Abstract
This study aimed whether the uptake of amino tracer positron emission tomography (PET) can be used as an additional imaging biomarker to estimate the prognosis of glioma. Participants comprised 56 adult patients with newly diagnosed and untreated World Health Organization (WHO) grade II–IV astrocytic glioma who underwent surgical excision and were evaluated by 11C-methionine PET prior to the surgical excision at Osaka City University Hospital from July 2011 to March 2018. Clinical and imaging studies were retrospectively reviewed based on medical records at our institution. Preoperative Karnofsky Performance Status (KPS) only influenced progression-free survival (hazard ratio [HR] 0.20; 95% confidence interval [CI] 0.10–0.41, p < 0.0001), whereas histology (anaplastic astrocytoma: HR 5.30, 95% CI 1.23–22.8, p = 0.025; glioblastoma: HR 11.52, 95% CI 2.27–58.47, p = 0.0032), preoperative KPS ≥ 80 (HR 0.23, 95% CI 0.09–0.62, p = 0.004), maximum lesion-to-contralateral normal brain tissue (LN max) ≥ 4.03 (HR 0.24, 95% CI 0.08–0.71, p = 0.01), and isocitrate dehydrogenase (IDH) status (HR 14.06, 95% CI 1.81–109.2, p = 0.011) were factors influencing overall survival (OS) in multivariate Cox regression. OS was shorter in patients with LN max ≥ 4.03 (29.3 months) than in patients with LN max < 4.03 (not reached; p = 0.03). OS differed significantly between patients with IDH mutant/LN max < 4.03 and patients with IDH mutant/LN max ≥ 4.03. LN max using 11C-methionine PET may be used in prognostic markers for newly identified and untreated WHO grade II–IV astrocytic glioma.
Similar content being viewed by others
Introduction
Gliomas are the second most common primary brain tumors according to the 2012–2016 Central Brain Tumor Registry of the United States1. Approximately 48.3% of primary malignant brain tumors are glioblastomas, 16.7% are other astrocytomas, and 4.5% are oligodendrogliomas1.
Although magnetic resonance imaging (MRI) has been one of the basic and less-invasive imaging modalities used in the management of glioma, brain PET imaging has recently been recommended2, 3. We have previously reported a positive correlation between WHO grade and accumulation of 11C-methionine among astrocytomas, but that study did not analyze the relationship with prognosis4. Additional analysis was thus performed in the current study. Moreover, the clinical studies investigating the relationship between molecular analysis and uptake of amino acid PET in glioma patients are sparse, and detailed prognostic analyses of associations with molecular profiles and 11C-methionine PET uptake in glioma patients have not been fully completed. This study aimed to evaluate the association between 11C-methionine uptakes, and prognosis in cases of newly diagnosed and untreated adult astrocytic glioma.
Methods
Patients
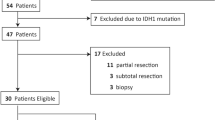
From July 2011 to March 2018, there were 66 adult patients and two patients under 18 years old with newly diagnosed and untreated WHO grade II–IV glioma who underwent surgical tumor resection and preoperative 11C-methionine PET examination, as previously reported4. From this previous cohort, we included adult astrocytic glioma patients with IDH mutated- TERT promoter wild-type, or those with IDH wild-type in the present study. Finally, a total of 56 patients with astrocytic tumor were included in the present cohort. The 56 patients were comprised of 36 male and 20 female patients, with a mean age of 54.0 years (range, 21–82 years). All 11C-methionine PET was performed within one month prior to tumor resection in glioblastoma patients and within six months in patients with lower-grade glioma. Pathological diagnosis was determined according to the 2016 WHO classification for central nervous system tumors5. This study was approved by the institutional review boards at the Graduate School of Medicine, Osaka City University (Approval Numbers: 2047 and 2020-115), and Osaka National Hospital (Approval Number: 0713). Genetic analyses were performed after obtaining written consent. This study was complied with all tenets of the Declaration of Helsinki.
11C-methionine PET
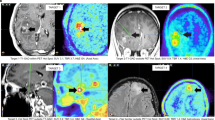
An Eminence B PET scanner (Shimadzu, Kyoto, Japan) or Biograph-16 PET scanner (Siemens, Bon, Germany) was used for 11C-methionine PET, according to previously reported procedures4, 6. Mean and maximum lesion-to-contralateral normal brain tissue (L/N) ratios were determined by dividing the tumor standardized uptake value by the mean standardized uptake value of the normal contralateral region of the brain, as previously reported4.
Genetic analysis
Genetic analysis was performed as previously described4. Genomic DNA was extracted from surgically resected tumor specimens using the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, USA) or NucleoSpin Tissue (Machery-Nagel, Duren, Germany). Hotspot mutations of IDH1/2 (codon 132 of IDH1 and codon 172 of IDH2) and TERT promoter (termed C228 and C250) were examined using Sanger sequencing with a 3130xLGenetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA) and Big-Dye® Terminator v1.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA). The methylation status of O6-methylguanine-DNA methyltransferase (MGMT) promoter was analyzed using quantitative methylation-specific PCR after bisulfite modification of tumor genomic DNA, as previously reported7.
Survival times
Progression-free survival (PFS) was defined as the time in months between evaluation with 11C-methionine PET and tumor progression according to the Response Assessment in Neuro-oncology working group8. Overall survival was defined as the time in months between evaluation with 11C-methionine PET and death.
Statistical analysis
Patients were subdivided into several groups on the basis of age (≥ 70 or < 70 years), preoperative KPS(≥ 80 or < 80), LN mean(≥ 2.46 or < 2.46), LN max(≥ 4.03 or < 4.03), and extent of resection (biopsy or partial removal, < 90%; subtotal removal, ≥ 90% or gross total removal, ≥ 95%) for statistical analysis.
To compare the patients background characteristics of each group classified according to IDH status or LN max or both, we performed statistical analysis using Pearson’s chi-square test. PFS and OS were analyzed using the Kaplan–Meier method. Survival date were evaluated using univariate and multivariate Cox regression analyses. Prognostic factors with a p < 0.05 in the univariate analysis were included in the multivariate analysis. The stepwise method was used to evaluate PFS and OS multivariate Cox regression analyses. Statistical significance was defined at the level of p < 0.05. All statistical analyses were conducted using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan)9.
Ethical approval
This study was approved by the institutional review boards at the Graduate School of Medicine, Osaka City University (approval numbers: 2047 and 2020-115), and Osaka National Hospital (Approval Number: 0713).
Consent to participate
Patient informed consents were waived due to the retrospective nature of the study.
Consent for publication
All authors have approved the manuscript and agree with publication.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Ten patients were classified into IDH mutant diffuse astrocytoma, 2 patients with IDH mutant anaplastic astrocytoma, 3 patients with IDH mutant glioblastoma, 9 patients with IDH wild-type diffuse astrocytoma, 10 patients with IDH wild-type anaplastic astrocytoma, and 22 patients with IDH wild-type glioblastoma. Median LN mean was 2.46 (interquartile range, 1.68–3.04), and median LN max was 4.03 (interquartile range, 2.56–4.89).
Univariate and multivariate analyses for PFS and OS
In univariate analysis, age, enhancement on MRI, preoperative KPS, histology, IDH status, and TERT promoter status influenced PFS, whereas age, enhancement on MRI, preoperative KPS, LN mean, LN max, histology, adjuvant therapy, and IDH status influenced OS (Table 2, Fig. 1). In multivariate Cox regression analysis, preoperative KPS only influenced PFS (HR 0.20, 95% CI 0.1–0.41, p < 0.0001), whereas histology (anaplastic astrocytoma: HR 5.3, 95% CI 1.23–22.8, p = 0.025; glioblastoma: HR 11.52, 95% CI 2.27–58.47, p = 0.0032), preoperative KPS ≥ 80 (HR 0.23,95% CI 0.09–0.62, p = 0.004), LN max ≥ 4.03 (HR 0.24, 95% CI 0.08–0.71, p = 0.01), and IDH status (HR 14.06, 95% CI 1.81–109.2, p = 0.011) were influential factors on OS (Table 3).
Median PFS in patients with diffuse astrocytoma, anaplastic astrocytoma, and glioblastoma were 37.2 months, 9.6 months, and 4.7 months, respectively (p = 0.0003, Table 2). Median OS was more favorable in patients with preoperative KPS ≥ 80 (83.3 months) than in patients with preoperative KPS < 80 (12.6 months, p < 0.0001; Table 2, Fig. 2A). Median OS was not reached for patients with diffuse astrocytoma, 27.1 months for those with anaplastic astrocytoma, and 20.5 months for those with glioblastoma (p < 0.0001, Table 2, Fig. 2B). Median OS was more favorable in patients with IDH mutation than that in patients with IDH wild-type (not reached vs. 26.1 months, respectively, p < 0.0001, Fig. 2C). Furthermore, OS appeared shorter in patients with LN max ≥ 4.03 (29.3 months) than in patients with LN max < 4.03 (not reached, p = 0.03; Fig. 2D).
OS in patients classified according to the IDH status/LN max (Fig. 3, Table 2)
Kaplan–Meier plot of the OS in relation to the IDH status/LN max classification. A significant difference in OS existed between patients with IDH mutant/LN max < 4.03 and those with IDH mutant/LN max ≥ 4.03 (p = 0.034), although no significant difference in OS was evident between patients with IDH mutant/LN max ≥ 4.03 and those with IDH wild-type/LN max < 4.03 (p = 0.40), or between patients with IDH wild-type/LN max < 4.03 and those with IDH wild-type/LN max ≥ 4.03 (p = 0.84).
Median OS was not reached for patients with IDH mutant/LN max < 4.03, 30.1 (95% CI, 30.1-Not reached) months for those with IDH mutant/LN max ≥ 4.03, 20.5 (95% CI, 7.4–52.3) months for those with IDH wild-type/LN max < 4.03, and 27.1 (95% CI, 12.6–39.8) months for those with IDH wild-type/LN max ≥ 4.03, respectively (p = 0.001). A significant difference in OS was seen between patients with IDH mutant/LN max < 4.03 and those with IDH mutant/LN max ≥ 4.03 (p = 0.034), although no significant difference in OS was seen between patients with IDH mutant/LN max ≥ 4.03 and those with IDH wild-type/LN max < 4.03 (p = 0.40), or between patients with IDH wild-type/LN max < 4.03 and those with IDH wild-type/LN max ≥ 4.03 (p = 0.84).
Discussion
The revised WHO 2016 classification of the central nervous system tumor requires the pathological diagnosis with molecular analysis to reach a diagnosis of glioma5. This molecular information has been said to correlate with prognosis, whereas there is still a matter of debate whether imaging biomarkers help estimation of prognosis. Although MRI remains the gold standard for diagnosing glioma, its role in estimating prognosis is limited10. On the other hand, 11C-methionine PET using amino tracer might be useful to detect the tumor, predict the grade or genetic status or both4, 7, 11,12,13, and distinguish tumor recurrence from radiation necrosis14,15,16 in glioma patients, although 11C-methionine PET can only be used in limited institutions that have a cyclotron since 11C-methionine has a short half-life about 20 min. However, relatively few reports have investigated the relationship between the uptake of amino tracer using PET and prognosis in glioma. Moreover, reports investigating prognosis of glioma patients in association with molecular analysis and PET in glioma have been limited17,18,19,20,21. Thus, our goal in the present study was to determine whether 11C-methionine PET can be used as an additional imaging biomarker of prognosis.
In the present study, we excluded patients with oligodendroglioma, or those with IDH mutated- TERT promoter mutated, or both because oligodendroglioma is considered to show better prognosis than astrocytoma and is often accompanied by both IDH and TERT promoter mutations. Although TERT promoter mutation is often seen in oligodendroglioma and primary glioblastoma, prognoses differ markedly between oligodendroglioma and glioblastoma22, 23. An argument has also been made regarding the association between uptake of 11C-methionine and oligodendroglioma13, 24,25,26,27. We have previously reported a positive correlation between WHO grade and the accumulation of 11C-methionine among astrocytomas, and a statistically higher uptake of 11C-methionine in oligodendroglioma than in diffuse astrocytoma4. In the current study, median PFS was 37.2 months for patients with diffuse astrocytoma, 9.6 months for those with anaplastic astrocytoma, and 4.7 months for those with glioblastoma, respectively. Median OS was not reached for patients with diffuse astrocytoma, 27.1 months for those with anaplastic astrocytoma, and 20.5 months for those with glioblastoma, respectively. Reuss et al. reported that 139 of 152 patients with diffuse astrocytoma diagnosed according to the WHO 2007 classification of the central nervous system tumors showed IDH mutant diffuse astrocytoma, whereas more than half of patients with diffuse astrocytoma were IDH wild-type in our cohort28. Minniti et al. reported that IDH mutant anaplastic astrocytoma was found in 56% of their anaplastic astrocytoma patients29. OS in patients with IDH wild-type was 2.8 years29. The relatively shorter PFS and OS of patients with diffuse astrocytoma and anaplastic astrocytoma in the current study were probably attributable to the fact that the present cohort included more patients with IDH wild-type astrocytoma than the previous study. On the other hand, Wakabayashi et al. reported that the median OS in patients with glioblastoma who received Stupp’s regimen was 20.3 months30, similar to our result in the current study.
Brain PET imaging has recently been recommended for use in addition to MRI in the management of glioma2, 3. Takano et al. reported that PFS was worse with LN max ≥ 2.0 than with LN max < 2.0 using 11C-methionine PET among patients with untreated, lower-grade, non-enhancing gliomas31. Discrimination of high-grade glioma from low-grade glioma is usually difficult using MRI alone prior to tumor resection in patients with non-enhancing, lower-grade glioma, so we considered whether 11C-methionine PET can be used to predict the prognosis of glioma. However, we could not find significant differences in PFS between astrocytoma patients with LN max ≥ 4.03 and LN max < 4.03 or between those with LN mean ≥ 2.46 and LN mean < 2.46 in the current study.
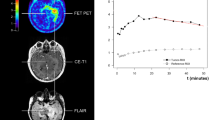
Recently, some reports have investigated the relationship between prognosis from molecular analysis and uptake of PET using 18F-fluoro-ethyl-tyrosine (18F-FET) PET17,18,19, 32 and 3,4-dihydroxy-6-18F-fluoro-ethyl-L-phenylalanine (18F-FDOPA) PET33. Galldiks et al. in a study of photopenic IDH mutant gliomas reported that glioma with 18F-FET accumulation below the level of background healthy brain showed unfavorable outcomes, and thus should be treated more actively18. The utility of dynamic 18F-FET PET has also been reported19. Suchorska et al. reported that longer minimal time-to-peak analysis using 18F-FET PET was associated with a favorable prognosis in IDH mutant astrocytomas19. A time-to-peak analysis≥ 25 min was associated with longer PFS and OS in patients with IDH wild-type high-grade astrocytoma according to Bauer et al.32. Kunz et al. reported homogeneous decreases in intratumoral uptake of 18F-FET over time as a factor associated with poor prognosis in non-enhancing glioma17. Using continuous measures of 18F-FDOPA PET, Patel et al. reported LN max and age as prognostic factors for OS in WHO grade I–IV gliomas, and that IDH or MGMT status did not correlate with uptake of 18F-FDOPA. In this study, we concluded that patients with LN max ≥ 4.03 displayed unfavorable OS compared to patients with LN max < 4.03 among patients with WHO grade II-IV astrocytoma. We also concluded that patients with LN max ≥ 4.03 showed unfavorable OS compared those with LN max < 4.03 among patients with WHO grade II-IV IDH mutant astrocytoma, although no significant difference in OS was evident between IDH wild-type WHO grade II-IV astrocytoma with LN max ≥ 4.03 and those with LN max < 4.03. Thus, another molecular imaging markers might be needed to estimate prognosis in IDH wild-type astrocytoma.
Some limitations need to be considered for the current study. First, the relatively small cohort of the current study might have influenced statistical analyses. For example, TERT promoter status did not influence OS in our cohort, although Arita et al. reported the usefulness of TERT promoter status in addition to the IDH status34. Further study with a larger cohort is thus needed to assess the correlation between prognosis and molecular/imaging biomarkers with amino-tracer PET in patients with astrocytoma. Second, we did not take volumetric analyses into consideration in the current study, although some reports have suggested that metabolic tumor volume did not correlate with survival outcomes17, 19, 32, 33, 35.
Conclusion
LN max using 11C-methionine PET offers a markers for estimating OS in patients with grade II-IV astrocytoma. LN max can also be used as a prognostic imaging biomarker to estimate OS in addition to IDH status in IDH-mutated astrocytoma.
Data availability
The date in the current study are available from the corresponding author on reasonable request.
Abbreviations
- PET:
-
Positron emission tomography
- WHO:
-
World Health Organization
- KPS:
-
Karnofsky Performance Status
- PFS:
-
Progression-free survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- TERT:
-
Telomerase reverse transcriptase
- LN:
-
Lesion-to-contralateral normal brain tissue
- IDH:
-
Isocitrate dehydrogenase
- OS:
-
Overall survival
- MRI:
-
Magnetic resonance imaging
- MGMT:
-
O6-methylguanine-DNA methyltransferase
- 18F-FET:
-
18F-fluoro-ethyl-tyrosine
- 18F-FDOPA:
-
3,4-Dihydroxy-6-18F-fluoro-ethyl-L-phenylalanine
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 21, v1–v100. https://doi.org/10.1093/neuonc/noz150 (2019).
Albert, N. L. et al. Response assessment in neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 18, 1199–1208. https://doi.org/10.1093/neuonc/now058 (2016).
Law, I. et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging 46, 540–557. https://doi.org/10.1007/s00259-018-4207-9 (2019).
Nakajo, K. et al. Diagnostic performance of 11C-methionine PET in newly diagnosed and untreated glioma based on the revised WHO 2016 classification. World Neurosurgery https://doi.org/10.1016/j.wneu.2021.01.012 (2021).
Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 131, 803–820. https://doi.org/10.1007/s00401-016-1545-1 (2016).
Tsuyuguchi, N., Terakawa, Y., Uda, T., Nakajo, K. & Kanemura, Y. Diagnosis of brain tumors using amino acid transport PET imaging with (18)F-fluciclovine: a comparative study with L-methyl-(11)C-methionine PET imaging. Asia Oceania J Nucl Med Biol 5, 85–94. https://doi.org/10.22038/aojnmb.2017.8843 (2017).
Okita, Y. et al. The association between (11)C-methionine uptake, IDH gene mutation, and MGMT promoter methylation in patients with grade II and III gliomas. Clin. Radiol. 75, 622–628. https://doi.org/10.1016/j.crad.2020.03.033 (2020).
van den Bent, M. J. et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 12, 583–593. https://doi.org/10.1016/s1470-2045(11)70057-2 (2011).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 48, 452–458. https://doi.org/10.1038/bmt.2012.244 (2013).
Kanazawa, T. et al. Imaging scoring systems for preoperative molecular diagnoses of lower-grade gliomas. Neurosurg. Rev. 42, 433–441. https://doi.org/10.1007/s10143-018-0981-x (2019).
Falk Delgado, A. & Falk Delgado, A. Discrimination between primary low-grade and high-grade glioma with (11)C-methionine PET: a bivariate diagnostic test accuracy meta-analysis. Br. J. Radiol. 91, 20170426. https://doi.org/10.1259/bjr.20170426 (2018).
Nariai, T. et al. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J. Neurosurg. 103, 498–507. https://doi.org/10.3171/jns.2005.103.3.0498 (2005).
Shinozaki, N. et al. Discrimination between low-grade oligodendrogliomas and diffuse astrocytoma with the aid of 11C-methionine positron emission tomography. J. Neurosurg. 114, 1640–1647. https://doi.org/10.3171/2010.11.Jns10553 (2011).
Terakawa, Y. et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49, 694–699. https://doi.org/10.2967/jnumed.107.048082 (2008).
Tsuyuguchi, N. et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible?. J. Neurosurg. 98, 1056–1064. https://doi.org/10.3171/jns.2003.98.5.1056 (2003).
Van Laere, K. et al. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur. J. Nucl. Med. Mol. Imaging 32, 39–51. https://doi.org/10.1007/s00259-004-1564-3 (2005).
Kunz, M. et al. Dynamic 18F-FET PET is a powerful imaging biomarker in gadolinium-negative gliomas. Neuro Oncol. 21, 274–284. https://doi.org/10.1093/neuonc/noy098 (2019).
Galldiks, N. et al. Photopenic defects on O-(2-[18F]-fluoroethyl)-L-tyrosine PET: clinical relevance in glioma patients. Neuro Oncol. 21, 1331–1338. https://doi.org/10.1093/neuonc/noz083 (2019).
Suchorska, B. et al. Identification of time-to-peak on dynamic 18F-FET-PET as a prognostic marker specifically in IDH1/2 mutant diffuse astrocytoma. Neuro Oncol. 20, 279–288. https://doi.org/10.1093/neuonc/nox153 (2018).
Bauer, E. K. B. et al. Prediction of survival in patients with IDH-wildtype astrocytic gliomas using dynamic O-(2-[(18)F]-fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging 47, 1486–1495. https://doi.org/10.1007/s00259-020-04695-0 (2020).
Tatekawa, H. et al. Maximum uptake and hypermetabolic volume of 18F-FDOPA PET estimate molecular status and overall survival in low-grade gliomas: a PET and MRI study. Clin. Nucl. Med. 45, e505–e511. https://doi.org/10.1097/rlu.0000000000003318 (2020).
Arita, H. et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 126, 267–276. https://doi.org/10.1007/s00401-013-1141-6 (2013).
Killela, P. J. et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA. 110, 6021–6026. https://doi.org/10.1073/pnas.1303607110 (2013).
Iwadate, Y., Shinozaki, N., Matsutani, T., Uchino, Y. & Saeki, N. Molecular imaging of 1p/19q deletion in oligodendroglial tumours with 11C-methionine positron emission tomography. J. Neurol. Neurosurg. Psychiatry 87, 1016–1021. https://doi.org/10.1136/jnnp-2015-311516 (2016).
Kebir, S. et al. Comparison of L-methyl-11C-methionine PET with magnetic resonance spectroscopy in detecting newly diagnosed glioma. Clin. Nucl. Med. 44, e375–e381. https://doi.org/10.1097/rlu.0000000000002577 (2019).
Saito, T. et al. 11C-methionine uptake correlates with combined 1p and 19q loss of heterozygosity in oligodendroglial tumors. AJNR Am. J. Neuroradiol. 34, 85–91. https://doi.org/10.3174/ajnr.A3173 (2013).
Takei, H. et al. Usefulness of positron emission tomography for differentiating gliomas according to the 2016 World Health Organization classification of tumors of the central nervous system. J Neurosurg https://doi.org/10.3171/2019.5.Jns19780 (2019).
Reuss, D. E. et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 129, 133–146. https://doi.org/10.1007/s00401-014-1370-3 (2015).
Minniti, G. et al. IDH1 mutation and MGMT methylation status predict survival in patients with anaplastic astrocytoma treated with temozolomide-based chemoradiotherapy. J. Neurooncol. 118, 377–383. https://doi.org/10.1007/s11060-014-1443-0 (2014).
Wakabayashi, T. et al. JCOG0911 INTEGRA study: a randomized screening phase II trial of interferonβ plus temozolomide in comparison with temozolomide alone for newly diagnosed glioblastoma. J. Neurooncol. 138, 627–636. https://doi.org/10.1007/s11060-018-2831-7 (2018).
Takano, K. et al. Diagnostic and prognostic value of 11C-methionine PET for nonenhancing gliomas. AJNR Am. J. Neuroradiol. 37, 44–50. https://doi.org/10.3174/ajnr.A4460 (2016).
Bauer, E. K. et al. Prediction of survival in patients with IDH-wildtype astrocytic gliomas using dynamic O-(2-[(18)F]-fluoroethyl)-L-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 47, 1486–1495. https://doi.org/10.1007/s00259-020-04695-0 (2020).
Patel, C. B. et al. (18)F-FDOPA PET and MRI characteristics correlate with degree of malignancy and predict survival in treatment-naïve gliomas: a cross-sectional study. J. Neurooncol. 139, 399–409. https://doi.org/10.1007/s11060-018-2877-6 (2018).
Arita, H. et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol. Commun. 4, 79. https://doi.org/10.1186/s40478-016-0351-2 (2016).
Oughourlian, T. C. et al. Rate of change in maximum (18)F-FDOPA PET uptake and non-enhancing tumor volume predict malignant transformation and overall survival in low-grade gliomas. J. Neurooncol. 147, 135–145. https://doi.org/10.1007/s11060-020-03407-w (2020).
Acknowledgements
We would like to thank Drs. Susumu Shiomi and Shigeaki Higashiyama for their cooperation with this study, and the technologists, especially Takashi Yamanaga and Hideki Kawabata, at the Central Radiology Department, School of Medical Sciences of Osaka City University, for their support with PET. We also wish to thank Tomoko Shofuda, Ema Yoshioka, and Daisuke Kanematsu, at the National Hospital Organization Osaka National Hospital for genetic analysis.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
K.N.: Conceptualization, Investigation, Writing-original draft. T.U.: Investigation, Supervision. T.K.: Writing-original draft. Y.T.: Investigation, Supervision. K.I.: Investigation, Supervision. N.T.: Conceptualization, Investigation, Supervision. Y.T.: Writing-original draft. A.N.: Writing-original draft. H.U.: Writing-original draft. S.K.: Writing-original draft. T.S.: Writing-original draft. K.O.: Supervision. Y.K.: Investigation, Resources, Supervision. T.G.: Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakajo, K., Uda, T., Kawashima, T. et al. Maximum 11C-methionine PET uptake as a prognostic imaging biomarker for newly diagnosed and untreated astrocytic glioma. Sci Rep 12, 546 (2022). https://doi.org/10.1038/s41598-021-04216-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04216-5
This article is cited by
-
A comparison study of dynamic [18F]Alfatide II imaging and [11C]MET in orthotopic rat models of glioblastoma
Journal of Cancer Research and Clinical Oncology (2024)
-
Imaging biomarkers for clinical applications in neuro-oncology: current status and future perspectives
Biomarker Research (2023)
-
PET radiotracers in glioma: a review of clinical indications and evidence
Clinical and Translational Imaging (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.