-GCSF: OR 2.86; 95% CI 1.30–6.25; hCG: OR 1.86; 95% CI 1.05–3.28) (Fig. 6). Furthermore, PRP significantly improved the IR as compared with LMWH (OR 2.81; 95% CI 1.07–7.4).
The I2 values were 37.4% for the CPR, 16.1% for the LBR, and 50.8% for the IR (Figures S1–S3). Comparison-adjusted funnel plots of the network meta-analysis of each outcome suggested that there was no publication bias (Fig. 7). Furthermore, the node-splitting method was used for comparing the differences between direct and indirect evidence to assess inconsistency. No significant inconsistencies were found in the results (all P > 0.05), indicating that the results are reliable (details shown in Table S1).
The ranking probability of SUCRA
The ranking probability of SUCRA for each treatment included in the network was shown in Table 2. In terms of the CPR, SC-GCSF was the most effective therapy (78.9%), while LMWH was the least effective therapy (18.3%). As far as the LBR is concerned, PRP was the most effective treatment (94.8%), and LMWH was the least effective (29.4%). Finally, regarding the IR, the most effective treatment was IU-GCSF (77.6%), and the least effective was LMWH (16.2%).
Discussion
The pregnancy rate has increased each year owing to the development of assisted reproductive technology, but there are still a number of patients who suffer from RIF32,33,34. Previous studies showed that uterine abnormalities; spermatic factor anomalies; genetic, hormonal, and metabolic pathologies; acquired thrombophilia; and autoimmune disorders are all possible causes of RIF35. However, RIF remains unexplained in approximately 30% of instances36. It has been reported that immune factors are crucial in the process of embryo implantation, and immunomodulatory therapies can improve the pregnancy outcomes of some patients with RIF36. Recently, there have been many studies conducted on the immune factors involved in the pathogenesis of RIF and immunotherapeutic methods, but there are differences in the efficacy and mechanisms of different preparations. Therefore, this study evaluated the efficacy of immunomodulatory therapies for improving the CPR and LBR of RIF patients through a network meta-analysis. Based on the outcomes of treated RIF patients, it was found that PBMCs and PRP are effective therapies for boosting the CPR and LBR. In comparison with the control group, treatment with PBMCs, PRP, SC-GCSF, or hCG significantly increased the CPR and IR, and PBMCs and PRP were significantly related with a higher LBR.
Previous research has demonstrated that RIF patients can benefit from immunomodulatory therapies, but there was still no direct or indirect comparison of the efficacy of different immunomodulatory therapies15,37,38,39,40. The present study evaluated the efficacy of five immunomodulatory therapies via a network meta-analysis system and found that SC-GCSF is the best therapy for improving the CPR, while IU-GCSF is the best option for improving the IR. Our results confirm the conclusions of Zhao et al. and Xie et al. Zhao et al. showed that the administration of G-CSF may have a favorable clinical effect on pregnancy outcomes. In addition, the best route by which to administer G-CSF may be a subcutaneous injection41. G-CSF, as a glycoprotein, belongs to the growth factor family. It was discovered to regulate the growth of the endometrium and to be involved in the occurrence of early endometriosis42. G-CSF has been shown to promote endometrial stem cells, mobilize bone marrow stem cells, and enhance endometrial development43. Xie et al. found that an intrauterine perfusion of G-CSF could significantly improve the IR as compared with control group44. However, there remains controversy regarding the ideal route of G-CSF administration, and the reasons for the different effects of these two administration routes have not yet been fully clarified. Therefore, more higher-quality studies are needed to clarify these phenomena.
In terms of the LBR, PRP has the best efficacy among the five assessed immunomodulatory therapies. We also discovered that PRP had a significantly better effect on the LBR than did IU-G-CSF, LMWH, and intralipid. Moreover, PRP can also increase the CPR and IR of RIF patients as compared with control group. PRP is composed of a high concentration of autologous platelets, normally 5–7 times greater than the platelet concentration in peripheral blood, which was collected by centrifuging peripheral whole blood45. PRP contains a variety of growth factors and cytokines, which may help regulate endometrial cell migration, attachment, proliferation, differentiation, and neovascularization, thereby having a beneficial effect on endometrial receptivity46,47. Amable et al. showed that, compared with whole blood plasma or platelet-poor plasma, the levels of 12 proteins (including six growth factors, three anti-inflammatory cytokines, and three pro-inflammatory cytokines) in activated PRP increased48. These cytokines and growth factors may boost the endometrium receptivity. Additionally, a mouse experiment showed that an autologous PRP intrauterine infusion accelerated and enhanced the regeneration of impaired endometrium and reduced endometrial fibrosis49. Owing to the limitation of the quantity of the studies, currently there is no meta-analysis to analyze the effect of PRP on the LBR, so additional high-standard studies are required to verify the benefits of PRP on the LBR.
An intrauterine infusion of PBMCs is also a good choice for RIF patients. PBMCs are mainly composed of T lymphocytes, B lymphocytes, and monocytes50. It has been reported that an infusion of PBMCs was able to regulate the production of a variety of cytokines and also promote the spread and invasion of blastocysts to the endometrium as well as the receptivity of the endometrium in vitro39. The results of a recent RCT indicate that PBMC infusion was an effective treatment strategy for RIF-related infertility25. Additionally, consistent with the results of our research, Maleki-Hajiagha et al. found that a PBMC infusion could increase the CPR and LBR of RIF patients14. Their study uncovered that PBMCs could significantly increase the CPR, LBR, and IR of RIF patients, as compared with the control group. The implantation promotion effect of PBMCs can be explained by a variety of mechanisms. It was reported that PBMCs can regulate the production of several cytokines, such as IL-1α, IL-1β, and TNF-α, and can promote the spread and invasion of blastocysts to the endometrium as well as the receptivity of the endometrium in vitro51. In addition, in vivo studies showed that the administration of PBMCs could promote implantation and clinical pregnancy rates and may optimize the in vitro fertilization results of patients with multiple failures from in vitro fertilization/ICSI24,52. Although our research indicates that its clinical effects were positive, adverse reactions should also be considered and will require further research for evaluation.
Our study has some limitations. First, no protocol was registered for this study. Second, conference abstracts and non-English language studies were excluded from this meta-analysis, and relatively few studies were included, with only one study on hCG. Therefore, there might be some potential local or other biases in the results. Third, the included studies may be biased, and undetermined hypercoagulative and immunological abnormalities were not investigated and intervened appropriately. Fourth, very few qualified studies reported the adverse events of their tested interventions, so it was lack of safety evaluation for the different drugs used in RIF treatment. Finally, there were differences in the dose of the same drug among different studies, but it was not feasible to further divide the studies into subgroups for analysis because of the restricted sample size.
Conclusions
This network meta-analysis showed that PBMC, PRP, SC-GCSF, and hCG administration can each significantly increase the CPR and IR as compared to the control group. Furthermore, PBMC and PRP administration led to a higher LBR as compared with the control group. Our findings suggest that, among the different available immunotherapeutic medications for treating RIF, PBMC and PRP might provide the best therapeutic efficacy. Additional high-quality studies are necessary to verify the conclusions drawn from this research owing to its restricted number of included studies.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary materials.
References
Bashiri, A., Halper, K. I. & Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 16, 121. https://doi.org/10.1186/s12958-018-0414-2 (2018).
Thornhill, A. R. et al. ESHRE PGD Consortium “Best practice guidelines for clinical preimplantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS)”. Hum. Reprod. 20, 35–48. https://doi.org/10.1093/humrep/deh579 (2005).
Coughlan, C. et al. Recurrent implantation failure: definition and management. Reprod. Biomed. Online 28, 14–38. https://doi.org/10.1016/j.rbmo.2013.08.011 (2014).
Cakiroglu, Y. & Tiras, B. Determining diagnostic criteria and cause of recurrent implantation failure. Curr. Opin. Obstet. Gynecol. 32, 198–204. https://doi.org/10.1097/gco.0000000000000620 (2020).
Robertson, S. A., Care, A. S. & Moldenhauer, L. M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Invest. 128, 4224–4235. https://doi.org/10.1172/JCI122182 (2018).
Bidarimath, M., Khalaj, K., Wessels, J. M. & Tayade, C. MicroRNAs, immune cells and pregnancy. Cell. Mol. Immunol. 11, 538–547. https://doi.org/10.1038/cmi.2014.45 (2014).
Kany, S., Vollrath, J. T. & Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 20, 6008. https://doi.org/10.3390/ijms20236008 (2019).
Wojdasiewicz, P., Poniatowski, ŁA. & Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 561459. https://doi.org/10.1155/2014/561459 (2014).
Lu, H. Q. & Hu, R. The role of immunity in the pathogenesis and development of pre-eclampsia. Scand J. Immunol. 90, e12756. https://doi.org/10.1111/sji.12756 (2019).
Liang, P. Y. et al. The pro-inflammatory and anti-inflammatory cytokine profile in peripheral blood of women with recurrent implantation failure. Reprod. Biomed. Online 31, 823–826. https://doi.org/10.1016/j.rbmo.2015.08.009 (2015).
Kofod, L. et al. Endometrial immune markers are potential predictors of normal fertility and pregnancy after in vitro fertilization. Am. J. Reprod. Immunol. https://doi.org/10.1111/aji.12684 (2017).
Ho, Y. K. et al. Peripheral CD56(+)CD16(+) NK cell populations in the early follicular phase are associated with successful clinical outcomes of intravenous immunoglobulin treatment in women with repeated implantation failure. Front. Endocrinol. Lausanne 10, 937. https://doi.org/10.3389/fendo.2019.00937 (2019).
Busnelli, A., Somigliana, E., Cirillo, F., Baggiani, A. & Levi-Setti, P. E. Efficacy of therapies and interventions for repeated embryo implantation failure: A systematic review and meta-analysis. Sci Rep 11, 1747. https://doi.org/10.1038/s41598-021-81439-6 (2021).
Maleki-Hajiagha, A. et al. Intrauterine administration of autologous peripheral blood mononuclear cells in patients with recurrent implantation failure: A systematic review and meta-analysis. J. Reprod. Immunol. 131, 50–56. https://doi.org/10.1016/j.jri.2019.01.001 (2019).
Cavalcante, M. B., Cavalcante, C., Sarno, M. & Barini, R. Intrauterine perfusion immunotherapies in recurrent implantation failures: Systematic review. Am. J. Reprod. Immunol. 83, e13242. https://doi.org/10.1111/aji.13242 (2020).
Al-Zebeidi, J. et al. Effect of empiric intravenous intralipid therapy on pregnancy outcome in women with unexplained recurrent implantation failure undergoing intracytoplasmic sperm injection-embryo transfer cycle: A randomized controlled trial. Gynecol. Endocrinol. 36, 131–134. https://doi.org/10.1080/09513590.2019.1631280 (2020).
Aleyasin, A., Abediasl, Z., Nazari, A. & Sheikh, M. Granulocyte colony-stimulating factor in repeated IVF failure, a randomized trial. Reproduction 151, 637–642. https://doi.org/10.1530/REP-16-0046 (2016).
Arefi, S. et al. Granulocyte-colony stimulating factor may improve pregnancy outcome in patients with history of unexplained recurrent implantation failure: An RCT. Int. J. Reprod. Biomed. 16, 299–304 (2018).
Berker, B. et al. The role of low-molecular-weight heparin in recurrent implantation failure: A prospective, quasi-randomized, controlled study. Fertil. Steril. 95, 2499–2502. https://doi.org/10.1016/j.fertnstert.2010.12.033 (2011).
Davari-Tanha, F., Shahrokh Tehraninejad, E., Ghazi, M. & Shahraki, Z. The role of G-CSF in recurrent implantation failure: A randomized double blind placebo control trial. Int. J. Reprod Biomed 14, 737–742 (2016).
Eftekhar, M., Miraj, S., Farid Mojtahedi, M. & Neghab, N. Efficacy of Intrauterine infusion of granulocyte colony stimulating factor on patients with history of implantation failure: A randomized control trial. Int. J. Reprod. Biomed. 14, 687–690 (2016).
Huang, P., Yao, C., Wei, L. & Lin, Z. The intrauterine perfusion of granulocyte-colony stimulating factor (G-CSF) before frozen-thawed embryo transfer in patients with two or more implantation failures. Hum. Fertil. (Camb.) https://doi.org/10.1080/14647273.2020.1811904 (2020).
Kalem, Z. et al. Intrauterine G-CSF Administration in Recurrent Implantation Failure (RIF): An Rct. Sci. Rep. https://doi.org/10.1038/s41598-020-61955-7 (2020).
Madkour, A. et al. Intrauterine insemination of cultured peripheral blood mononuclear cells prior to embryo transfer improves clinical outcome for patients with repeated implantation failures. Zygote 24, 58–69. https://doi.org/10.1017/s0967199414000719 (2016).
Nobijari, F. F. et al. Endometrium immunomodulation by intrauterine insemination administration of treated peripheral blood mononuclear cell prior frozen/thawed embryos in patients with repeated implantation failure. Zygote 27, 214–218. https://doi.org/10.1017/S0967199419000145 (2019).
Pourmoghadam, Z. et al. Intrauterine administration of autologous hCG- activated peripheral blood mononuclear cells improves pregnancy outcomes in patients with recurrent implantation failure; A double-blind, randomized control trial study. J Reprod Immunol 142, 103182. https://doi.org/10.1016/j.jri.2020.103182 (2020).
Salehpour, L. N. S., Hosseini, M. S. & Moghanjoughi, P. H. The effects of autologous platelet-rich plasma in repeated implantation failure: a randomized controlled trial. Hum. Fertil. 23, 209–213. https://doi.org/10.1080/14647273.2019.1569268 (2020).
Urman, B. et al. Luteal phase empirical low molecular weight heparin administration in patients with failed ICSI embryo transfer cycles: A randomized open-labeled pilot trial. Hum. Reprod. 24, 1640–1647. https://doi.org/10.1093/humrep/dep086 (2009).
Wang, M., Deng, H. & Ye, H. Intrauterine injection of human chorionic gonadotropin improves pregnancy outcome in patients with repeated implantation failure in frozen-thawed embryo transfer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 44, 1247–1251. https://doi.org/10.11817/j.issn.1672-7347.2019.180469 (2019).
Yu, N. et al. Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) activated by HCG improves the implantation and pregnancy rates in patients with repeated implantation failure: a prospective randomized study. Am. J. Reprod. Immunol. 76, 212–216. https://doi.org/10.1111/aji.12542 (2016).
Zamaniyan, M. et al. Effect of platelet-rich plasma on pregnancy outcomes in infertile women with recurrent implantation failure: a randomized controlled trial. Gynecol. Endocrinol. 37, 141–145. https://doi.org/10.1080/09513590.2020.1756247 (2021).
Kushnir, V. A., Barad, D. H., Albertini, D. F., Darmon, S. K. & Gleicher, N. Systematic review of worldwide trends in assisted reproductive technology 2004–2013. Reprod. Biol. Endocrinol. 15, 6. https://doi.org/10.1186/s12958-016-0225-2 (2017).
Bromer, J. G. & Seli, E. Assessment of embryo viability in assisted reproductive technology: shortcomings of current approaches and the emerging role of metabolomics. Curr. Opin. Obstet. Gynecol. 20, 234–241. https://doi.org/10.1097/GCO.0b013e3282fe723d (2008).
Mak, J. S. M. et al. The effect of endometrial scratch on natural-cycle cryopreserved embryo transfer outcomes: A randomized controlled study. Reprod. Biomed. Online 35, 28–36. https://doi.org/10.1016/j.rbmo.2017.04.004 (2017).
Mekinian, A. et al. Unexplained recurrent miscarriage and recurrent implantation failure: Is there a place for immunomodulation?. Am. J. Reprod. Immunol. 76, 8–28. https://doi.org/10.1111/aji.12493 (2016).
Kolanska, K. et al. Unexplained recurrent implantation failures: Predictive factors of pregnancy and therapeutic management from a French multicentre study. J. Reprod. Immunol. 145, 103313. https://doi.org/10.1016/j.jri.2021.103313 (2021).
Potdar, N., Gelbaya, T. A., Konje, J. C. & Nardo, L. G. Adjunct low-molecular-weight heparin to improve live birth rate after recurrent implantation failure: A systematic review and meta-analysis. Hum. Reprod. Update 19, 674–684. https://doi.org/10.1093/humupd/dmt032 (2013).
Pourmoghadam, Z. et al. Efficacy of intrauterine administration of autologous peripheral blood mononuclear cells on the pregnancy outcomes in patients with recurrent implantation failure: A systematic review and meta-analysis. J. Reprod. Immunol. 137, 103077. https://doi.org/10.1016/j.jri.2019.103077 (2020).
Yakin, K., Oktem, O. & Urman, B. Intrauterine administration of peripheral mononuclear cells in recurrent implantation failure: a systematic review and meta-analysis. Sci. Rep. 9, 3897. https://doi.org/10.1038/s41598-019-40521-w (2019).
Zhang, L. et al. Therapeutic role of granulocyte colony-stimulating factor (G-CSF) for infertile women under in vitro fertilization and embryo transfer (IVF-ET) treatment: A meta-analysis. Arch. Gynecol. Obstet. 298, 861–871. https://doi.org/10.1007/s00404-018-4892-4 (2018).
Zhao, J., Xu, B., Xie, S., Zhang, Q. & Li, Y. P. Whether G-CSF administration has beneficial effect on the outcome after assisted reproductive technology? A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 14, 62. https://doi.org/10.1186/s12958-016-0197-2 (2016).
Jensen, J. R., Witz, C. A., Schenken, R. S. & Tekmal, R. R. A potential role for colony-stimulating factor 1 in the genesis of the early endometriotic lesion. Fertil. Steril. 93, 251–256. https://doi.org/10.1016/j.fertnstert.2008.09.050 (2010).
Xu, B., Zhang, Q., Hao, J., Xu, D. & Li, Y. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reprod. Biomed. Online 30, 349–358. https://doi.org/10.1016/j.rbmo.2014.12.006 (2015).
Xie, Y. et al. Efficacy of intrauterine perfusion of granulocyte colony-stimulating factor (G-CSF) for Infertile women with thin endometrium: A systematic review and meta-analysis. Am. J. Reprod. Immunol. https://doi.org/10.1111/aji.12701 (2017).
Alves, R. & Grimalt, R. A review of platelet-rich plasma: History, biology, mechanism of action, and classification. Skin Appendage Disord. 4, 18–24. https://doi.org/10.1159/000477353 (2018).
Magdi, Y. et al. Revisiting the management of recurrent implantation failure through freeze-all policy. Fertil. Steril. 108, 72–77. https://doi.org/10.1016/j.fertnstert.2017.04.020 (2017).
Chang, Y. et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 8, 1286–1290 (2015).
Amable, P. R. et al. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 4, 67. https://doi.org/10.1186/scrt218 (2013).
Jang, H. Y. et al. Effects of autologous platelet-rich plasma on regeneration of damaged endometrium in female rats. Yonsei Med. J. 58, 1195–1203. https://doi.org/10.3349/ymj.2017.58.6.1195 (2017).
Chaudhary, N., Que Nguyen, T. N., Maguire, A., Wynne, C. & Meade, A. D. Comparison of sample preparation methodologies towards optimisation of Raman spectroscopy for peripheral blood mononuclear cells. Anal. Methods 13, 1019–1032. https://doi.org/10.1039/d0ay02040k (2021).
Yu, N. et al. HCG-activated human peripheral blood mononuclear cells (PBMC) promote trophoblast cell invasion. PLoS ONE 10, e0125589. https://doi.org/10.1371/journal.pone.0125589 (2015).
Li, S. et al. Intrauterine administration of hCG-activated autologous human peripheral blood mononuclear cells (PBMC) promotes live birth rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J. Reprod. Immunol. 119, 15–22. https://doi.org/10.1016/j.jri.2016.11.006 (2017).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: M.L., D.Y. (II) Administrative support: Y.Y., P.Y. (III) Provision of study materials: Y.T., X.S. (IV) Collection and assembly of data: M.L., Y.Y. (V) Data analysis and interpretation: D.Y., M.L. (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, M., Yuan, Y., Qiao, Y. et al. The effectiveness of immunomodulatory therapies for patients with repeated implantation failure: a systematic review and network meta-analysis. Sci Rep 12, 18434 (2022). https://doi.org/10.1038/s41598-022-21014-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21014-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.