Abstract
The association between nonalcoholic fatty liver (NAFL) or liver fibrosis and diabetic peripheral neuropathy (DPN) has not been well studied. We aimed to investigate the association of NAFL or liver fibrosis indices and DPN in individuals with type 2 diabetes. In this observational study, we included 264 individuals with type 2 diabetes, and calculated non-alcoholic fatty liver disease (NAFLD) liver fat score, NAFLD fibrosis score, and Fibrosis-4 (FIB-4) index to evaluate the status of NAFLD or liver fibrosis. DPN was diagnosed when the Michigan Neuropathy Screening Instrument—Physical Examination score was ≥ 2.5. The NAFLD fibrosis score and FIB-4 index were significantly higher in individuals with DPN than in those without DPN. Logistic analyses showed that the NAFLD fibrosis score and FIB-4 index were associated with DPN after adjustment for covariates (adjusted odds ratio 1.474 and 1.961, respectively). In the subgroup analysis, this association was only significant in the group with a high NAFLD liver fat score (> − 0.640). Serum levels of fetuin-A, a hepatokine, were decreased in individuals with abnormal vibration perception or 10-g monofilament tests compared with their counterparts. The present study suggests that liver fibrosis might be associated with DPN in individuals with type 2 diabetes.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease worldwide, affecting approximately 25% of the global population1. It encompasses a spectrum of diseases, extending from nonalcoholic fatty liver (NAFL) through nonalcoholic steatohepatitis (NASH), advanced fibrosis to cirrhosis, and end-stage liver disease. The natural history of NAFL is variable, with the majority of individuals having benign disease with steatosis without inflammation2. However, 40% of individuals with NAFL develop advanced fibrosis, which can result in cirrhosis3. The prevalence of NAFLD in individuals with type 2 diabetes is higher than that in the general population, ranging from 40 to 70%2. In addition, individuals with type 2 diabetes showed an increased risk of developing NASH, advanced fibrosis, and cirrhosis4,5,6,7. NAFLD in individuals with type 2 diabetes is associated with an increased risk of developing cardiovascular disease (CVD)8,9 and an increased risk of microvascular complications, such as nephropathy and retinopathy10,11,12.
Diabetic peripheral neuropathy (DPN) is the most common form of diabetic neuropathy and affects approximately 50% of individuals with diabetes13. Risk factors for DPN include hypertension, smoking, hyperglycaemia, diabetes duration, age, dyslipidaemia, and obesity, which are also known risk factors for CVD14. Considering that NAFLD, CVD, and DPN share risk factors and that NAFLD is associated with an increased risk of other microvascular complications, it is reasonable to hypothesize that individuals with diabetes and NAFL or liver fibrosis would also have a high risk for DPN.
In the present study, we examined the association of NAFL or liver fibrosis with DPN using noninvasive methods for evaluating NAFLD and liver fibrosis. In addition, we tested the association between DPN and fetuin-A, a hepatokine that is known to be elevated in individuals with NAFLD15.
Results
Clinical and biochemical characteristics of the study participants
The present study included the data of 264 individuals with type 2 diabetes but without chronic liver diseases; 38.2% had DPN. Body mass index (BMI), fasting plasma glucose (FPG), haemoglobin A1c (HbA1c), and aspartate aminotransferase (AST) levels were significantly higher in individuals with DPN than in those without DPN (Table 1). The prevalence of suspected NAFLD based on NAFLD liver fat score > − 0.640 was compatible between individuals with and without DPN (73.3% vs. 69.3%, p = 0.493). However, both the NAFLD fibrosis score and Fibrosis-4 (FIB-4) index were significantly higher in individuals with DPN than in those without DPN.
We stratified individuals by NAFLD liver fat score (≤ − 0.640 or > − 0.640) and the presence of DPN. Compared with individuals with a low NAFLD liver fat score (≤ − 0.640), individuals with a high NAFLD liver fat score (> − 0.640) were more obese and had higher blood pressure (BP), triglyceride levels, AST, alanine aminotransferase (ALT), and homeostatic model assessment-insulin resistance (HOMA-IR) (Table 2). However, there were no significant differences in neuropathy examination results between individuals with a low NAFLD liver fat score (≤ − 0.640) and those with a high NAFLD liver fat score (> − 0.640). In contrast to the subgroup with a low NAFLD liver fat score (≤ − 0.640), the NAFLD fibrosis score and FIB-4 index were significantly higher in individuals with DPN than in those without DPN among individuals with a high NAFLD liver fat score (> − 0.640).
Association of NAFLD fibrosis score and FIB-4 index with DPN
Logistic regression analyses showed that the NAFLD liver fat score was not associated with DPN. However, the NAFLD fibrosis score and FIB-4 index were significantly associated with DPN: adjusted odds ratio (aOR) 1.474 (95% confidence interval [CI] 1.055, 2.058), and aOR 1.961 (95% CI 1.209, 3.183), respectively (Table 3). In the subgroup analysis, this association was only observed in individuals with a high NAFLD liver fat score (> − 0.640). The aORs for the NAFLD fibrosis score and FIB-4 index were 1.501 (95% CI 1.006, 2.239) and 2.272 (95% CI 1.271, 4.059), respectively.
Association between fetuin-A and DPN features
Among individuals with a high NAFLD liver fat score (> − 0.640), serum fetuin-A levels were 613.5 ± 181.0 µg/ml in individuals without DPN and 611.3 ± 182.7 µg/ml in those with DPN (p = 0.956). Serum fetuin-A levels were significantly lower in individuals with abnormal vibration perception (542.2 ± 144.9 µg/ml vs. 639.0 ± 183.0 µg/ml, p = 0.014) and in those with an abnormal 10-g monofilament test (494.2 ± 121.0 µg/ml vs. 625.2 ± 182.1 µg/ml, p = 0.029) compared with their counterparts. The area under the receiver operating characteristic curve (AUROC) of the fetuin-A levels for the absence of abnormal vibration perception was 0.671 (95% CI 0.531, 0.811) and for the absence of abnormal 10-g monofilament test was 0.736 (95% CI 0.561, 0.911) (Fig. 1).
Serum fetuin-A levels and ROC curves for the detection of abnormalities in DPN examination in individuals with a high NAFLD liver fat score (≥ − 0.640). (A) Normal (n = 63) and abnormal (n = 19) vibration perception. (B) ROC curve of fetuin-A for the absence of abnormal vibration perception. (C) Normal (n = 74) and abnormal (n = 8) 10-g monofilament tests. (D) ROC curve of fetuin-A for the absence of an abnormal 10-g monofilament test. *p < 0.05. Data are the mean ± standard deviation. AUROC, area under the ROC curve; DPN, diabetic peripheral neuropathy; NAFLD, nonalcoholic fatty liver disease; ROC, receiver operating characteristic.
Discrimination power of liver fibrosis indices and fetuin-A for DPN
Supplementary Table S1 shows the discrimination power and various cut-off values of the NAFLD fibrosis score, FIB-4 index, and fetuin-A for DPN. Overall, the performance was not sufficient for use as a diagnostic tool for DPN.
Discussion
In this cross-sectional study, there was a lack of significant association between NAFLD liver fat score and DPN, but liver fibrosis indices such as NAFLD fibrosis index and FIB-4 index were higher in individuals with DPN than in individuals without DPN. In addition, even after adjustment for known DPN risk factors, the NAFLD fibrosis score and FIB-4 index were independently associated with DPN.
Previous studies have shown conflicting results regarding the association between NAFLD and DPN. Mantovani et al.16 used ultrasonography for the diagnosis of NAFLD, and they used the Michigan Neuropathy Screening Instrument (MNSI) method and a vibration perception threshold (VPT) assessment for the diagnosis of DPN. They showed a positive association between NAFLD and DPN in Italian individuals with type 1 diabetes (mean age 43.4 years, mean HbA1c 8.0%, and median diabetes duration 17 years). Lv et al.17 used ultrasonography for the diagnosis of NAFLD, and diagnosed DPN based on physical examination. They showed a negative association between NAFLD and DPN in hospitalised Chinese individuals with type 2 diabetes (mean age 63.4 years, mean HbA1c 8.7%, and mean diabetes duration 9.6 years). Kim et al.18 used ultrasonography for the diagnosis of NAFLD, and used a nerve conduction study, a current perception threshold test, and physical examination for the diagnosis of DPN. They showed no association between NAFLD and DPN in Korean individuals with type 2 diabetes (mean age 57.7 years, mean HbA1c 8.4%, and mean diabetes duration 6.2 years), which was consistent with our results. Potential explanations for these differences are the different characteristics of participants in each study and different diagnostic criteria for DPN. Otherwise, considering that the majority of individuals with NAFLD in previous studies were estimated to have NAFL, which is an early stage of NAFLD, other medical conditions or more severe stages of NAFLD might be more important contributors to DPN than NAFL per se.
A previous cohort study reported that an elevated lower-limb vibration perception threshold was associated with markers of liver fibrosis, such as the NAFLD fibrosis score, and with liver stiffness measurement in individuals with type 2 diabetes19. Our study is consistent with previous observations, and we further found that the association between DPN and liver fibrosis indices was only significant in individuals with a high NAFLD liver fat score (> − 0.640). This can be explained by increased vulnerability of the liver to injuries such as oxidative stress or cytokines, as reflected by higher BMI, AST, ALT, and HOMA-IR levels in individuals with a high NAFLD liver fat score (> − 0.640) compared with those with low NAFLD liver fat score (≤ − 0.640).
The ‘multiple hit hypothesis’ suggests that multiple insults might be generated in individuals with type 2 diabetes due to altered inter-organ crosstalk between the intestine, adipose tissue, skeletal muscle, liver, and pancreas, and that these insults would synergistically result in the development and progression of NAFLD20. During the development of NAFLD, hypercaloric diets can induce intestinal dysbiosis and excess fat storage in adipose tissue, skeletal muscle, and liver, which result in inflammation and insulin resistance. Insulin resistance results in hyperglycaemia and hyperinsulinaemia. During the progression of NAFLD, glucolipotoxicity increases reactive oxygen species (ROS) generation and endoplasmic reticulum stress, resulting in cell death. Together, these dead cells combined with infiltrated inflammatory cells in the liver, free fatty acids, intestine-derived lipopolysaccharides, and transforming growth factor (TGF)-β from Kupffer cells activate hepatic stellate cells (HSCs). Activated HSCs increase the extracellular matrix, leading to liver fibrosis. Among these insults related to the progression of NAFLD, hyperglycaemia, insulin resistance, oxidative stress, and inflammation are also involved in the pathogenesis of DPN21.
Advanced glycation end products (AGEs) are implicated in the pathogenesis of DPN. The formation of AGEs increases under chronic hyperglycaemia in diabetes. Interaction of AGEs with their receptors (RAGEs) activates intracellular signalling pathways and increases oxidative stress and inflammation, ultimately resulting in neuronal injuries22,23. Interestingly, patients with NASH exhibited higher hepatic and serum glyceraldehyde-derived AGEs levels than those with simple steatosis or healthy controls24. In addition, glyceraldehyde-derived AGEs increase ROS generation and upregulate fibrogenic genes such as α-smooth muscle actin, TGF-β1, and collagen type Iα2 in human hepatic stellate cell line in vitro25. These results suggest that glyceraldehyde-derived AGEs may contribute to the pathogenesis of NASH. Considering the potential role of AGEs and RAGEs in the pathogenesis of both DPN and NAFLD, our finding that the NAFLD fibrosis score and FIB-4 index were associated with DPN appears reasonable.
The progression of NAFLD alters the secretion of hepatokines such as fetuin-A, fetuin-B, and dipeptidyl peptidase-426,27, and we evaluated an association between fetuin-A and DPN. Serum fetuin-A levels were negatively associated with abnormal vibration perception and abnormal 10-g monofilament tests. Considering a previous study that showed TGF-β1 signalling suppression by fetuin-A28, and a previous study that showed high TGF-β1 levels in individuals with DPN29, our results seem to suggest a possibility of link between fetuin-A and DPN. Although, fetuin-A cannot be used as a diagnostic tool for DPN, this link suggests the possibility of loss of protection sensation.
This study has several limitations. First, it cannot establish a causal relationship because of its cross-sectional nature. Second, liver biopsy, the gold standard method for the diagnosis of NAFLD and liver fibrosis, was not performed. Third, neurophysiological studies were not used for the diagnosis of DPN. Despite these limitations, this study provides valuable insight implying that the progression of NAFL to liver fibrosis might affect the development of DPN and suggests the possible role of fetuin-A in specific feature of DPN, a loss of protection sensation.
In conclusion, liver fibrosis might be associated with DPN in individuals with type 2 diabetes and suspected NAFLD. Notably, this association was independent of previously known risk factors. The present study suggests the need for special attention to DPN in individuals with type 2 diabetes and NAFLD, especially those with a high NAFLD fibrosis score or FIB-4 index. Future studies to investigate the molecular mechanism of the association between liver fibrosis and DPN are necessary.
Methods
Study population
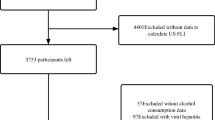
A prospective observational study is ongoing to discover reliable screening tools and biomarkers for DPN. The inclusion criteria were age ≥ 19 years, diagnosis of type 2 diabetes, and no change in glucose-lowering drugs in the last 3 months. The exclusion criteria were stage 4 or 5 chronic kidney disease (estimated glomerular filtration rate [eGFR] < 30 mL min−1 [1.73 m]−2), pregnancy, and severe diabetic foot ulcers or previous amputation. This is a subset study analysing data from individuals who were enrolled during the initial 3-year period (January 2017 to January 2020). We recruited 300 individuals with type 2 diabetes from Seoul National University Bundang Hospital (SNUBH), a tertiary academic hospital. In the present study, the following individuals were excluded: (1) individuals (n = 19) with cirrhosis of any etiology or chronic liver disease due to excessive alcohol consumption (alcohol consumption > 30 g/day for men and > 20 g/day for women) or viral hepatitis based on a medical history and medications; (2) individuals (n = 15) with incomplete data needed to calculate the NAFLD liver fat score, NAFLD fibrosis score, or FIB-4 index; and (3) individuals (n = 2) aged under 35 years due to poor performance of NAFLD fibrosis score and FIB-4 index for a diagnosis of liver fibrosis in those aged ≤ 35 years30. The remaining 264 individuals with type 2 diabetes were included in the final analysis (Fig. 2). The study was approved by the Institutional Review Board of SNUBH (no. B-2012-657-106), and was performed in accordance with relevant guidelines and regulations. All participants provided written informed consent.
Flow chart of the selection of individuals for the analysis. Among 264 individuals with type 2 diabetes, individuals with suspected NAFLD (n = 187) were selected based on the NAFLD liver fat score. They were divided into 2 groups according to the presence of DPN. DPN, diabetic peripheral neuropathy; NAFLD, nonalcoholic fatty liver disease.
Anthropometric and biochemical analyses
Anthropometric indices and neurologic tests were measured by a well-trained research nurse. BMI was calculated as weight (kg) divided by the square of the height (m). Waist circumference was measured at the midpoint between the margin of the lowest rib and the iliac crest. Systolic BP and diastolic BP were measured by an electronic blood pressure metre after 10 min of rest in a sitting position. Alcohol consumption was assessed by two questions from the Alcohol Use Disorders Identification Test-Consumption: (1) the usual frequency of drinking, (2) the typical quantity of drinking31. We defined drinkers as those who drink any alcoholic beverage more than once a month. Smoking status was classified as never smoker (< 100 cigarettes in lifetime and currently a nonsmoker), ex-smoker (≥ 100 cigarettes in lifetime and currently a nonsmoker), and current smoker (≥ 100 cigarettes in lifetime and currently a smoker). Positive exercise was defined as exercising for > 150 min/week. Blood samples were collected after an overnight fast. FPG levels were measured by the hexokinase method, and HbA1c levels were measured by high-performance liquid chromatography (Bio-Rad, Hercules, CA, USA). Serum insulin levels were measured by immunoradiometric assay (DIAsource, Nivelles, Belgium). Total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, and LDL cholesterol were measured by enzymatic colorimetric assay. Liver function tests, including AST and ALT, and renal function tests were measured by the protocol of the central laboratory of SNUBH. HOMA-IR was calculated using the following formula32: HOMA-IR = (fasting insulin [μIU/ml] × FPG [mg/dl]/405). Among individuals with a high NAFLD liver fat score (> -0.640), serum fetuin-A levels of 41 individuals with DPN and 41 individuals without DPN were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, no. DFTA00, Minneapolis, MN, USA).
Assessment of microvascular complications of diabetes
DPN was assessed using the MNSI, which includes two separate assessments: a 15-item self-administered questionnaire (MNSI-Q) and a lower extremity physical examination (MNSI-PE)33. The MNSI-PE is scored for abnormalities of foot appearance such as deformities, dry skin, calluses, infections and fissures (normal = 0, abnormal = 1), ulceration (absent = 0, present = 1), vibration perception at great toe (absent = 1, reduced = 0.5, present = 0), and ankle reflexes (absent = 1, present with reinforcement = 0.5, present = 0). The total possible score is 8 points for both feet. DPN was diagnosed when the MNSI-PE score was ≥ 2.5, based on prior studies34,35. A 10-g monofilament test was considered abnormal when an individual had a sensation of fewer than seven points on one of the two feet36. Abnormal appearance was defined as the presence of any abnormality except ulceration, as ulceration was defined separately. Ankle reflexes were tested using a tendon hammer at the Achilles tendon. Abnormal vibration perception was defined as the absence of vibration perception on either side of the great toe using a 128-Hz tuning fork. A trained nurse performed all neurologic examinations.
Noninvasive methods for evaluating NAFLD and liver fibrosis
The NAFLD liver fat score was calculated according to the following formula: − 2.89 + 1.18 × metabolic syndrome (yes = 1, no = 0) + 0.45 × type 2 diabetes (yes = 2, no = 0) + 0.15 × fasting serum insulin (IU/l) + 0.04 × AST (U/l) − 0.94 × AST/ALT. A NAFLD liver fat score > − 0.640 was used to identify suspected NAFLD according to a previous report that a score > − 0.640 detected NAFLD with a sensitivity of 86% and specificity of 71%37. The NAFLD fibrosis score was calculated according to the following formula: − 1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose or diabetes (yes = 1, no = 0) + 0.99 × AST/ALT − 0.013 × platelets (109/l) − 0.66 × albumin (g/dl). A NAFLD fibrosis score > 0.676 was used to identify liver fibrosis38. The FIB-4 index was calculated according to the following formula: (age [years] × AST [U/l])/(platelets [109/l] × ALT1/2 [U/l]). A FIB-4 index ≥ 1.3 was used to identify liver fibrosis39. However, for individuals aged ≥ 65 years, a FIB-4 index ≥ 2.0 was used to identify liver fibrosis as previously reported30.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) or number (%). Variables with a nonnormal distribution were log-transformed prior to analysis. Comparisons of continuous variables between individuals with and without DPN were performed using Student’s unpaired t tests. Categorical variables were compared using χ2 tests. The associations between the presence of DPN and NAFLD liver fat score, NAFLD fibrosis score, and FIB-4 index were analysed using logistic regression models. Multivariable logistic regression analysis was performed including known risk factors for DPN. The prediction performance of liver fibrosis indices and serum fetuin-A levels for DPN and for the absence of abnormal vibration perception or absence of abnormal 10-g monofilament test was assessed by analysing receiver operating characteristic (ROC) curves, and the AUROC was calculated. Based on various cut-off values, we calculated the sensitivity, specificity, positive predictive value, and negative predictive value. In all cases, p < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS version 25.0 (IBM, Armonk, NY, USA). Figures were drawn using GraphPad Prism software (version 9.1.2; GraphPad Software Inc., San Diego, CA, USA).
References
Marjot, T., Moolla, A., Cobbold, J. F., Hodson, L. & Tomlinson, J. W. Nonalcoholic fatty liver disease in adults: Current concepts in etiology, outcomes, and management. Endocr. Rev. https://doi.org/10.1210/endrev/bnz009 (2020).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344. https://doi.org/10.1038/nrgastro.2013.41 (2013).
McPherson, S. et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 62, 1148–1155. https://doi.org/10.1016/j.jhep.2014.11.034 (2015).
Caldwell, S. H. et al. Cryptogenic cirrhosis: Clinical characterization and risk factors for underlying disease. Hepatology 29, 664–669. https://doi.org/10.1002/hep.510290347 (1999).
Hossain, N. et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 1224–1229. https://doi.org/10.1016/j.cgh.2009.06.007 (2009).
Lee, B. W. et al. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a position statement of the fatty liver research group of the Korean diabetes association. Diabetes Metab. J. 44, 382–401. https://doi.org/10.4093/dmj.2020.0010 (2020).
Ciardullo, S. & Perseghin, G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabolism 121, 154752. https://doi.org/10.1016/j.metabol.2021.154752 (2021).
Targher, G. et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 30, 2119–2121. https://doi.org/10.2337/dc07-0349 (2007).
Targher, G. et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30, 1212–1218. https://doi.org/10.2337/dc06-2247 (2007).
Targher, G. et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 51, 444–450. https://doi.org/10.1007/s00125-007-0897-4 (2008).
Targher, G. et al. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J. Am. Soc. Nephrol. 19, 1564–1570. https://doi.org/10.1681/ASN.2007101155 (2008).
Han, E., Kim, M. K., Jang, B. K. & Kim, H. S. Albuminuria is associated with steatosis burden in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Diabetes Metab. J https://doi.org/10.4093/dmj.2020.0118 (2021).
Tesfaye, S. et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33, 2285–2293. https://doi.org/10.2337/dc10-1303 (2010).
Papanas, N. & Ziegler, D. Risk factors and comorbidities in diabetic neuropathy: An update 2015. Rev. Diabet. Stud. 12, 48–62. https://doi.org/10.1900/RDS.2015.12.48 (2015).
Haukeland, J. W. et al. Fetuin a in nonalcoholic fatty liver disease: In vivo and in vitro studies. Eur. J. Endocrinol. 166, 503–510. https://doi.org/10.1530/EJE-11-0864 (2012).
Mantovani, A. et al. Nonalcoholic fatty liver disease is associated with an increased prevalence of distal symmetric polyneuropathy in adult patients with type 1 diabetes. J. Diabetes Complicat 31, 1021–1026. https://doi.org/10.1016/j.jdiacomp.2017.01.024 (2017).
Lv, W. S. et al. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J. Gastroenterol. 19, 3134–3142. https://doi.org/10.3748/wjg.v19.i20.3134 (2013).
Kim, B. Y., Jung, C. H., Mok, J. O., Kang, S. K. & Kim, C. H. Prevalences of diabetic retinopathy and nephropathy are lower in Korean type 2 diabetic patients with non-alcoholic fatty liver disease. J. Diabetes Investig. 5, 170–175. https://doi.org/10.1111/jdi.12139 (2014).
Williams, K. H. et al. An association of large-fibre peripheral nerve dysfunction with non-invasive measures of liver fibrosis secondary to non-alcoholic fatty liver disease in diabetes. J. Diabetes Complicat. 29, 1240–1247. https://doi.org/10.1016/j.jdiacomp.2015.06.015 (2015).
Dewidar, B., Kahl, S., Pafili, K. & Roden, M. Metabolic liver disease in diabetes: From mechanisms to clinical trials. Metabolism 111S, 154299. https://doi.org/10.1016/j.metabol.2020.154299 (2020).
Sloan, G., Selvarajah, D. & Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 17, 400–420. https://doi.org/10.1038/s41574-021-00496-z (2021).
Rhee, S. Y. & Kim, Y. S. The role of advanced glycation end products in diabetic vascular complications. Diabetes Metab. J. 42, 188–195. https://doi.org/10.4093/dmj.2017.0105 (2018).
Vincent, A. M. et al. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology 148, 548–558. https://doi.org/10.1210/en.2006-0073 (2007).
Hyogo, H. et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol 22, 1112–1119. https://doi.org/10.1111/j.1440-1746.2007.04943.x (2007).
Iwamoto, K. et al. Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J Gastroenterol 43, 298–304. https://doi.org/10.1007/s00535-007-2152-7 (2008).
Peter, A. et al. The hepatokines fetuin-A and fetuin-B are upregulated in the state of hepatic steatosis and may differently impact on glucose homeostasis in humans. Am. J. Physiol. Endocrinol. Metab. 314, E266–E273. https://doi.org/10.1152/ajpendo.00262.2017 (2018).
Barchetta, I. et al. Circulating dipeptidyl peptidase-4 is independently associated with the presence and severity of NAFLD/NASH in individuals with and without obesity and metabolic disease. J. Endocrinol. Invest. https://doi.org/10.1007/s40618-020-01392-5 (2020).
Sato, M. et al. Fetuin-A negatively correlates with liver and vascular fibrosis in nonalcoholic fatty liver disease subjects. Liver Int 35, 925–935. https://doi.org/10.1111/liv.12478 (2015).
Ybarra, J., Pou, J. M., Romeo, J. H., Merce, J. & Jurado, J. Transforming growth factor beta 1 as a biomarker of diabetic peripheral neuropathy: Cross-sectional study. J. Diabetes Complicat. 24, 306–312. https://doi.org/10.1016/j.jdiacomp.2009.07.007 (2010).
McPherson, S. et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. J. Gastroenterol. 112, 740–751. https://doi.org/10.1038/ajg.2016.453 (2017).
Bush, K., Kivlahan, D. R., McDonell, M. B., Fihn, S. D. & Bradley, K. A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory care quality improvement project (ACQUIP). Alcohol use disorders identification test. Arch. Intern. Med. 158, 1789–1795. https://doi.org/10.1001/archinte.158.16.1789 (1998).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. https://doi.org/10.1007/BF00280883 (1985).
Feldman, E. L. et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 17, 1281–1289. https://doi.org/10.2337/diacare.17.11.1281 (1994).
Herman, W. H. et al. Use of the michigan neuropathy screening instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: Results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabet Med. 29, 937–944. https://doi.org/10.1111/j.1464-5491.2012.03644.x (2012).
Lunetta, M., Le Moli, R., Grasso, G. & Sangiorgio, L. A simplified diagnostic test for ambulatory screening of peripheral diabetic neuropathy. Diabetes Res. Clin. Pract. 39, 165–172. https://doi.org/10.1016/s0168-8227(98)00005-9 (1998).
Won, J. C. & Park, T. S. Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol. Metab. (Seoul) 31, 230–238. https://doi.org/10.3803/EnM.2016.31.2.230 (2016).
Kotronen, A. et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 137, 865–872. https://doi.org/10.1053/j.gastro.2009.06.005 (2009).
Angulo, P. et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45, 846–854. https://doi.org/10.1002/hep.21496 (2007).
Vallet-Pichard, A. et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 46, 32–36. https://doi.org/10.1002/hep.21669 (2007).
Acknowledgements
The authors sincerely thank all the participants for their cooperation. We thank Yoojung Song (Seoul National University Bundang Hospital, Seongnam, Korea) for assistance with data registration and analysis.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (No. NRF-2020R1C1C1013766).
Author information
Authors and Affiliations
Contributions
K.K. conducted the study, acquired and analysed the data, and drafted the manuscript. H.C.C., Y.K.L., C.H.A., B.K.K., J.H.M., S.H.C., and H.C.J. contributed to the conception and design of the study and contributed to the production and interpretation of data. T.J.O. is the guarantor of the manuscript. All authors critically reviewed the manuscript and approved this version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, K., Oh, T.J., Cho, H.C. et al. Liver fibrosis indices are related to diabetic peripheral neuropathy in individuals with type 2 diabetes. Sci Rep 11, 24372 (2021). https://doi.org/10.1038/s41598-021-03870-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03870-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.